An Accelerated Release Study to Evaluate Long-Acting Contraceptive Levonorgestrel-Containing in Situ Forming Depot Systems
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Preparation of Injectable ISD Formulations
2.3. Real-Time (Long-Term) in Vitro Drug Release Study
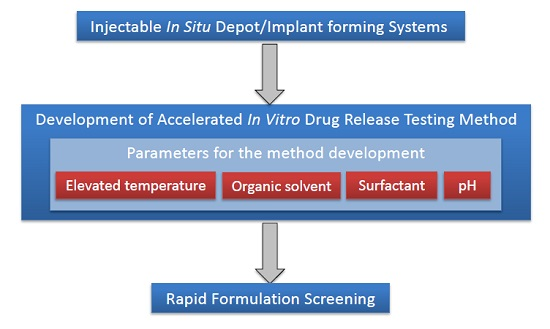
2.4. Accelerated (Short-Term) in Vitro Release Study
2.5. Drug Analysis
3. Results and Discussion
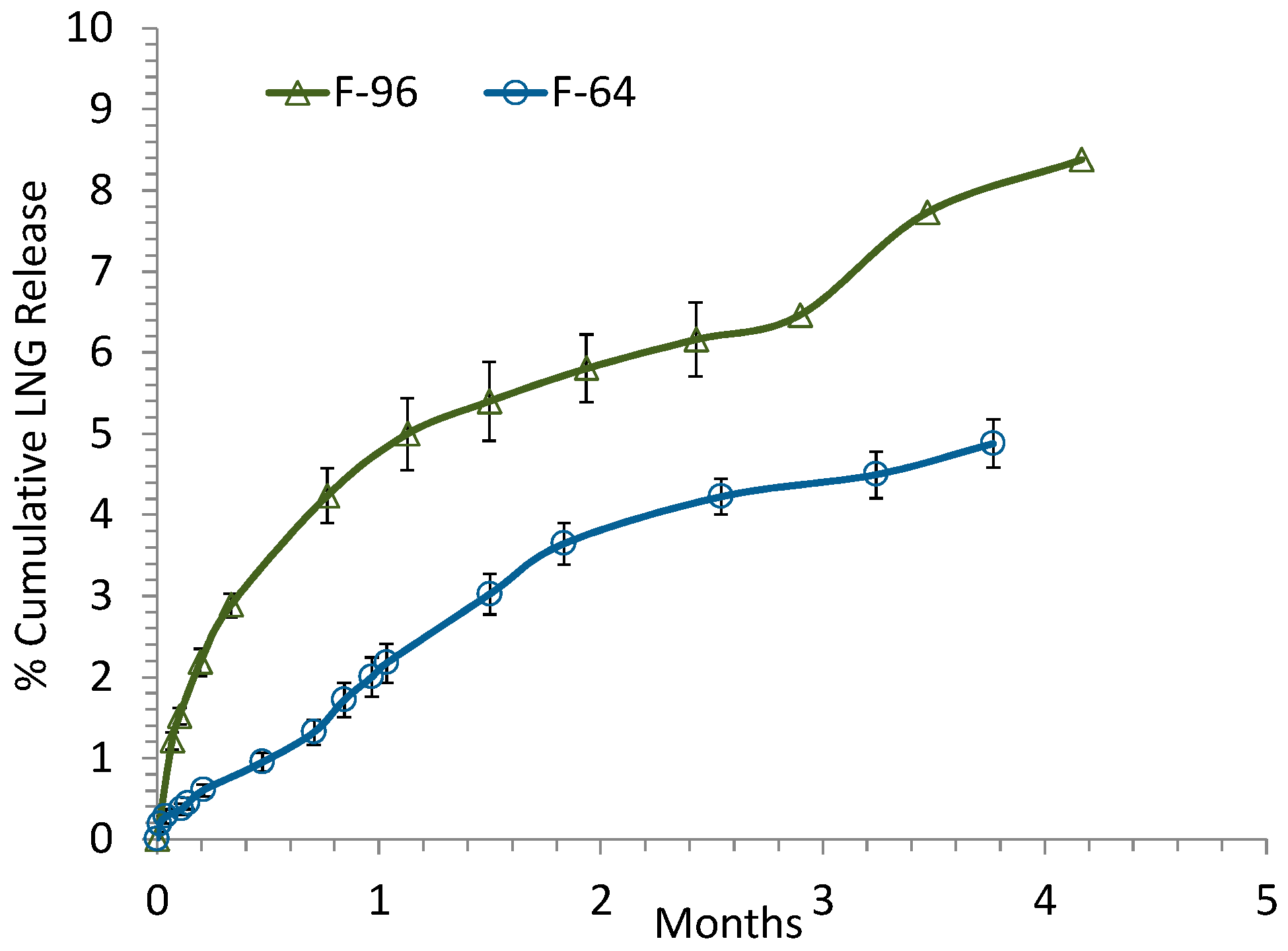
3.1. Real-Time (Long-Term) in Vitro Release
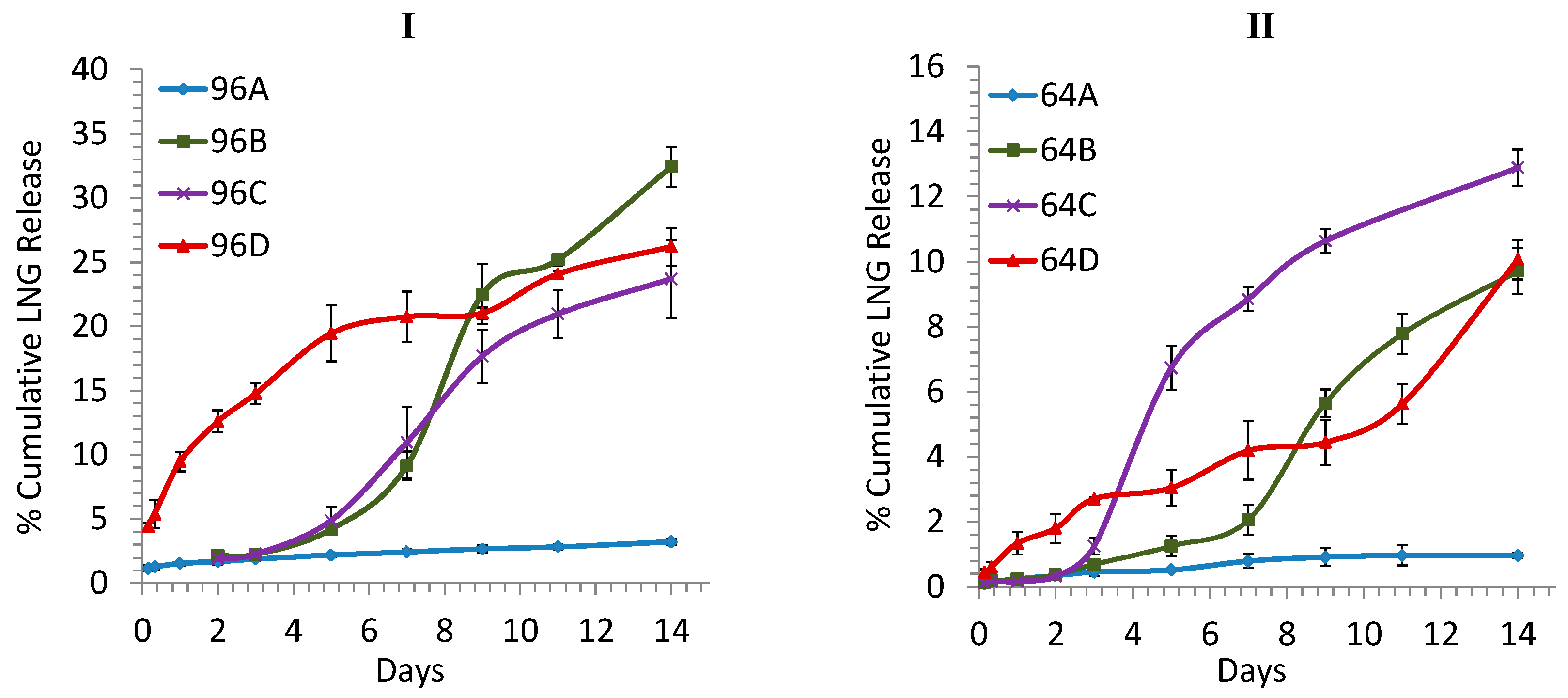
3.2. Accelerated (Short-Term) in Vitro Release
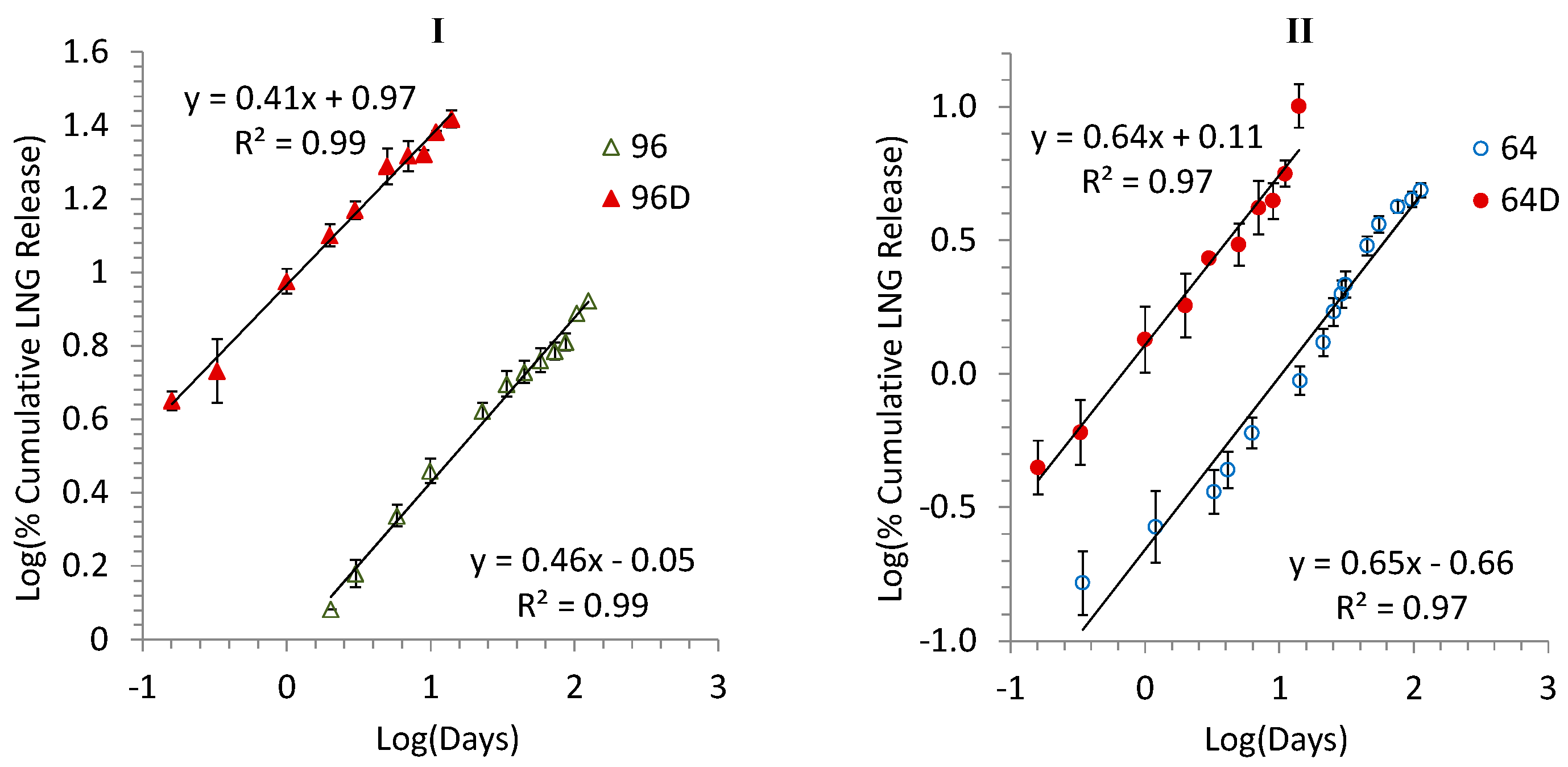
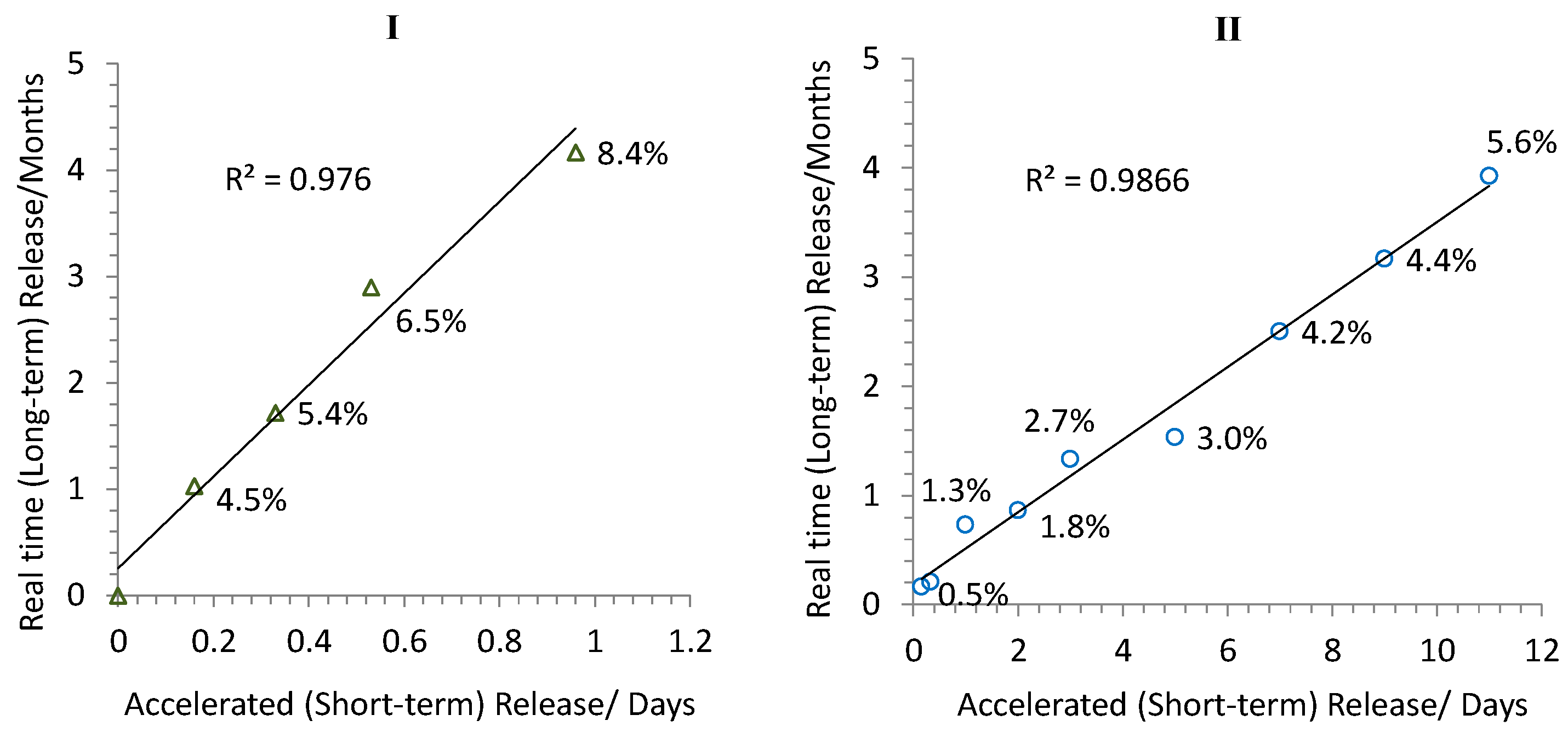
3.3. Release Kinetics and Correlation between Accelerated and Real-Time Releases
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Dunn, R.L.; English, J.P.; Cowsar, D.R.; Vanderbilt, D.P. Biodegradable in-Situ Forming Implants and Methods of Producing the Same. U.S. Patent 4,938,763 A, 3 July 1990. [Google Scholar]
- Dunn, R.L.; English, J.P.; Cowsar, D.R.; Vanderbilt, D.D. Biodegradable in-Situ Forming Implants and Methods of Producing the Aame. U.S. Patent 5,990,194 A, 23 November 1999. [Google Scholar]
- Graham, P.; Brodbeck, K.; McHugh, A. Phase inversion dynamics of PLGA solutions related to drug delivery. J. Control. Release 1999, 58, 233–245. [Google Scholar] [CrossRef]
- Kempe, S.; Mäder, K. In situ forming implants—an attractive formulation principle for parenteral depot formulations. J. Control. Release 2012, 161, 668–679. [Google Scholar] [CrossRef] [PubMed]
- Hatefi, A.; Amsden, B. Biodegradable injectable in situ forming drug delivery systems. J. Control. Release 2002, 80, 9–28. [Google Scholar] [CrossRef]
