Liposome-Based Adjuvants for Subunit Vaccines: Formulation Strategies for Subunit Antigens and Immunostimulators
Abstract
:1. Introduction
2. The Physicochemical Properties of Liposomes Affect the Immune Responses
2.1. The Effects of Particle Size
2.2. Effect of Surface Charge
2.3. Effect of Surface Modification
2.4. Effect of Lipid Bilayer Fluidity
3. Pathogen-Derived Immunostimulators Are Ligands for Pattern-Recognition Receptors
4. Incorporation Strategies for Amphiphilic Lipids and Hydrophobic Compounds
5. Incorporation Strategies for Nucleic Acids
5.1. RNA/DNA-Based Immunostimulators
5.2. DNA–Antigen Vectors
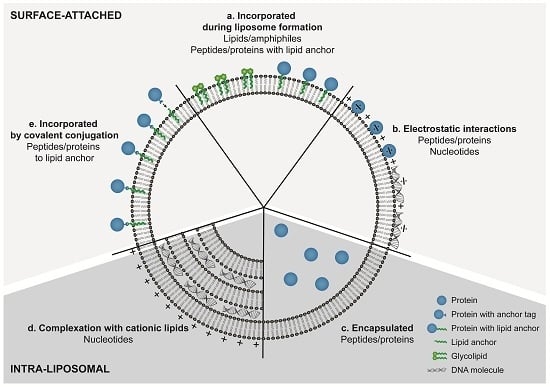
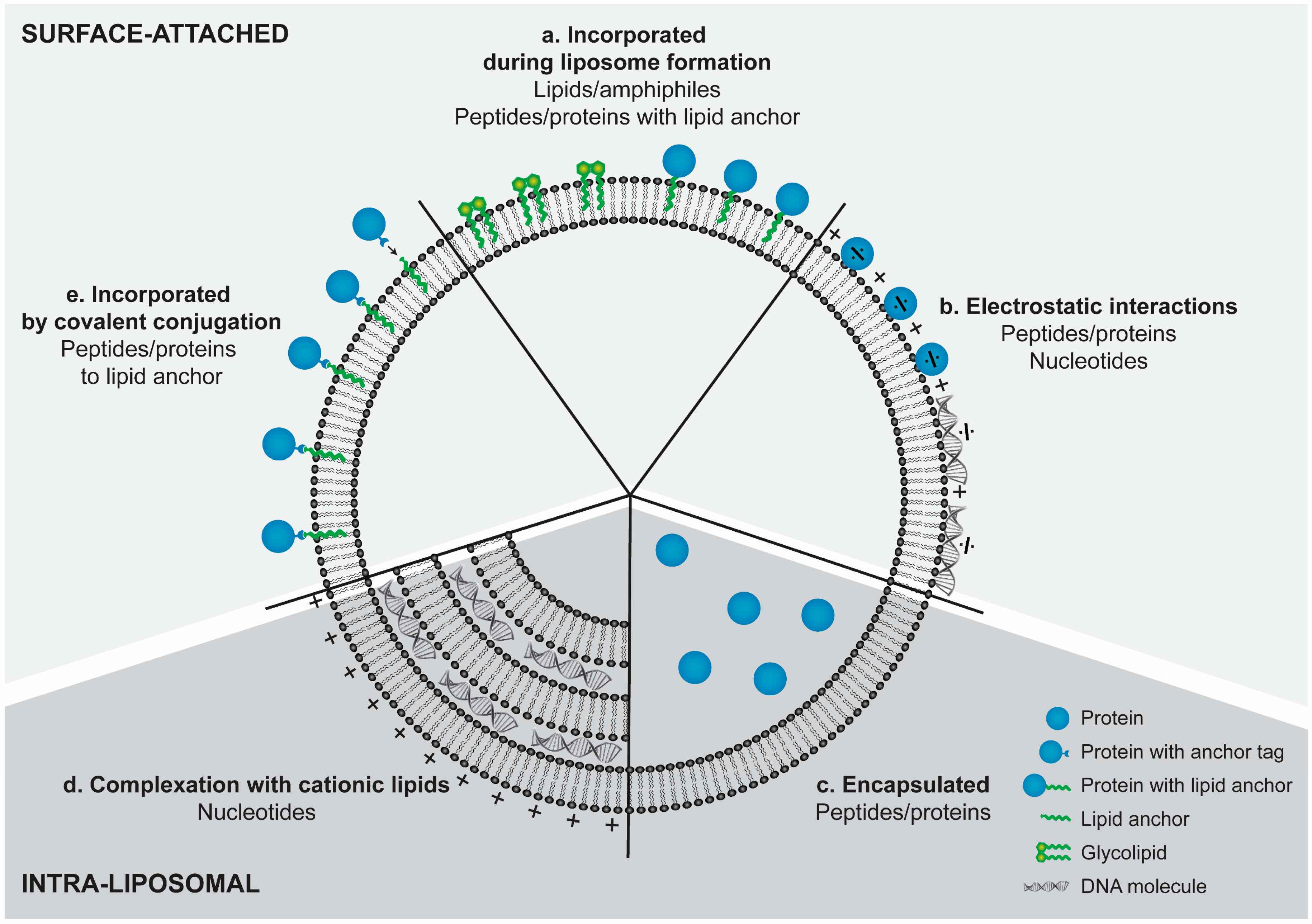
6. Incorporation Strategies for Peptides and Proteins
7. Clinical Experience with Liposome-Based Adjuvants
8. Future Perspectives: Challenges in the Further Development of Liposome-Based Adjuvants
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| APC | Antigen-presenting cell |
| Chol | Cholesterol |
| CLR | C-type lectin receptor |
| CMI | Cell-mediated immunity |
| CpG-ODN | CpG oligodeoxynucleotide |
| CTL | Cytotoxic T-lymphocyte |
| DC | Dendritic cell |
| DCP | Dicetyl phosphate |
| DDA | Dimethyldioctadecylammonium bromide |
| DMPA | Dimyristoyl phosphatidic acid |
| DMPC | Dimyristoyl phosphatidylcholine |
| DMPG | Dimyristoyl phosphatidylglycerol |
| DMPS | Dimyristoyl phosphatidylserine |
| DMSO | Dimethyl sulfoxide |
| DMTAP | Dimyristoyl trimethylammonium-propane |
| DODAC | Dioleyldimethylammonium chloride |
| DODAP | Dioleoyl dimethylammonium propanediol |
| DOPC | Dioleolyl phosphatidylcholine |
| DOPE | Dioleoyl phosphoethanolamine |
| DOPS | Dioleolyl phosphatidylserine |
| DOTAP | Dioleoyl trimethylammonium-propane |
| DPPC | Dipalmitoyl phosphatidylcholine |
| DSPC | Distearoyl phosphatidylcholine |
| DSPE | Distearoyl phosphoethanolamine |
| DSPG | Distearoyl phosphatidylglycerol |
| dsRNA | Double-stranded RNA |
| DT | Diphtheria toxoid |
| EPC | Egg-phosphatidylcholine |
| EPG | Egg-phosphatidylglycerol |
| GLA | Glucopyranosyl lipid adjuvant |
| HA | Hemagglutinin |
| IFN | Interferon |
| i.m. | Intramuscular |
| LPS | Lipopolysaccharide |
| MHC | Major histocompatibility complex |
| MMG | Monomycoloyl glycerol |
| MPL | Monophosphoryl lipid-A |
| NA | Neuraminidase |
| NLR | NOD-like receptor |
| OVA | Chicken egg ovalbumin |
| PA | Phosphatidic acid |
| PAMP | Pathogen-associated molecular pattern |
| PC | Phosphatidylcholine |
| PDI | Polydispersity index |
| pDNA | Plasmid DNA |
| PE | Phosphatidylethanolamine |
| PEG | Polyethylene glycol |
| pI | Isoelectric point |
| Poly(I:C) | Polyinosinic:polycytidylic acid |
| PPD | Purified protein derivative |
| PRR | Pattern recognition receptor |
| RLR | RIG-I-like receptor |
| SA | Stearylamine |
| s.c. | Subcutaneous |
| SOI | Site of injection |
| TDB | Trehalose-6,6´-dibehenate |
| Th | T-helper cell |
| Tfh | Follicular T-helper cell |
| TLR | Toll-like receptor |
| Tm | Main phase transition temperature |
| Zp | Zeta-potential |
References
- Rappuoli, R.; Miller, H.I.; Falkow, S. The intangible value of vaccination. Science 2002, 297, 937–939. [Google Scholar] [CrossRef] [PubMed]
- Rappuoli, R. Bridging the knowledge gaps in vaccine design. Nat. Biotech. 2007, 25, 1361–1366. [Google Scholar] [CrossRef] [PubMed]
- Di Pasquale, A.; Preiss, S.; Tavares Da Silva, F.; Garçon, N. Vaccine adjuvants: From 1920 to 2015 and beyond. Vaccines 2015, 3, 320–343. [Google Scholar] [CrossRef] [PubMed]
- Zepp, F. Principles of vaccine design—lessons from nature. Vaccine 2010, 28 (Suppl. 3), C14–C24. [Google Scholar] [CrossRef] [PubMed]
- Dey, A.K.; Srivastava, I.K. Novel adjuvants and delivery systems for enhancing immune responses induced by immunogens. Expert Rev. Vaccines 2010, 10, 227–251. [Google Scholar] [CrossRef] [PubMed]
- Petrovsky, N.; Aguilar, J.C. Vaccine adjuvants: Current state and future trends. Immunol. Cell Biol. 2004, 82, 488–496. [Google Scholar] [CrossRef] [PubMed]
- Perrie, Y.; Mohammed, A.R.; Kirby, D.J.; McNeil, S.E.; Bramwell, V.W. Vaccine adjuvant systems: Enhancing the efficacy of sub-unit protein antigens. Int. J. Pharm. 2008, 364, 272–280. [Google Scholar] [CrossRef] [PubMed]
- Mohan, T.; Verma, P.; Rao, D.N. Novel adjuvants & delivery vehicles for vaccines development: A road ahead. Indian J. Med. Res. 2013, 138, 779–795. [Google Scholar] [PubMed]
- Kamath, A.T.; Mastelic, B.; Christensen, D.; Rochat, A.-F.; Agger, E.M.; Pinschewer, D.D.; Andersen, P.; Lambert, P.-H.; Siegrist, C.-A. Synchronization of dendritic cell activation and antigen exposure is required for the induction of th1/th17 responses. J. Immunol. 2012, 188, 4828–4837. [Google Scholar] [CrossRef] [PubMed]
