Characterization of Commercially Available Vaginal Lubricants: A Safety Perspective
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials
2.2. Tested Products
2.3. pH and Buffering Capacity Measurements
2.4. Osmolality Assessment
2.5. Cytotoxicity Studies
2.6. Statistical Analysis
3. Results and Discussion
| Product | pH a | Osmolality (mOsm/kg) b |
|---|---|---|
| Fillergyn® | 4.5 ± 0.1 | 991 ± 6 |
| Geliofil® Classic | 3.8 ± 0.1 | 3,582 ± 11 |
| GelSea® | 5.7 ± 0.1 | 3,797 ± 16 |
| Ginix® | 5.0 ± 0.1 | 989 ± 9 |
| Ginix® Plus | 5.0 ± 0.1 | 977 ± 8 |
| Hyalo Gyn® | 4.8 ± 0.1 | 1,336 ± 7 |
| K-Y® Jelly | 3.5 ± 0.2 | 3,631 ± 13 |
| Phyto Soya® | 4.6 ± 0.1 | 1,226 ± 6 |
| RepHresh® | 3.4 ± 0.1 | 1,439 ± 6 |
| Replens® | 3.0 ± 0.1 | 1,177 ± 5 |
| Velastisa® Intim VG | 3.7 ± 0.1 | 1,151 ± 7 |
| Vidermina® | 4.9 ± 0.1 | 3,707 ± 16 |
| Universal Placebo | 4.4 ± 0.1 | 298 ± 2 |
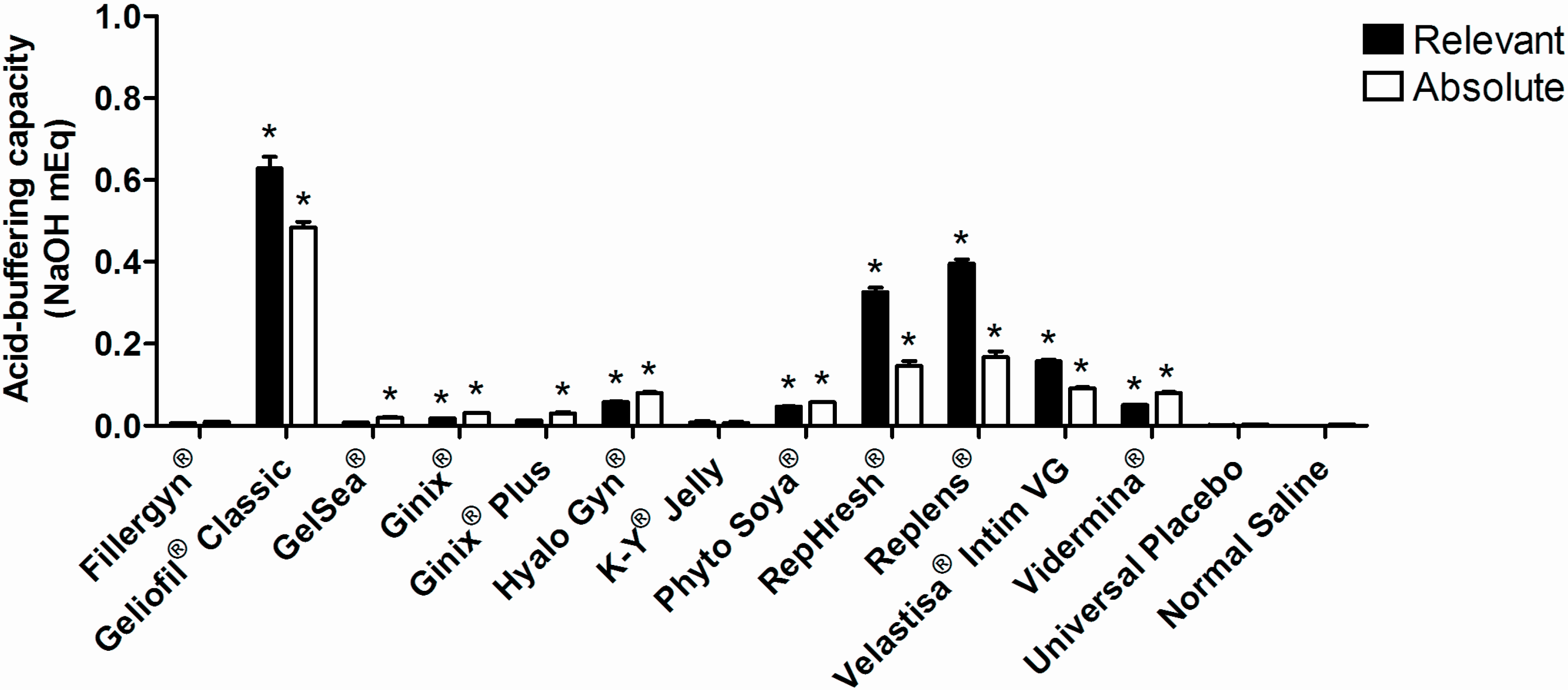



4. Conclusions
Supplementary Files
Supplementary File 1Acknowledgments
Author Contributions
Conflicts of Interest
References
- das Neves, J.; Amaral, M.H.; Bahia, M.F. Vaginal drug delivery. In Pharmaceutical Manufacturing Handbook: Production and Processes; Gad, S.C., Ed.; Wiley: Hoboken, NJ, USA, 2008; pp. 809–878. [Google Scholar]
- das Neves, J.; Palmeira-de-Oliveira, R.; Palmeira-de-Oliveira, A.; Rodrigues, F.; Sarmento, B. Vaginal mucosa and drug delivery. In Mucoadhesive Materials and Drug Delivery Systems; Khutoryanskiy, V.V., Ed.; Wiley: Chichester, UK, 2014; pp. 99–131. [Google Scholar]
- Braunstein, S.; van de Wijgert, J. Preferences and practices related to vaginal lubrication: Implications for microbicide acceptability and clinical testing. J. Womens Health 2005, 14, 424–433. [Google Scholar] [CrossRef]
- Pavelić, Ž.; Škalko-Basnet, N.; Jalšenjak, I. Characterisation and in vitro evaluation of bioadhesive liposome gels for local therapy of vaginitis. Int. J. Pharm. 2005, 301, 140–148. [Google Scholar]