- Wu, L.; Janagam, D.R.; Mandrell, T.D.; Johnson, J.R.; Lowe, T.L. Long-acting injectable hormonal dosage forms for contraception. Pharm. Res. 2015, 32, 2180–2191. [Google Scholar] [CrossRef] [PubMed]
- Thakur, R.R.S.; McMillan, H.L.; Jones, D.S. Solvent induced phase inversion-based in situ forming controlled release drug delivery implants. J. Control. Release 2014, 176, 8–23. [Google Scholar] [CrossRef] [PubMed]
- Patel, R.B.; Solorio, L.; Wu, H.; Krupka, T.; Exner, A.A. Effect of injection site on in situ implant formation and drug release in vivo. J. Control. Release 2010, 147, 350–358. [Google Scholar] [CrossRef] [PubMed]
- Patel, R.B.; Carlson, A.N.; Solorio, L.; Exner, A.A. Characterization of formulation parameters affecting low molecular weight drug release from in situ forming drug delivery systems. J. Biomed. Mater. Res. A 2010, 94, 476–484. [Google Scholar]
- Parent, M.; Nouvel, C.; Koerber, M.; Sapin, A.; Maincent, P.; Boudier, A. PLGA in situ implants formed by phase inversion: Critical physicochemical parameters to modulate drug release. J. Control. Release 2013, 172, 292–304. [Google Scholar] [CrossRef] [PubMed]
- Zolnik, B.S.; Leary, P.E.; Burgess, D.J. Elevated temperature accelerated release testing of PLGA microspheres. J. Control. Release 2006, 112, 293–300. [Google Scholar] [CrossRef] [PubMed]
- Shen, J.; Burgess, D.J. Accelerated in-vitro release testing methods for extended-release parenteral dosage forms. J. Pharm. Pharmacol. 2012, 64, 986–996. [Google Scholar] [CrossRef] [PubMed]