- de Titta, A.; Ballester, M.; Julier, Z.; Nembrini, C.; Jeanbart, L.; van der Vlies, A.J.; Swartz, M.A.; Hubbell, J.A. Nanoparticle conjugation of cpg enhances adjuvancy for cellular immunity and memory recall at low dose. Proc. Natl. Acad. Sci. USA 2013, 110, 19902–19907. [Google Scholar] [CrossRef] [PubMed]
- Tighe, H.; Takabayashi, K.; Schwartz, D.; Marsden, R.; Beck, L.; Corbeil, J.; Richman, D.D.; Eiden, J.J., Jr.; Spiegelberg, H.L.; Raz, E. Conjugation of protein to immunostimulatory DNA results in a rapid, long-lasting and potent induction of cell-mediated and humoral immunity. Eur. J. Immunol. 2000, 30, 1939–1947. [Google Scholar] [CrossRef]
- Nordly, P.; Madsen, H.B.; Nielsen, H.M.; Foged, C. Status and future prospects of lipid-based particulate delivery systems as vaccine adjuvants and their combination with immunostimulators. Expert Opin. Drug Deliv. 2009, 6, 657–672. [Google Scholar] [CrossRef] [PubMed]
- Bangham, A.D. Lipid bilayers and biomembranes. Annu. Rev. Biochem. 1972, 41, 753–776. [Google Scholar] [CrossRef] [PubMed]
- Lasic, D.D. Novel applications of liposomes. Trends Biotechnol. 1998, 16, 307–321. [Google Scholar] [CrossRef]
- Allison, A.C.; Gregoriadis, G. Liposomes as immunological adjuvants. Nature 1974, 252, 252–252. [Google Scholar] [CrossRef] [PubMed]
- Perrie, Y.; Crofts, F.; Devitt, A.; Griffiths, H.R.; Kastner, E.; Nadella, V. Designing liposomal adjuvants for the next generation of vaccines. Adv. Drug Deliv. Rev. 2015. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schwendener, R.A. Liposomes as vaccine delivery systems: A review of the recent advances. Ther. Adv. Vaccines 2014, 2, 159–182. [Google Scholar] [CrossRef] [PubMed]
- Badiee, A.; Khamesipour, A.; Samiei, A.; Soroush, D.; Shargh, V.H.; Kheiri, M.T.; Barkhordari, F.; Robert Mc Master, W.; Mahboudi, F.; Jaafari, M.R. The role of liposome size on the type of immune response induced in balb/c mice against leishmaniasis: Rgp63 as a model antigen. Exp. Parasitol. 2012, 132, 403–409. [Google Scholar] [CrossRef] [PubMed]
- Henriksen-Lacey, M.; Devitt, A.; Perrie, Y. The vesicle size of dda:Tdb liposomal adjuvants plays a role in the cell-mediated immune response but has no significant effect on antibody production. J. Control. Release 2011, 154, 131–137. [Google Scholar] [CrossRef] [PubMed]
- Nakanishi, T.; Kunisawa, J.; Hayashi, A.; Tsutsumi, Y.; Kubo, K.; Nakagawa, S.; Fujiwara, H.; Hamaoka, T.; Mayumi, T. Positively charged liposome functions as an efficient immunoadjuvant in inducing immune responses to soluble proteins. Biochem. Biophys. Res. Commun. 1997, 240, 793–797. [Google Scholar] [CrossRef] [PubMed]
- Miller, C.R.; Bondurant, B.; McLean, S.D.; McGovern, K.A.; O'Brien, D.F. Liposome−cell interactions in vitro: Effect of liposome surface charge on the binding and endocytosis of conventional and sterically stabilized liposomes. Biochemistry 1998, 37, 12875–12883. [Google Scholar] [CrossRef] [PubMed]
- Foged, C.; Arigita, C.; Sundblad, A.; Jiskoot, W.; Storm, G.; Frokjaer, S. Interaction of dendritic cells with antigen-containing liposomes: Effect of bilayer composition. Vaccine 2004, 22, 1903–1913. [Google Scholar] [CrossRef] [PubMed]
- Mazumdar, T.; Anam, K.; Ali, N. Influence of phospholipid composition on the adjuvanticity and protective efficacy of liposome-encapsulated leishmania donovani antigens. J. Parasitol. 2005, 91, 269–274. [Google Scholar] [CrossRef] [PubMed]
- Badiee, A.; Jaafari, M.R.; Khamesipour, A.; Samiei, A.; Soroush, D.; Kheiri, M.T.; Barkhordari, F.; McMaster, W.R.; Mahboudi, F. Enhancement of immune response and protection in balb/c mice immunized with liposomal recombinant major surface glycoprotein of leishmania (rgp63): The role of bilayer composition. Colloids Surf. B 2009, 74, 37–44. [Google Scholar] [CrossRef] [PubMed]
- Brunner, R.; Jensen-Jarolim, E.; Pali-Schöll, I. The abc of clinical and experimental adjuvants—A brief overview. Immunol. Lett. 2010, 128, 29–35. [Google Scholar] [CrossRef] [PubMed]
- Brewer, J.M.; Tetley, L.; Richmond, J.; Liew, F.Y.; Alexander, J. Lipid vesicle size determines the th1 or th2 response to entrapped antigen. J. Immunol. 1998, 161, 4000–4007. [Google Scholar] [PubMed]
- Oussoren, C.; Zuidema, J.; Crommelin, D.J.A.; Storm, G. Lymphatic uptake and biodistribution of liposomes after subcutaneous injection: Ii. Influence of liposomal size, lipid composition and lipid dose. Biochim. Biophys. Acta 1997, 1328, 261–272. [Google Scholar] [CrossRef]