- das Neves, J.; Bahia, M.F. Gels as vaginal drug delivery systems. Int. J. Pharm. 2006, 318, 1–14. [Google Scholar]
- das Neves, J.; Amaral, M.H.; Bahia, M.F. Performance of an in vitro mucoadhesion testing method for vaginal semisolids: Influence of different testing conditions and instrumental parameters. Eur. J. Pharm. Biopharm. 2008, 69, 622–632. [Google Scholar]
- das Neves, J.; da Silva, M.V.; Gonçalves, M.P.; Amaral, M.H.; Bahia, M.F. Rheological properties of vaginal hydrophilic polymer gels. Curr. Drug Deliv. 2009, 6, 83–92. [Google Scholar]
- Geibel, S. Condoms and condiments: Compatibility and safety of personal lubricants and their use in Africa. J. Int. AIDS Soc. 2013, 16, 18531. [Google Scholar] [CrossRef] [PubMed]
- Voeller, B.; Coulson, A.H.; Bernstein, G.S.; Nakamura, R.M. Mineral oil lubricants cause rapid deterioration of latex condoms. Contraception 1989, 39, 95–102. [Google Scholar] [CrossRef] [PubMed]
- Fevola, M.J.; Gentner, L.; Ahmad, N.; Librizzi, J.J. Getting intimate with polymers: Personal lubricants. Cosmet. Toilet. 2008, 123, 59–68. [Google Scholar]
- Nicole, W. A question for women’s health: Chemicals in feminine hygiene products and personal lubricants. Environ. Health Perspect. 2014, 122, A70–A75. [Google Scholar] [CrossRef] [PubMed]
- Maguire, R.A.; Bergman, N.; Phillips, D.M. Comparison of microbicides for efficacy in protecting mice against vaginal challenge with herpes simplex virus type 2, cytotoxicity, antibacterial properties, and sperm immobilization. Sex. Transm. Dis. 2001, 28, 259–265. [Google Scholar] [CrossRef] [PubMed]
- Adriaens, E.; Remon, J.P. Mucosal irritation potential of personal lubricants relates to product osmolality as detected by the slug mucosal irritation assay. Sex. Transm. Dis. 2008, 35, 512–516. [Google Scholar] [CrossRef] [PubMed]