- Wheaton, C.; Savage, A.; Shukla, A.; Neiffer, D.; Qu, W.; Sun, Y.; Lasley, B. The use of long acting subcutaneous levonorgestrel (lng) gel depot as an effective contraceptive option for cotton-top tamarins (saguinus oedipus). Zoo Biol. 2011, 30, 498–522. [Google Scholar] [CrossRef] [PubMed]
- Iyer, S.S.; Barr, W.H.; Karnes, H.T. A ‘biorelevant’ approach to accelerated in vitro drug release testing of a biodegradable, naltrexone implant. Int. J. Pharm. 2007, 340, 119–125. [Google Scholar] [CrossRef] [PubMed]
- Shen, J.; Burgess, D.J. Accelerated in vitro release testing of implantable PLGA microsphere/PVA hydrogel composite coatings. Int. J. Pharm. 2012, 422, 341–348. [Google Scholar] [CrossRef] [PubMed]
- Alexis, F. Factors affecting the degradation and drug-release mechanism of poly(lactic acid) and poly[(lactic acid)-co-(glycolic acid)]. Polym. Int. 2005, 54, 36–46. [Google Scholar] [CrossRef]
- Agrawal, C.; Huang, D.; Schmitz, J.; Athanasiou, K. Elevated temperature degradation of a 50:50 copolymer of PLA-PGA. Tissue Eng. 1997, 3, 345–352. [Google Scholar] [CrossRef]
- Faisant, N.; Akiki, J.; Siepmann, F.; Benoit, J.; Siepmann, J. Effects of the type of release medium on drug release from PLGA-based microparticles: Experiment and theory. Int. J. Pharm. 2006, 314, 189–197. [Google Scholar] [CrossRef] [PubMed]
- Shen, J.; Lee, K.; Choi, S.; Qu, W.; Wang, Y.; Burgess, D.J. A reproducible accelerated in vitro release testing method for PLGA microspheres. Int. J. Pharm. 2016, 498, 274–282. [Google Scholar] [CrossRef] [PubMed]
- Duda, J.; Zielinski, J.M. Free-volume theory. Plast. Eng. N. Y. 1996, 32, 143–171. [Google Scholar]
- Aso, Y.; Yoshioka, S.; Po, A.L.W.; Terao, T. Effect of temperature on mechanisms of drug release and matrix degradation of poly(d,l-lactide) microspheres. J. Control. Release 1994, 31, 33–39. [Google Scholar] [CrossRef]