- Carstens, M.G.; Camps, M.G.M.; Henriksen-Lacey, M.; Franken, K.; Ottenhoff, T.H.M.; Perrie, Y.; Bouwstra, J.A.; Ossendorp, F.; Jiskoot, W. Effect of vesicle size on tissue localization and immunogenicity of liposomal DNA vaccines. Vaccine 2011, 29, 4761–4770. [Google Scholar] [CrossRef] [PubMed]
- Henriksen-Lacey, M.; Christensen, D.; Bramwell, V.W.; Lindenstrøm, T.; Agger, E.M.; Andersen, P.; Perrie, Y. Liposomal cationic charge and antigen adsorption are important properties for the efficient deposition of antigen at the injection site and ability of the vaccine to induce a cmi response. J. Control. Release 2010, 145, 102–108. [Google Scholar] [CrossRef] [PubMed]
- Kraaijeveld, C.A.; Schilham, M.; Jansen, J.; Benaissa-Trouw, B.; Harmsen, M.; van Houte, A.J.; Snippe, H. The effect of liposomal charge on the neutralizing antibody response against inactivated encephalomyocarditis and semliki forest viruses. Clin. Exp. Immunol. 1984, 56, 509–514. [Google Scholar] [PubMed]
- Aggarwal, P.; Hall, J.B.; McLeland, C.B.; Dobrovolskaia, M.A.; McNeil, S.E. Nanoparticle interaction with plasma proteins as it relates to particle biodistribution, biocompatibility and therapeutic efficacy. Adv. Drug Deliv. Rev. 2009, 61, 428–437. [Google Scholar] [CrossRef] [PubMed]
- Nel, A.E.; Madler, L.; Velegol, D.; Xia, T.; Hoek, E.M.V.; Somasundaran, P.; Klaessig, F.; Castranova, V.; Thompson, M. Understanding biophysicochemical interactions at the nano-bio interface. Nat. Mater. 2009, 8, 543–557. [Google Scholar] [CrossRef] [PubMed]
- Owens III, D.E.; Peppas, N.A. Opsonization, biodistribution, and pharmacokinetics of polymeric nanoparticles. Int. J. Pharm. 2006, 307, 93–102. [Google Scholar] [CrossRef] [PubMed]
- Korsholm, K.S.; Petersen, R.V.; Agger, E.M.; Andersen, P. T-helper 1 and t-helper 2 adjuvants induce distinct differences in the magnitude, quality and kinetics of the early inflammatory response at the site of injection. Immunology 2010, 129, 75–86. [Google Scholar] [CrossRef] [PubMed]
- Badiee, A.; Jaafari, M.R.; Khamesipour, A.; Samiei, A.; Soroush, D.; Kheiri, M.T.; Barkhordari, F.; McMaster, W.R.; Mahboudi, F. The role of liposome charge on immune response generated in balb/c mice immunized with recombinant major surface glycoprotein of leishmania (rgp63). Exp. Parasitol. 2009, 121, 362–369. [Google Scholar] [CrossRef] [PubMed]
- Romberg, B.; Hennink, W.; Storm, G. Sheddable coatings for long-circulating nanoparticles. Pharm. Res. 2008, 25, 55–71. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wang, J.; Gao, Y.; Zhu, J.; Wientjes, M.; Au, J. Relationships between liposome properties, cell membrane binding, intracellular processing, and intracellular bioavailability. AAPS J. 2011, 13, 585–597. [Google Scholar] [CrossRef] [PubMed]
- Song, L.Y.; Ahkong, Q.F.; Rong, Q.; Wang, Z.; Ansell, S.; Hope, M.J.; Mui, B. Characterization of the inhibitory effect of peg-lipid conjugates on the intracellular delivery of plasmid and antisense DNA mediated by cationic lipid liposomes. Biochim. Biophys. Acta 2002, 1558, 1–13. [Google Scholar] [CrossRef]
- Kaur, R.; Bramwell, V.W.; Kirby, D.J.; Perrie, Y. Manipulation of the surface pegylation in combination with reduced vesicle size of cationic liposomal adjuvants modifies their clearance kinetics from the injection site, and the rate and type of t cell response. J. Control. Release 2012. [Google Scholar] [CrossRef] [PubMed]
- Kaur, R.; Bramwell, V.W.; Kirby, D.J.; Perrie, Y. Pegylation of dda:Tdb liposomal adjuvants reduces the vaccine depot effect and alters the th1/th2 immune responses. J. Control. Release 2012, 158, 72–77. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, Y.; Taneichi, M.; Kasai, M.; Kakiuchi, T.; Uchida, T. Liposome-coupled antigens are internalized by antigen-presenting cells via pinocytosis and cross-presented to cd8+ t cells. PLoS ONE 2010, 5, e15225. [Google Scholar] [CrossRef] [PubMed]
- Christensen, D.; Henriksen-Lacey, M.; Kamath, A.T.; Lindenstrøm, T.; Korsholm, K.S.; Christensen, J.P.; Rochat, A.-F.; Lambert, P.-H.; Andersen, P.; Siegrist, C.-A.; et al. A cationic vaccine adjuvant based on a saturated quaternary ammonium lipid have different in vivo distribution kinetics and display a distinct cd4 t cell-inducing capacity compared to its unsaturated analog. J. Control. Release 2012, 160, 468–476. [Google Scholar] [CrossRef] [PubMed]
- Maisonneuve, C.; Bertholet, S.; Philpott, D.J.; De Gregorio, E. Unleashing the potential of nod- and toll-like agonists as vaccine adjuvants. Proc. Natl. Acad. Sci. USA 2014, 111, 12294–12299. [Google Scholar] [CrossRef] [PubMed]