- Moench, T.R.; Mumper, R.J.; Hoen, T.E.; Sun, M.; Cone, R.A. Microbicide excipients can greatly increase susceptibility to genital herpes transmission in the mouse. BMC Infect. Dis. 2010, 10, 331. [Google Scholar] [CrossRef] [PubMed]
- Begay, O.; Jean-Pierre, N.; Abraham, C.J.; Chudolij, A.; Seidor, S.; Rodriguez, A.; Ford, B.E.; Henderson, M.; Katz, D.; Zydowsky, T.; et al. Identification of personal lubricants that can cause rectal epithelial cell damage and enhance HIV type 1 replication in vitro. AIDS Res. Hum. Retrovir. 2011, 27, 1019–1024. [Google Scholar] [CrossRef] [PubMed]
- Dezzutti, C.S.; Brown, E.R.; Moncla, B.; Russo, J.; Cost, M.; Wang, L.; Uranker, K.; Kunjara Na Ayudhya, R.P.; Pryke, K.; Pickett, J.; et al. Is wetter better? An evaluation of over-the-counter personal lubricants for safety and anti-HIV-1 activity. PLoS One 2012, 7, e48328. [Google Scholar] [CrossRef] [PubMed]
- WHO/UNFPA/FHI. Use and Procurement of Additional Lubricants for Male and Female Condoms: WHO/UNFPA/FHI; WHO: Geneva, Switzerland, 2012. [Google Scholar]
- Tien, D.; Schnaare, R.L.; Kang, F.; Cohl, G.; McCormick, T.J.; Moench, T.R.; Doncel, G.; Watson, K.; Buckheit, R.W.; Lewis, M.G.; et al. In vitro and in vivo characterization of a potential universal placebo designed for use in vaginal microbicide clinical trials. AIDS Res. Hum. Retrovir. 2005, 21, 845–853. [Google Scholar] [CrossRef]
- Schwartz, J.L.; Ballagh, S.A.; Kwok, C.; Mauck, C.K.; Weiner, D.H.; Rencher, W.F.; Callahan, M.M. Fourteen-day safety and acceptability study of the universal placebo gel. Contraception 2007, 75, 136–141. [Google Scholar] [CrossRef] [PubMed]
- Garg, S.; Anderson, R.A.; Chany, C.J., II; Waller, D.P.; Diao, X.H.; Vermani, K.; Zaneveld, L.J. Properties of a new acid-buffering bioadhesive vaginal formulation (ACIDFORM). Contraception 2001, 64, 67–75. [Google Scholar]
- CurveExpert Basic 1.4. Available online: http://www.curveexpert.net/curveexpert-basic/ (accessed on 30 June 2014).