- Li, S.; McCarthy, S. Further investigations on the hydrolytic degradation of poly(d,l-lactide). Biomaterials 1999, 20, 35–44. [Google Scholar] [CrossRef]
- Buchholz, B. Accelerated degradation test on resorbable polymers. In Degradation Phenomena on Polymeric Biomaterials; Springer: Berlin, Germany, 1992; pp. 67–76. [Google Scholar]
- Makino, K.; ARAKAwA, M.; Kondo, T. Preparation and in vitro degradation properties of polylactide microcapsules. In Membranes and Membrane Processes; Springer: Berlin, Germany, 1986; pp. 371–377. [Google Scholar]
- Bergsma, J.; Rozema, F.; Bos, R.; Boering, G.; Joziasse, C.; Pennings, A. In vitro predegradation at elevated temperatures of poly(lactide). J. Mater. Sci. Mater. Med. 1995, 6, 642–646. [Google Scholar] [CrossRef]
- D’Souza, S.S.; Faraj, J.A.; DeLuca, P.P. A model-dependent approach to correlate accelerated with real-time release from biodegradable microspheres. AAPS PharmSciTech 2005, 6, E553–E564. [Google Scholar] [CrossRef] [PubMed]
- Hakkarainen, M.; Albertsson, A.-C.; Karlsson, S. Weight losses and molecular weight changes correlated with the evolution of hydroxyacids in simulated in vivo degradation of homo-and copolymers of PLA and PGA. Polym. Degrad. Stab. 1996, 52, 283–291. [Google Scholar] [CrossRef]
- Kamberi, M.; Nayak, S.; Myo-Min, K.; Carter, T.P.; Hancock, L.; Feder, D. A novel accelerated in vitro release method for biodegradable coating of drug eluting stents: Insight to the drug release mechanisms. Eur. J. Pharm. Sci. 2009, 37, 217–222. [Google Scholar] [CrossRef] [PubMed]