- Kaisho, T.; Akira, S. Toll-like receptor function and signaling. J. Allergy Clin. Immunol. 2006, 117, 979–987. [Google Scholar] [CrossRef] [PubMed]
- Newton, K.; Dixit, V.M. Signaling in innate immunity and inflammation. Cold Spring Harb. Perspect. Biol. 2012. [Google Scholar] [CrossRef] [PubMed]
- Geijtenbeek, T.B.H.; Gringhuis, S.I. Signalling through c-type lectin receptors: Shaping immune responses. Nat. Rev. Immunol. 2009, 9, 465–479. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, S.; Agrawal, A.; Doughty, B.; Gerwitz, A.; Blenis, J.; Van Dyke, T.; Pulendran, B. Cutting edge: Different toll-like receptor agonists instruct dendritic cells to induce distinct th responses via differential modulation of extracellular signal-regulated kinase-mitogen-activated protein kinase and c-fos. J. Immunol. 2003, 171, 4984–4989. [Google Scholar] [CrossRef] [PubMed]
- Medzhitov, R. Toll-like receptors and innate immunity. Nat. Rev. Immunol. 2001, 1, 135–145. [Google Scholar] [CrossRef] [PubMed]
- Jin, B.; Sun, T.; Yu, X.-H.; Liu, C.-Q.; Yang, Y.-X.; Lu, P.; Fu, S.-F.; Qiu, H.-B.; Yeo, A.E.T. Immunomodulatory effects of dsrna and its potential as vaccine adjuvant. J. Biomed. Biotechnol. 2010, 2010, 690438. [Google Scholar] [CrossRef] [PubMed]
- Akira, S.; Uematsu, S.; Takeuchi, O. Pathogen recognition and innate immunity. Cell 2006, 124, 783–801. [Google Scholar] [CrossRef] [PubMed]
- Bangham, A.D.; Standish, M.M.; Watkins, J.C. Diffusion of univalent ions across the lamellae of swollen phospholipids. J. Mol. Biol. 1965, 13, 238–IN227. [Google Scholar] [CrossRef]
- Richards, R.L.; Rao, M.; Wassef, N.M.; Glenn, G.M.; Rothwell, S.W.; Alving, C.R. Liposomes containing lipid a serve as an adjuvant for induction of antibody and cytotoxic t-cell responses against rts,s malaria antigen. Infect. Immun. 1998, 66, 2859–2865. [Google Scholar] [PubMed]
- Nordly, P.; Agger, E.; Andersen, P.; Nielsen, H.; Foged, C. Incorporation of the tlr4 agonist monophosphoryl lipid a into the bilayer of dda/tdb liposomes: Physico-chemical characterization and induction of cd8+ t-cell responses in vivo. Pharm. Res. 2011, 28, 553–562. [Google Scholar] [CrossRef] [PubMed]
- Alving, C.R. Lipopolysaccharide, lipid a, and liposomes containing lipid a as immunologic adjuvants. Immunobiology 1993, 187, 430–446. [Google Scholar] [CrossRef]
- Davidsen, J.; Rosenkrands, I.; Christensen, D.; Vangala, A.; Kirby, D.; Perrie, Y.; Agger, E.M.; Andersen, P. Characterization of cationic liposomes based on dimethyldioctadecylammonium and synthetic cord factor from m. Tuberculosis (trehalose 6,6′-dibehenate)—A novel adjuvant inducing both strong cmi and antibody responses. Biochim. Biophys. Acta 2005, 1718, 22–31. [Google Scholar] [CrossRef] [PubMed]
- Agger, E.M.; Rosenkrands, I.; Hansen, J.; Brahimi, K.; Vandahl, B.S.; Aagaard, C.; Werninghaus, K.; Kirschning, C.; Lang, R.; Christensen, D.; et al. Cationic liposomes formulated with synthetic mycobacterial cordfactor (caf01): A versatile adjuvant for vaccines with different immunological requirements. PLoS ONE 2008, 3, e3116. [Google Scholar] [CrossRef] [PubMed]
- Andersen, C.A.S.; Rosenkrands, I.; Olsen, A.W.; Nordly, P.; Christensen, D.; Lang, R.; Kirschning, C.; Gomes, J.M.; Bhowruth, V.; Minnikin, D.E.; et al. Novel generation mycobacterial adjuvant based on liposome-encapsulated monomycoloyl glycerol from mycobacterium bovis bacillus calmette-guérin. J. Immunol. 2009, 183, 2294–2302. [Google Scholar] [CrossRef] [PubMed]
- Andersen, C.S.; Agger, E.M.; Rosenkrands, I.; Gomes, J.M.; Bhowruth, V.; Gibson, K.J.C.; Petersen, R.V.; Minnikin, D.E.; Besra, G.S.; Andersen, P. A simple mycobacterial monomycolated glycerol lipid has potent immunostimulatory activity. J. Immunol. 2009, 182, 424–432. [Google Scholar] [CrossRef] [PubMed]
- Nordly, P.; Korsholm, K.S.; Pedersen, E.A.; Khilji, T.S.; Franzyk, H.; Jorgensen, L.; Nielsen, H.M.; Agger, E.M.; Foged, C. Incorporation of a synthetic mycobacterial monomycoloyl glycerol analogue stabilizes dimethyldioctadecylammonium liposomes and potentiates their adjuvant effect in vivo. Eur. J. Pharm. Biopharm. 2011, 77, 89–98. [Google Scholar] [CrossRef] [PubMed]
- Rosenkrands, I.; Vingsbo-Lundberg, C.; Bundgaard, T.J.; Lindenstrøm, T.; Enouf, V.; van der Werf, S.; Andersen, P.; Agger, E.M. Enhanced humoral and cell-mediated immune responses after immunization with trivalent influenza vaccine adjuvanted with cationic liposomes. Vaccine 2011, 29, 6283–6291. [Google Scholar] [CrossRef] [PubMed]
- Copland, M.J.; Baird, M.A.; Rades, T.; McKenzie, J.L.; Becker, B.; Reck, F.; Tyler, P.C.; Davies, N.M. Liposomal delivery of antigen to human dendritic cells. Vaccine 2003, 21, 883–890. [Google Scholar] [CrossRef]
