Terahertz Pulsed Imaging and Magnetic Resonance Imaging as Tools to Probe Formulation Stability
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials
2.2. Accelerated Stability Testing Conditions
2.3. TPI Measurements
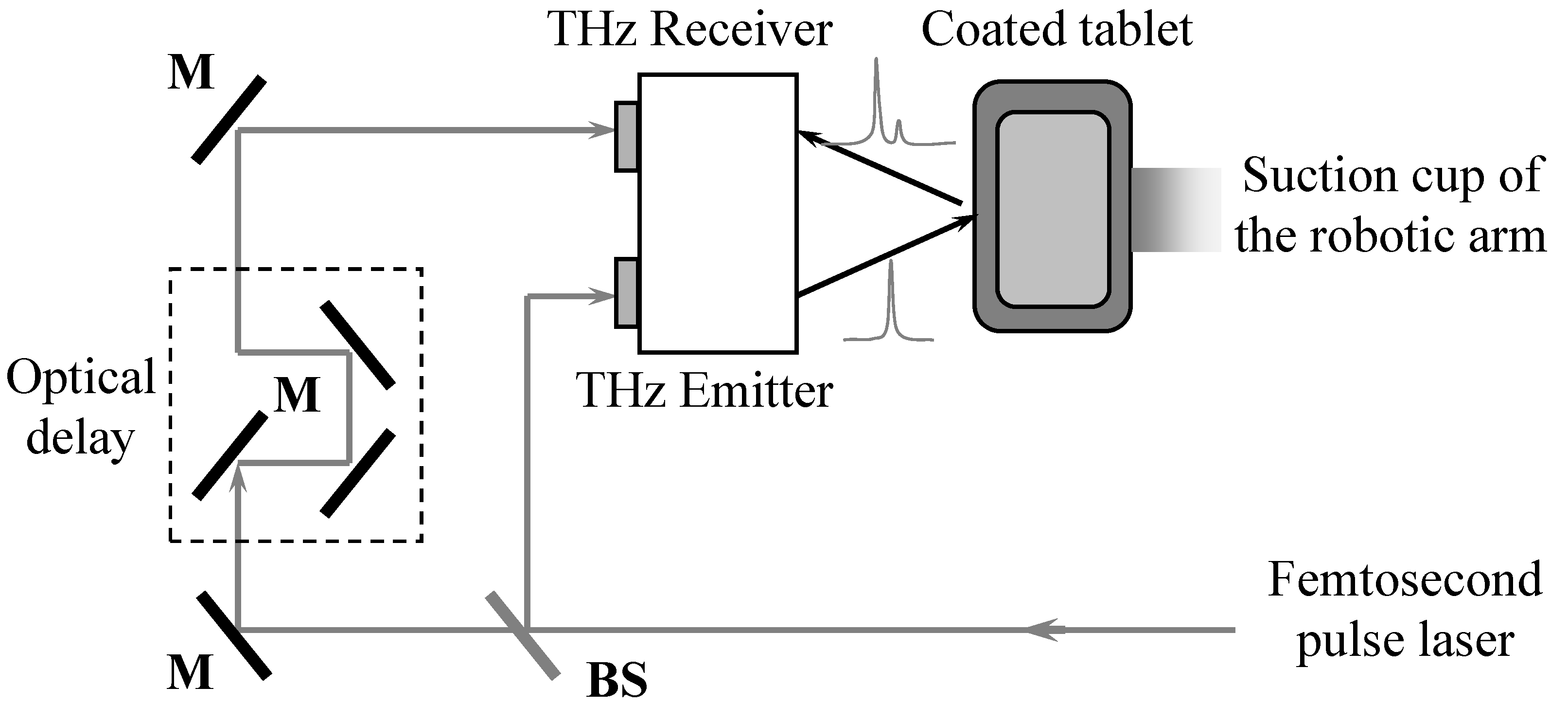
2.4. Dissolution Processes
2.5. Dissolution Protocol
2.6. UV Measurements
3. Results and Discussion
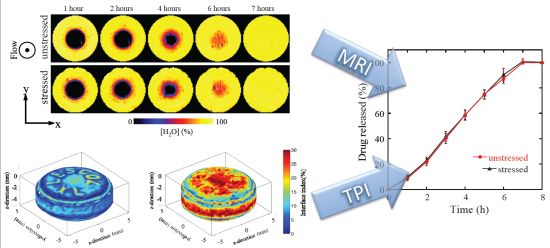
3.1. Results
3.1.1. Coating Characterization