- Makino, K.; Ohshima, H.; Kondo, T. Mechanism of hydrolytic degradation of poly(l-lactide) microcapsules: Effects of pH, ionic strength and buffer concentration. J. Microencapsul. 1986, 3, 203–212. [Google Scholar] [CrossRef] [PubMed]
- Nokhodchi, A.; Hassan-Zadeh, D.; Monajjem-Zadeh, F.; Taghi-Zadeh, N. Effect of various surfactants and their concentration on controlled release of captopril from polymeric matrices. Acta Pharm. 2008, 58, 151–162. [Google Scholar] [CrossRef] [PubMed]
- Nokhodchi, A.; Norouzi-Sani, S.; Siahi-Shadbad, M.-R.; Lotfipoor, F.; Saeedi, M. The effect of various surfactants on the release rate of propranolol hydrochloride from hydroxypropylmethylcellulose (HPMC)-eudragit matrices. Eur. J. Pharm. Biopharm. 2002, 54, 349–356. [Google Scholar] [CrossRef]
- Zambito, Y.; Pedreschi, E.; Di Colo, G. Is dialysis a reliable method for studying drug release from nanoparticulate systems?—A case study. Int. J. Pharm. 2012, 434, 28–34. [Google Scholar] [CrossRef] [PubMed]
- Messaritaki, A.; Black, S.J.; van der Walle, C.F.; Rigby, S.P. NMR and confocal microscopy studies of the mechanisms of burst drug release from PLGA microspheres. J. Control. Release 2005, 108, 271–281. [Google Scholar] [CrossRef] [PubMed]
- Shameem, M.; Lee, H.; DeLuca, P.P. A short-term (accelerated release) approach to evaluate peptide release from PLGA depot formulations. AAPS PharmSci. 1999, 1, 1–6. [Google Scholar] [CrossRef]
- Cort, T.L.; Song, M.-S.; Bielefeldt, A.R. Nonionic surfactant effects on pentachlorophenol biodegradation. Water Res. 2002, 36, 1253–1261. [Google Scholar] [CrossRef]
- Lee, D.-H.; Kim, E.-S.; Chang, H.-W. Effect of tween surfactant components for remediation of toluene-contaminated groundwater. Geosci. J. 2005, 9, 261–267. [Google Scholar] [CrossRef]