- East, L.; Isacke, C.M. The mannose receptor family. Biochim. Biophys. Acta 2002, 1572, 364–386. [Google Scholar] [CrossRef]
- Heurtault, B.; Gentine, P.; Thomann, J.-S.; Baehr, C.; Frisch, B.; Pons, F. Design of a liposomal candidate vaccine against pseudomonas aeruginosa and its evaluation in triggering systemic and lung mucosal immunity. Pharm. Res. 2009, 26, 276–285. [Google Scholar] [CrossRef] [PubMed]
- Hanson, M.C.; Abraham, W.; Crespo, M.P.; Chen, S.H.; Liu, H.; Szeto, G.L.; Kim, M.; Reinherz, E.L.; Irvine, D.J. Liposomal vaccines incorporating molecular adjuvants and intrastructural t-cell help promote the immunogenicity of hiv membrane-proximal external region peptides. Vaccine 2015, 33, 861–868. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Huang, L. Induction of cytotoxic t-lymphocytes and antitumor activity by a liposomal lipopeptide vaccine. Mol. Pharm. 2008, 5, 464–471. [Google Scholar] [CrossRef] [PubMed]
- Guan, H.H.; Budzynski, W.; Koganty, R.R.; Krantz, M.J.; Reddish, M.A.; Rogers, J.A.; Longenecker, B.M.; Samuel, J. Liposomal formulations of synthetic muc1 peptides: Effects of encapsulation versus surface display of peptides on immune responses. Bioconjug. Chem. 1998, 9, 451–458. [Google Scholar] [CrossRef] [PubMed]
- Yuseff, M.-I.; Pierobon, P.; Reversat, A.; Lennon-Dumenil, A.-M. How b cells capture, process and present antigens: A crucial role for cell polarity. Nat. Rev. Immunol. 2013, 13, 475–486. [Google Scholar] [CrossRef] [PubMed]
- Batista, F.D.; Harwood, N.E. The who, how and where of antigen presentation to b cells. Nat. Rev. Immunol. 2009, 9, 15–27. [Google Scholar] [CrossRef] [PubMed]
- Shahum, E.; Thérien, H.-M. Liposomal adjuvanticity: Effect of encapsulation and surface-linkage on antibody production and proliferative response. Int. J. Immunopharmacol. 1995, 17, 9–20. [Google Scholar] [CrossRef]
- Watson, D.S.; Huang, Z.; Szoka, F.C. All-trans retinoic acid potentiates the antibody response in mice to a lipopeptide antigen adjuvanted with liposomal lipid a. Immunol. Cell Biol. 2009, 87, 630–633. [Google Scholar] [CrossRef] [PubMed]
- Fox, C.B.; Sivananthan, S.J.; Duthie, M.S.; Vergara, J.; Guderian, J.A.; Moon, E.; Coblentz, D.; Reed, S.G.; Carter, D. A nanoliposome delivery system to synergistically trigger tlr4 and tlr7. J. Nanobiotechnol. 2014, 12, 17. [Google Scholar] [CrossRef] [PubMed]
- Jelinek, I.; Leonard, J.N.; Price, G.E.; Brown, K.N.; Meyer-Manlapat, A.; Goldsmith, P.K.; Wang, Y.; Venzon, D.; Epstein, S.L.; Segal, D.M. Tlr3-specific double-stranded rna oligonucleotide adjuvants induce dendritic cell cross-presentation, ctl responses, and antiviral protection. J. Immunol. 2011, 186, 2422–2429. [Google Scholar] [CrossRef] [PubMed]
- Hafner, A.M.; Corthésy, B.; Merkle, H.P. Particulate formulations for the delivery of poly(i:C) as vaccine adjuvant. Adv. Drug Deliv. Rev. 2013, 65, 1386–1399. [Google Scholar] [CrossRef] [PubMed]
- Klinman, D.M. Use of cpg oligodeoxynucleotides as immunoprotective agents. Expert Opin. Biol. Ther. 2004, 4, 937–946. [Google Scholar] [CrossRef] [PubMed]
- Bode, C.; Zhao, G.; Steinhagen, F.; Kinjo, T.; Klinman, D.M. Cpg DNA as a vaccine adjuvant. Expert Rev. Vaccines 2011, 10, 499–511. [Google Scholar] [CrossRef] [PubMed]
- Gursel, I.; Gursel, M.; Ishii, K.J.; Klinman, D.M. Sterically stabilized cationic liposomes improve the uptake and immunostimulatory activity of cpg oligonucleotides. J. Immunol. 2001, 167, 3324–3328. [Google Scholar] [CrossRef] [PubMed]
- Ludewig, B.; Barchiesi, F.; Pericin, M.; Zinkernagel, R.M.; Hengartner, H.; Schwendener, R.A. In vivo antigen loading and activation of dendritic cells via a liposomal peptide vaccine mediates protective antiviral and anti-tumour immunity. Vaccine 2000, 19, 23–32. [Google Scholar] [CrossRef]
- Andrews, C.D.; Huh, M.-S.; Patton, K.; Higgins, D.; Van Nest, G.; Ott, G.; Lee, K.-D. Encapsulating immunostimulatory cpg oligonucleotides in listeriolysin o-liposomes promotes a th1-type response and ctl activity. Mol. Pharm. 2012, 9, 1118–1125. [Google Scholar] [CrossRef] [PubMed]
- Perrie, Y.; Gregoriadis, G. Liposome-entrapped plasmid DNA: Characterisation studies. Biochim. Biophys. Acta 2000, 1475, 125–132. [Google Scholar] [CrossRef]
- Nordly, P.; Rose, F.; Christensen, D.; Nielsen, H.M.; Andersen, P.; Agger, E.M.; Foged, C. Immunity by formulation design: Induction of high cd8+ t-cell responses by poly(i:C) incorporated into the caf01 adjuvant via a double emulsion method. J. Control. Release 2011, 150, 307–317. [Google Scholar] [CrossRef] [PubMed]