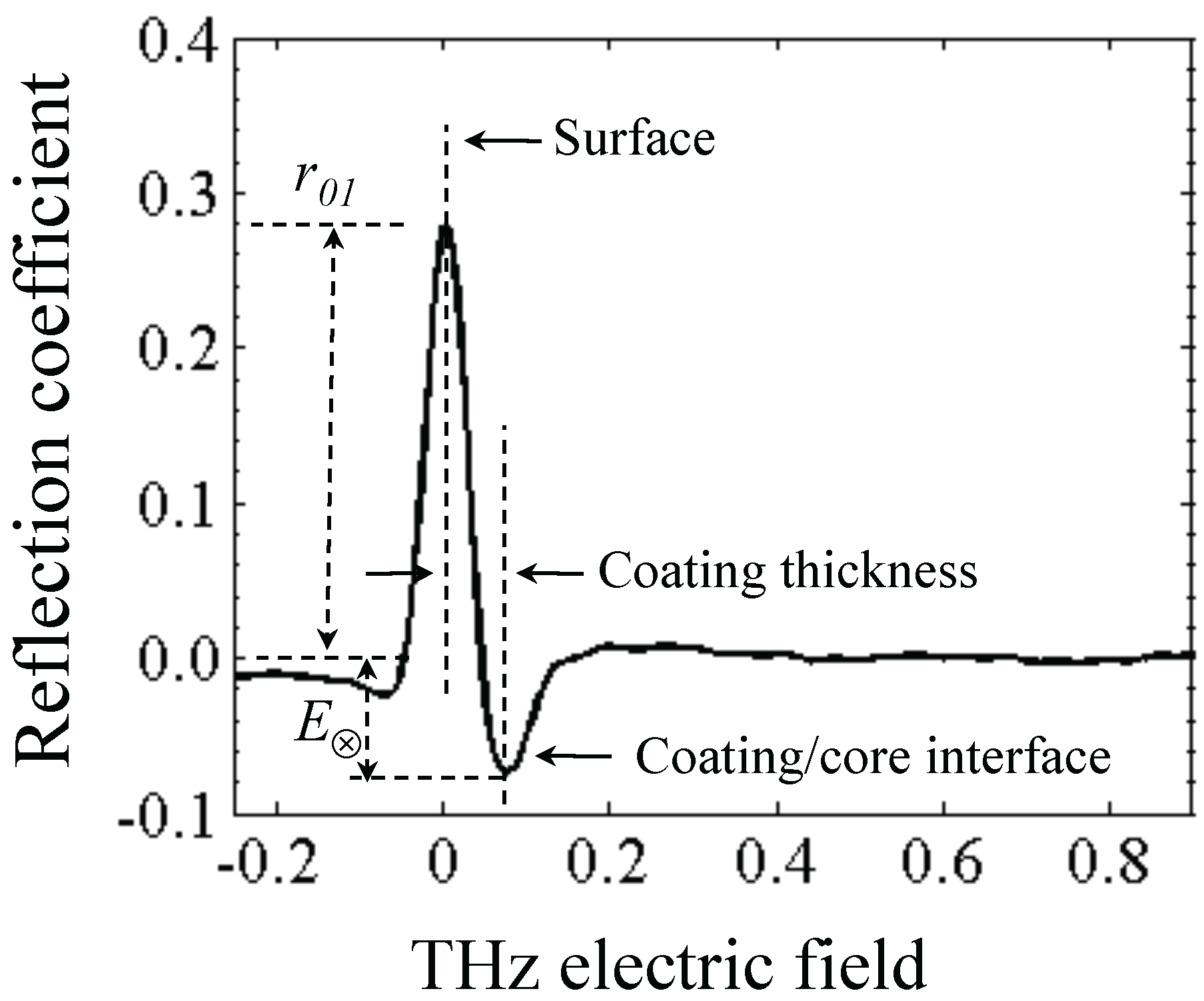
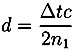
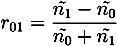
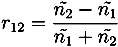

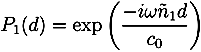
with ñ1 = n1 + iκ1
and
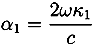
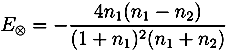
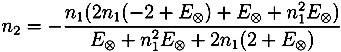
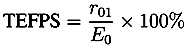
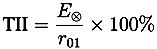