- Li, J.; Jiang, G.; Ding, F. The effect of pH on the polymer degradation and drug release from PLGA-MPEG microparticles. J. Appl. Polym. Sci. 2008, 109, 475–482. [Google Scholar] [CrossRef]
- Cardoso, J.; Queirós, Y.; Machado, K.; Costa, J.; Lucas, E. Synthesis, characterization, and in vitro degradation of poly(lactic acid) under petroleum production conditions. Braz. J. Pet. Gas 2013, 7. [Google Scholar] [CrossRef]
- Brown, C.K.; Friedel, H.D.; Barker, A.R.; Buhse, L.F.; Keitel, S.; Cecil, T.L.; Kraemer, J.; Morris, J.M.; Reppas, C.; Stickelmeyer, M.P. FIP/AAPS joint workshop report: Dissolution/in vitro release testing of novel/special dosage forms. AAPS PharmSciTech 2011, 12, 782–794. [Google Scholar] [CrossRef] [PubMed]
- Ritger, P.L.; Peppas, N.A. A simple equation for description of solute release II. Fickian and anomalous release from swellable devices. J. Control. Release 1987, 5, 37–42. [Google Scholar] [CrossRef]
- Huang, X.; Lowe, T.L. Biodegradable thermoresponsive hydrogels for aqueous encapsulation and controlled release of hydrophilic model drugs. Biomacromolecules 2005, 6, 2131–2139. [Google Scholar] [CrossRef] [PubMed]
- Colombo, P.; Bettini, R.; Catellani, P.L.; Santi, P.; Peppas, N.A. Drug volume fraction profile in the gel phase and drug release kinetics in hydroxypropylmethyl cellulose matrices containing a soluble drug. Eur. J. Pharm. Sci. 1999, 9, 33–40. [Google Scholar] [CrossRef]
| Formulation # | PLGA 50:50 wt % | PLA wt % | LNG wt % | Solvent (9:1) wt % |
|---|---|---|---|---|
| 96 | 4.1 (iv − 0.63) | 20.3 (iv − 0.47) | 2.5 | 73.1 NMP/BB (9:1) |
| 64 | 4.7 (iv − 0.63) | 18.8 (iv − 0.63) | 6 | 70.5 NMP/TEC (9:1) |