- Shargh, V.H.; Jaafari, M.R.; Khamesipour, A.; Jaafari, I.; Jalali, S.A.; Abbasi, A.; Badiee, A. Liposomal sla co-incorporated with po cpg odns or ps cpg odns induce the same protection against the murine model of leishmaniasis. Vaccine 2012, 30, 3957–3964. [Google Scholar] [CrossRef] [PubMed]
- Barnier-Quer, C.; Elsharkawy, A.; Romeijn, S.; Kros, A.; Jiskoot, W. Adjuvant effect of cationic liposomes for subunit influenza vaccine: Influence of antigen loading method, cholesterol and immune modulators. Pharmaceutics 2013, 5, 392–410. [Google Scholar] [CrossRef] [PubMed]
- Hansen, J.; Lindenstrøm, T.; Lindberg-Levin, J.; Aagaard, C.; Andersen, P.; Agger, E. Caf05: Cationic liposomes that incorporate synthetic cord factor and poly(i:C) induce ctl immunity and reduce tumor burden in mice. Cancer Immunol. Immunother. 2011, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Korsholm, K.S.; Hansen, J.; Karlsen, K.; Filskov, J.; Mikkelsen, M.; Lindenstrøm, T.; Schmidt, S.T.; Andersen, P.; Christensen, D. Induction of cd8+ t-cell responses against subunit antigens by the novel cationic liposomal caf09 adjuvant. Vaccine 2014, 32, 3927–3935. [Google Scholar] [CrossRef] [PubMed]
- Zaks, K.; Jordan, M.; Guth, A.; Sellins, K.; Kedl, R.; Izzo, A.; Bosio, C.; Dow, S. Efficient immunization and cross-priming by vaccine adjuvants containing tlr3 or tlr9 agonists complexed to cationic liposomes. J. Immunol. 2006, 176, 7335–7345. [Google Scholar] [CrossRef] [PubMed]
- Mansourian, M.; Badiee, A.; Jalali, S.A.; Shariat, S.; Yazdani, M.; Amin, M.; Jaafari, M.R. Effective induction of anti-tumor immunity using p5 her-2/neu derived peptide encapsulated in fusogenic dotap cationic liposomes co-administrated with cpg-odn. Immunol. Lett. 2014, 162, 87–93. [Google Scholar] [CrossRef] [PubMed]
- Varypataki, E.M.; van der Maaden, K.; Bouwstra, J.; Ossendorp, F.; Jiskoot, W. Cationic liposomes loaded with a synthetic long peptide and poly(i:C): A defined adjuvanted vaccine for induction of antigen-specific t cell cytotoxicity. AAPS J. 2015, 17, 216–226. [Google Scholar] [CrossRef] [PubMed]
- Neeland, M.R.; Elhay, M.J.; Meeusen, E.N.T.; de Veer, M.J. Vaccination with liposomal poly(i:C) induces discordant maturation of migratory dendritic cell subsets and anti-viral gene signatures in afferent lymph cells. Vaccine 2014, 32, 6183–6192. [Google Scholar] [CrossRef] [PubMed]
- Hemmi, H.; Kaisho, T.; Takeuchi, O.; Sato, S.; Sanjo, H.; Hoshino, K.; Horiuchi, T.; Tomizawa, H.; Takeda, K.; Akira, S. Small anti-viral compounds activate immune cells via the tlr7 myd88-dependent signaling pathway. Nat. Immunol. 2002, 3, 196–200. [Google Scholar] [CrossRef] [PubMed]
- Grunwald, T.; Ulbert, S. Improvement of DNA vaccination by adjuvants and sophisticated delivery devices: Vaccine-platforms for the battle against infectious diseases. Clin. Exp. Vaccine Res. 2015, 4, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Gregoriadis, G.; Saffie, R.; de Souza, J.B. Liposome-mediated DNA vaccination. FEBS Lett. 1997, 402, 107–110. [Google Scholar] [CrossRef]
- Perrie, Y.; Frederik, P.M.; Gregoriadis, G. Liposome-mediated DNA vaccination: The effect of vesicle composition. Vaccine 2001, 19, 3301–3310. [Google Scholar] [CrossRef]
- Rodriguez, A.E.; Zamorano, P.; Wilkowsky, S.; Torrá, F.; Ferreri, L.; Dominguez, M.; Florin-Christensen, M. Delivery of recombinant vaccines against bovine herpesvirus type 1 gd and babesia bovis msa-2c to mice using liposomes derived from egg yolk lipids. Vet. J. 2013, 196, 550–551. [Google Scholar] [CrossRef] [PubMed]
- Balbino, T.A.; Azzoni, A.R.; de la Torre, L.G. Microfluidic devices for continuous production of pdna/cationic liposome complexes for gene delivery and vaccine therapy. Colloids Surf. B 2013, 111, 203–210. [Google Scholar] [CrossRef] [PubMed]
- Watson, D.S.; Endsley, A.N.; Huang, L. Design considerations for liposomal vaccines: Influence of formulation parameters on antibody and cell-mediated immune responses to liposome associated antigens. Vaccine 2012, 30, 2256–2272. [Google Scholar] [CrossRef] [PubMed]
- Kedar, E.; Gur, H.; Babai, I.; Samira, S.; Even-Chen, S.; Barenholz, Y. Delivery of cytokines by liposomes: Hematopoietic and immunomodulatory activity of interleukin-2 encapsulated in conventional liposomes and in long-circulating liposomes. J. Immunother. 2000, 23, 131–145. [Google Scholar] [CrossRef] [PubMed]
- Hamborg, M.; Jorgensen, L.; Bojsen, A.; Christensen, D.; Foged, C. Protein antigen adsorption to the dda/tdb liposomal adjuvant: Effect on protein structure, stability, and liposome physicochemical characteristics. Pharm. Res. 2013, 30, 140–155. [Google Scholar] [CrossRef] [PubMed]