- Owen, D.H.; Katz, D.F. A vaginal fluid simulant. Contraception 1999, 59, 91–95. [Google Scholar] [CrossRef] [PubMed]
- das Neves, J.; Michiels, J.; Ariën, K.K.; Vanham, G.; Amiji, M.; Bahia, M.F.; Sarmento, B. Polymeric nanoparticles affect the intracellular delivery, antiretroviral activity and cytotoxicity of the microbicide drug candidate dapivirine. Pharm. Res. 2012, 29, 1468–1484. [Google Scholar] [CrossRef] [PubMed]
- Grimes, D.A.; Lopez, L.M.; Raymond, E.G.; Halpern, V.; Nanda, K.; Schulz, K.F. Spermicide used alone for contraception. Cochrane Database Syst. Rev. 2013, 12, CD005218. [Google Scholar] [PubMed]
- Wu, J.P.; Fielding, S.L.; Fiscella, K. The effect of polycarbophil gel (Replens™) on bacterial vaginosis: A pilot study. Eur. J. Obstet. Gynecol. Reprod. Biol. 2007, 130, 132–136. [Google Scholar] [CrossRef] [PubMed]
- Fiorilli, A.; Molteni, B.; Milani, M. Successful treatment of bacterial vaginosis with a policarbophil-carbopol acidic vaginal gel: Results from a randomised double-blind, placebo-controlled trial. Eur. J. Obstet. Gynecol. Reprod. Biol. 2005, 120, 202–205. [Google Scholar] [CrossRef] [PubMed]
- Sobel, J.D. Vaginitis. N. Engl. J. Med. 1997, 337, 1896–1903. [Google Scholar] [CrossRef] [PubMed]
- Kaminsky, M.; Willigan, D.A. pH and the potential irritancy of douche formulations to the vaginal mucosa of the albino rabbit and rat. Food Chem. Toxicol. 1982, 20, 193–196. [Google Scholar] [CrossRef] [PubMed]
- Simões, J.A.; Bahamondes, L.G.; Camargo, R.P.; Alves, V.M.; Zaneveld, L.J.; Waller, D.P.; Schwartz, J.; Callahan, M.M.; Mauck, C.K. A pilot clinical trial comparing an acid-buffering formulation (ACIDFORM gel) with metronidazole gel for the treatment of symptomatic bacterial vaginosis. Br. J. Clin. Pharmacol. 2006, 61, 211–217. [Google Scholar] [CrossRef] [PubMed]
- Sinha, A.; Ewies, A.A. Non-hormonal topical treatment of vulvovaginal atrophy: An up-to-date overview. Climacteric 2013, 16, 305–312. [Google Scholar] [CrossRef]
- Boskey, E.R.; Cone, R.A.; Whaley, K.J.; Moench, T.R. Origins of vaginal acidity: High D/L lactate ratio is consistent with bacteria being the primary source. Hum. Reprod. 2001, 16, 1809–1813. [Google Scholar] [CrossRef] [PubMed]
- Bonferoni, M.C.; Giunchedi, P.; Scalia, S.; Rossi, S.; Sandri, G.; Caramella, C. Chitosan gels for the vaginal delivery of lactic acid: Relevance of formulation parameters to mucoadhesion and release mechanisms. AAPS PharmSciTech 2006, 7, 104. [Google Scholar] [CrossRef] [PubMed]
- Lacey, C.J.; Woodhall, S.; Qi, Z.; Sawant, S.; Cowen, M.; McCormack, S.; Jiang, S. Unacceptable side-effects associated with a hyperosmolar vaginal microbicide in a phase 1 trial. Int. J. STD AIDS 2010, 21, 714–717. [Google Scholar] [CrossRef] [PubMed]
- Kutteh, W.H.; Chao, C.H.; Ritter, J.O.; Byrd, W. Vaginal lubricants for the infertile couple: Effect on sperm activity. Int. J. Fertil. Menopausal. Stud. 1996, 41, 400–404. [Google Scholar] [PubMed]
- Agarwal, A.; Deepinder, F.; Cocuzza, M.; Short, R.A.; Evenson, D.P. Effect of vaginal lubricants on sperm motility and chromatin integrity: A prospective comparative study. Fertil. Steril. 2008, 89, 375–379. [Google Scholar] [CrossRef] [PubMed]
- Mahalingam, A.; Simmons, A.P.; Ugaonkar, S.R.; Watson, K.M.; Dezzutti, C.S.; Rohan, L.C.; Buckheit, R.W., Jr.; Kiser, P.F. Vaginal microbicide gel for delivery of IQP-0528, a pyrimidinedione analog with a dual mechanism of action against HIV-1. Antimicrob. Agents Chemother. 2011, 55, 1650–1660. [Google Scholar] [CrossRef] [PubMed]