| Condition | PBS (% v/v) | Ethanol (% v/v) | Tween 20 (% w/v) | pH | Temperature (°C) |
|---|---|---|---|---|---|
| A | 100% | 0 | 0 | 7.4 | 50 |
| B | Adjusted to 100% | 25% | 0 | 7.4 | 50 |
| C | Adjusted to 100% | 25% | 0.5% | 7.4 | 50 |
| D | Adjusted to 100% | 25% | 0.5% | 9.0 | 50 |
| Formulation (Release Condition) | Power Law (Korsmeyer–Peppas Model) | ||
|---|---|---|---|
| r2 (Coefficient of Determination) | k (Rate Constant) | n (Release Exponent) | |
| 96(A) | 0.96 | 1.62 | 0.22 |
| 96(B) | 0.94 | 0.50 | 1.59 |
| 96(C) | 0.97 | 0.57 | 1.15 |
| 96(D) | 0.99 | 9.23 | 0.41 |
| 96 (long-term) | 0.99 | 0.90 | 0.46 |
| 64(A) | 0.99 | 0.26 | 0.52 |
| 64(B) | 0.86 | 0.41 | 0.97 |
| 64(C) | 0.88 | 0.49 | 1.24 |
| 64(D) | 0.97 | 1.28 | 0.64 |
| 64 (long-term) | 0.97 | 0.22 | 0.65 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Janagam, D.R.; Wang, L.; Ananthula, S.; Johnson, J.R.; Lowe, T.L. An Accelerated Release Study to Evaluate Long-Acting Contraceptive Levonorgestrel-Containing in Situ Forming Depot Systems. Pharmaceutics 2016, 8, 28. https://doi.org/10.3390/pharmaceutics8030028
Janagam DR, Wang L, Ananthula S, Johnson JR, Lowe TL. An Accelerated Release Study to Evaluate Long-Acting Contraceptive Levonorgestrel-Containing in Situ Forming Depot Systems. Pharmaceutics. 2016; 8(3):28. https://doi.org/10.3390/pharmaceutics8030028
Chicago/Turabian StyleJanagam, Dileep R., Lizhu Wang, Suryatheja Ananthula, James R. Johnson, and Tao L. Lowe. 2016. "An Accelerated Release Study to Evaluate Long-Acting Contraceptive Levonorgestrel-Containing in Situ Forming Depot Systems" Pharmaceutics 8, no. 3: 28. https://doi.org/10.3390/pharmaceutics8030028