- Hamborg, M.; Rose, F.; Jorgensen, L.; Bjorklund, K.; Pedersen, H.B.; Christensen, D.; Foged, C. Elucidating the mechanisms of protein antigen adsorption to the caf/naf liposomal vaccine adjuvant systems: Effect of charge, fluidity and antigen-to-lipid ratio. Biochim. Biophys. Acta 2014, 1838, 2001–2010. [Google Scholar] [CrossRef] [PubMed]
- Shek, P.N.; Heath, T.D. Immune response mediated by liposome-associated protein antigens. Iii. Immunogenicity of bovine serum albumin covalently coupled to vesicle surface. Immunology 1983, 50, 101–106. [Google Scholar] [PubMed]
- Lockner, J.W.; Ho, S.O.; McCague, K.C.; Chiang, S.M.; Do, T.Q.; Fujii, G.; Janda, K.D. Enhancing nicotine vaccine immunogenicity with liposomes. Bioorg. Med. Chem. Lett. 2013, 23, 975–978. [Google Scholar] [CrossRef] [PubMed]
- Faham, A.; Altin, J.G. Antigen-containing liposomes engrafted with flagellin-related peptides are effective vaccines that can induce potent antitumor immunity and immunotherapeutic effect. J. Immunol. 2010, 185, 1744–1754. [Google Scholar] [CrossRef] [PubMed]
- Powers, D.C.; Hanscome, P.J.; Freda Pietrobon, P.J. In previously immunized elderly adults inactivated influenza a (h1n1) virus vaccines induce poor antibody responses that are not enhanced by liposome adjuvant. Vaccine 1995, 13, 1330–1335. [Google Scholar] [CrossRef]
- Ambrosch, F.; Wiedermann, G.; Jonas, S.; Althaus, B.; Finkel, B.; Glück, R.; Herzog, C. Immunogenicity and protectivity of a new liposomal hepatitis a vaccine. Vaccine 1997, 15, 1209–1213. [Google Scholar] [CrossRef]
- Childers, N.K.; Tong, G.; Mitchell, S.; Kirk, K.; Russell, M.W.; Michalek, S.M. A controlled clinical study of the effect of nasal immunization with a streptococcus mutans antigen alone or incorporated into liposomes on induction of immune responses. Infect. Immun. 1999, 67, 618–623. [Google Scholar] [PubMed]
- Alvarez, M.J.; Echechipía, S.; García, B.; Tabar, A.I.; Martín, S.; Rico, P.; Olaguibel, J.M. Liposome-entrapped d. Pteronyssinus vaccination in mild asthma patients: Effect of 1-year double-blind, placebo-controlled trial on inflammation, bronchial hyper-responsiveness and immediate and late bronchial responses to the allergen. Clin. Exp. Allergy 2002, 32, 1574–1582. [Google Scholar] [CrossRef] [PubMed]
- Ben-Yehuda, A.; Joseph, A.; Barenholz, Y.; Zeira, E.; Even-Chen, S.; Louria-Hayon, I.; Babai, I.; Zakay-Rones, Z.; Greenbaum, E.; Galprin, I.; et al. Immunogenicity and safety of a novel il-2-supplemented liposomal influenza vaccine (influsome-vac) in nursing-home residents. Vaccine 2003, 21, 3169–3178. [Google Scholar] [CrossRef]
- Ben-Yehuda, A.; Joseph, A.; Zeira, E.; Even-Chen, S.; Louria-Hayon, I.; Babai, I.; Zakay-Rones, Z.; Greenbaum, E.; Barenholz, Y.; Kedar, E. Immunogenicity and safety of a novel liposomal influenza subunit vaccine (influsome-vac) in young adults. J. Med. Virol. 2003, 69, 560–567. [Google Scholar] [CrossRef] [PubMed]
- Lell, B.; Agnandji, S.; von Glasenapp, I.; Haertle, S.; Oyakhiromen, S.; Issifou, S.; Vekemans, J.; Leach, A.; Lievens, M.; Dubois, M.-C.; et al. A randomized trial assessing the safety and immunogenicity of as01 and as02 adjuvanted rts,s malaria vaccine candidates in children in gabon. PLoS ONE 2009, 4, e7611. [Google Scholar] [CrossRef] [PubMed]
- Kester, K.E.; Cummings, J.F.; Ofori-Anyinam, O.; Ockenhouse, C.F.; Krzych, U.; Moris, P.; Schwenk, R.; Nielsen, R.A.; Debebe, Z.; Pinelis, E.; et al. Randomized, double-blind, phase 2a trial of falciparum malaria vaccines rts,s/as01b and rts,s/as02a in malaria-naive adults: Safety, efficacy, and immunologic associates of protection. J. Infect. Dis. 2009, 200, 337–346. [Google Scholar] [CrossRef] [PubMed]
- Polhemus, M.E.; Remich, S.A.; Ogutu, B.R.; Waitumbi, J.N.; Otieno, L.; Apollo, S.; Cummings, J.F.; Kester, K.E.; Ockenhouse, C.F.; Stewart, A.; et al. Evaluation of rts,s/as02a and rts,s/as01b in adults in a high malaria transmission area. PLoS ONE 2009, 4, e6465. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.-L.; Park, K.; Soo, R.A.; Sun, Y.; Tyroller, K.; Wages, D.; Ely, G.; Yang, J.C.-H.; Mok, T. Inspire: A phase iii study of the blp25 liposome vaccine (l-blp25) in asian patients with unresectable stage iii non-small cell lung cancer. BMC Cancer 2011, 11, 430–430. [Google Scholar] [CrossRef] [PubMed]
- Zollinger, W.D.; Babcock, J.G.; Moran, E.E.; Brandt, B.L.; Matyas, G.R.; Wassef, N.M.; Alving, C.R. Phase i study of a neisseria meningitidis liposomal vaccine containing purified outer membrane proteins and detoxified lipooligosaccharide. Vaccine 2012, 30, 712–721. [Google Scholar] [CrossRef] [PubMed]