- Rohan, L.C.; Moncla, B.J.; Kunjara Na Ayudhya, R.P.; Cost, M.; Huang, Y.; Gai, F.; Billitto, N.; Lynam, J.D.; Pryke, K.; Graebing, P.; et al. In vitro and ex vivo testing of tenofovir shows it is effective as an HIV-1 microbicide. PLoS One 2010, 5, e9310. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dezzutti, C.S.; Rohan, L.C.; Wang, L.; Uranker, K.; Shetler, C.; Cost, M.; Lynam, J.D.; Friend, D. Reformulated tenofovir gel for use as a dual compartment microbicide. J. Antimicrob. Chemother. 2012, 67, 2139–2142. [Google Scholar] [CrossRef] [PubMed]
- Grammen, C.; Arien, K.K.; Venkatraj, M.; Joossens, J.; van der Veken, P.; Heeres, J.; Lewi, P.J.; Haenen, S.; Augustyns, K.; Vanham, G.; et al. Development and in vitro evaluation of a vaginal microbicide gel formulation for UAMC01398, a novel diaryltriazine NNRTI against HIV-1. Antivir. Res. 2014, 101, 113–121. [Google Scholar] [CrossRef] [PubMed]
- Decker, T.; Lohmann-Matthes, M.L. A quick and simple method for the quantitation of lactate dehydrogenase release in measurements of cellular cytotoxicity and tumor necrosis factor (TNF) activity. J. Immunol. Methods 1988, 115, 61–69. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Geng, L.; Song, X.; Li, H.; Giordan, N.; Liao, Q. Evaluation of the efficacy and safety of hyaluronic acid vaginal gel to ease vaginal dryness: A multicenter, randomized, controlled, open-label, parallel-group, clinical trial. J. Sex. Med. 2013, 10, 1575–1584. [Google Scholar] [CrossRef] [PubMed]
- Van Damme, L.; Ramjee, G.; Alary, M.; Vuylsteke, B.; Chandeying, V.; Rees, H.; Sirivongrangson, P.; Mukenge-Tshibaka, L.; Ettiegne-Traore, V.; Uaheowitchai, C.; et al. Effectiveness of COL-1492, a nonoxynol-9 vaginal gel, on HIV-1 transmission in female sex workers: A randomised controlled trial. Lancet 2002, 360, 971–977. [Google Scholar] [CrossRef] [PubMed]
- Hillier, S.L.; Moench, T.; Shattock, R.; Black, R.; Reichelderfer, P.; Veronese, F. In vitro and in vivo: The story of nonoxynol 9. J. Acquir. Immune Defic. Syndr. 2005, 39, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Gali, Y.; Delezay, O.; Brouwers, J.; Addad, N.; Augustijns, P.; Bourlet, T.; Hamzeh-Cognasse, H.; Ariën, K.K.; Pozzetto, B.; Vanham, G. In vitro evaluation of viability, integrity and inflammation in genital epithelia upon exposure to pharmaceutical excipients and candidate microbicides. Antimicrob. Agents Chemother. 2010, 54, 5105–5114. [Google Scholar] [CrossRef] [PubMed]
- Poudrier, J.K. Final report on the safety assessment of phenoxyethanol. J. Am. Coll. Toxicol. 1990, 9, 259–277. [Google Scholar] [CrossRef]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Cunha, A.R.; Machado, R.M.; Palmeira-de-Oliveira, A.; Martinez-de-Oliveira, J.; Das Neves, J.; Palmeira-de-Oliveira, R. Characterization of Commercially Available Vaginal Lubricants: A Safety Perspective. Pharmaceutics 2014, 6, 530-542. https://doi.org/10.3390/pharmaceutics6030530
Cunha AR, Machado RM, Palmeira-de-Oliveira A, Martinez-de-Oliveira J, Das Neves J, Palmeira-de-Oliveira R. Characterization of Commercially Available Vaginal Lubricants: A Safety Perspective. Pharmaceutics. 2014; 6(3):530-542. https://doi.org/10.3390/pharmaceutics6030530
Chicago/Turabian StyleCunha, Ana Raquel, Rita M. Machado, Ana Palmeira-de-Oliveira, José Martinez-de-Oliveira, José Das Neves, and Rita Palmeira-de-Oliveira. 2014. "Characterization of Commercially Available Vaginal Lubricants: A Safety Perspective" Pharmaceutics 6, no. 3: 530-542. https://doi.org/10.3390/pharmaceutics6030530