- Hamilton, E.; Blackwell, K.; Hobeika, A.C.; Clay, T.M.; Broadwater, G.; Ren, X.-R.; Chen, W.; Castro, H.; Lehmann, F.; Spector, N.; et al. Phase i clinical trial of her2-specific immunotherapy with concomitant her2 kinase inhibtion. J. Transl. Med. 2012, 10, 28–28. [Google Scholar] [CrossRef] [PubMed]
- Román, V.R.G.; Jensen, K.J.; Jensen, S.S.; Leo-Hansen, C.; Jespersen, S.; Té, D.d.S.; Rodrigues, C.M.; Janitzek, C.M.; Vinner, L.; Katzenstein, T.L.; et al. Therapeutic vaccination using cationic liposome-adjuvanted hiv type 1 peptides representing hla-supertype-restricted subdominant t cell epitopes: Safety, immunogenicity, and feasibility in guinea-bissau. AIDS Res. Hum. Retrovir. 2013, 29, 1504–1512. [Google Scholar] [CrossRef] [PubMed]
- Montoya, J.; Solon, J.A.; Cunanan, S.R.C.; Acosta, L.; Bollaerts, A.; Moris, P.; Janssens, M.; Jongert, E.; Demoitié, M.-A.; Mettens, P.; et al. A randomized, controlled dose-finding phase ii study of the m72/as01 candidate tuberculosis vaccine in healthy ppd-positive adults. J. Clin. Immunol. 2013, 33, 1360–1375. [Google Scholar] [CrossRef] [PubMed]
- van Dissel, J.T.; Joosten, S.A.; Hoff, S.T.; Soonawala, D.; Prins, C.; Hokey, D.A.; O’Dee, D.M.; Graves, A.; Thierry-Carstensen, B.; Andreasen, L.V.; et al. A novel liposomal adjuvant system, caf01, promotes long-lived mycobacterium tuberculosis-specific t-cell responses in human. Vaccine 2014, 32, 7098–7107. [Google Scholar] [CrossRef] [PubMed]
- Marty-Roix, R.; Vladimer, G.I.; Pouliot, K.; Weng, D.; Buglione-Corbett, R.; West, K.; MacMicking, J.D.; Chee, J.D.; Wang, S.; Lu, S.; et al. Identification of qs-21 as an inflammasome-activating molecular component of saponin adjuvants. J. Biol. Chem. 2015, 291, 1123–1136. [Google Scholar] [CrossRef] [PubMed]
- Merck KGaA. Merck discontinues clinical development program of tecemotide as a monotherapy in stage iii non-small cell lung cancer. http://www.merckgroup.com/en/media/extNewsDetail.html?newsId=8475BA17A3F51470C1257D50006901B4&newsType=1 (accessed on 16 November 2015).
- De Gregorio, E.; Rappuoli, R. From empiricism to rational design: A personal perspective of the evolution of vaccine development. Nat. Rev. Immunol. 2014, 14, 505–514. [Google Scholar] [CrossRef] [PubMed]

| Year Published | Target | Lipids in Liposomes | Immunomodulators/Potentiators | Antigen | Prophylactic/Therapeutic | Phase | NCT | Refs. |
|---|---|---|---|---|---|---|---|---|
| 1995 | Influenza | DMPC:Chol | - | H1N1 Split virus | P | I | - | [102] |
| 1997 | Hepatitis A | Phospholipids | HA, NA | Inactivated hepatitis A virus particles | P | I | - | [103] |
| 1999 | Streptococcus mutans | DPPC:DCP: Chol | - | C-GTF | P | I | - | [104] |
| 2002 | Mite allergy: D. pteronyssinus | DPPC:Chol | Tocopheryl acid succinate (vitamin E) | Mite body extract | T | I | - | [105] |
| 2003 | Influenza | DMPC:DMPG | IL-2 | H1N1 Split virus | P | I/II | - | [106,107] |
| 2009 | Malaria | - | MPL, QS21 (AS01) | RTS,S | P | I/II | NCT00307021, NCT00075049, NCT00197054 | [108,109,110] |
| 2011 | Lung cancer | DMPG:DPPC:Chol | MPL | BLP25 | T | III | NCT01015443 | [111] |
| 2012 | Neisseria meningitidis | DMPG:DMPC:Chol | MPL | Outer membrane proteins and deacetylated lipooligosaccharide | P | I | - | [112] |
| 2012 | Breast cancer | - | MPL, QS21, CpG (AS15) | dHER2 protein | T | I | - | [113] |
| 2013 | HIV | DDA | TDB (CAF01) | Cocktail of peptides | T | I | NCT01141205 | [114] |
| 2013 | Mycobacterium tuberculosis | - | MPL, QS21 (AS01) | M72 | P | I | NCT00621322 | [115] |
| 2014 | Mycobacterium tuberculosis | DDA | TDB (CAF01) | H1 protein | P | I | NCT00922363 | [116] |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tandrup Schmidt, S.; Foged, C.; Smith Korsholm, K.; Rades, T.; Christensen, D. Liposome-Based Adjuvants for Subunit Vaccines: Formulation Strategies for Subunit Antigens and Immunostimulators. Pharmaceutics 2016, 8, 7. https://doi.org/10.3390/pharmaceutics8010007
Tandrup Schmidt S, Foged C, Smith Korsholm K, Rades T, Christensen D. Liposome-Based Adjuvants for Subunit Vaccines: Formulation Strategies for Subunit Antigens and Immunostimulators. Pharmaceutics. 2016; 8(1):7. https://doi.org/10.3390/pharmaceutics8010007
Chicago/Turabian StyleTandrup Schmidt, Signe, Camilla Foged, Karen Smith Korsholm, Thomas Rades, and Dennis Christensen. 2016. "Liposome-Based Adjuvants for Subunit Vaccines: Formulation Strategies for Subunit Antigens and Immunostimulators" Pharmaceutics 8, no. 1: 7. https://doi.org/10.3390/pharmaceutics8010007