| r01 | E⊗ | n0 | n1 | n2 | d/µm | ||
|---|---|---|---|---|---|---|---|
| Unstressed | Top | 0.21 | -0.01 | 1.00 | 1.54 | 1.57 | 50.6 |
| Bottom | 0.22 | -0.01 | 1.00 | 1.55 | 1.58 | 49.9 | |
| Centre | 0.18 | -0.01 | 1.00 | 1.43 | 1.46 | 57.3 | |
| Stressed | Top | 0.26 | -0.05 | 1.00 | 1.70 | 1.89 | 41.0 |
| Bottom | 0.26 | -0.06 | 1.00 | 1.72 | 1.89 | 43.1 | |
| Centre | 0.25 | -0,06 | 1.00 | 1.67 | 1.90 | 40.2 |
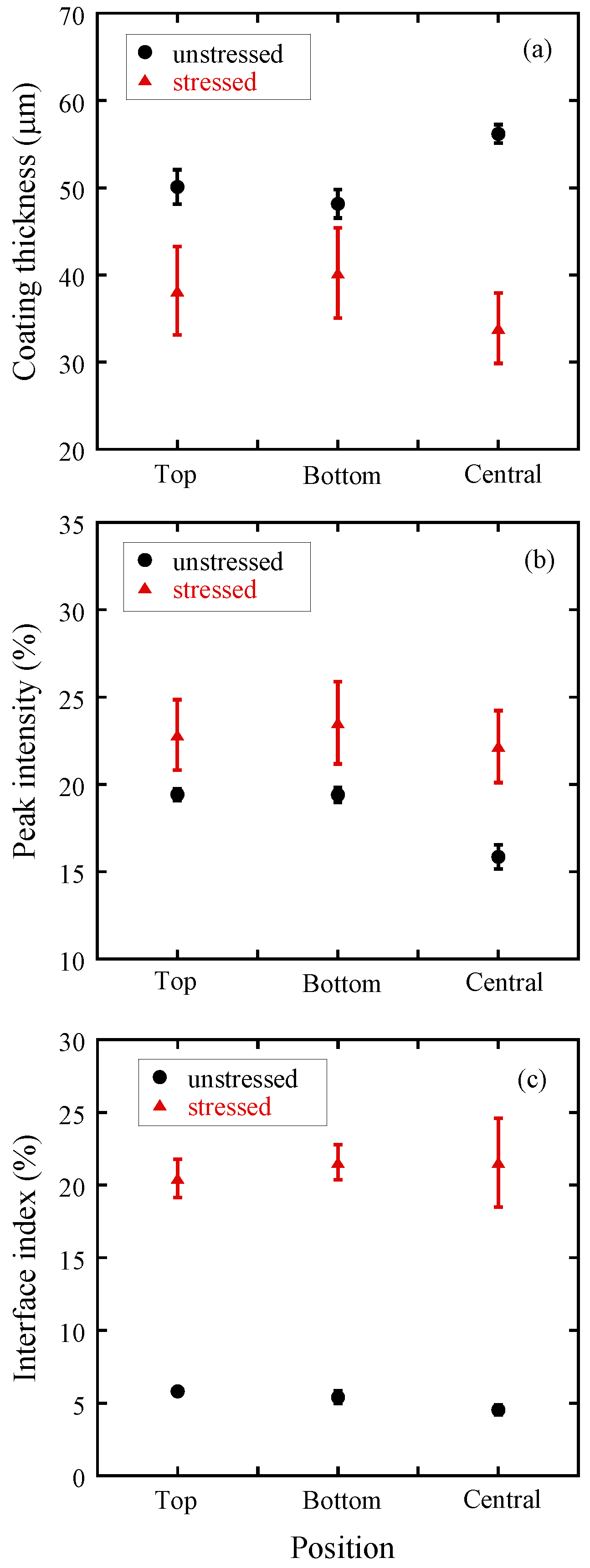
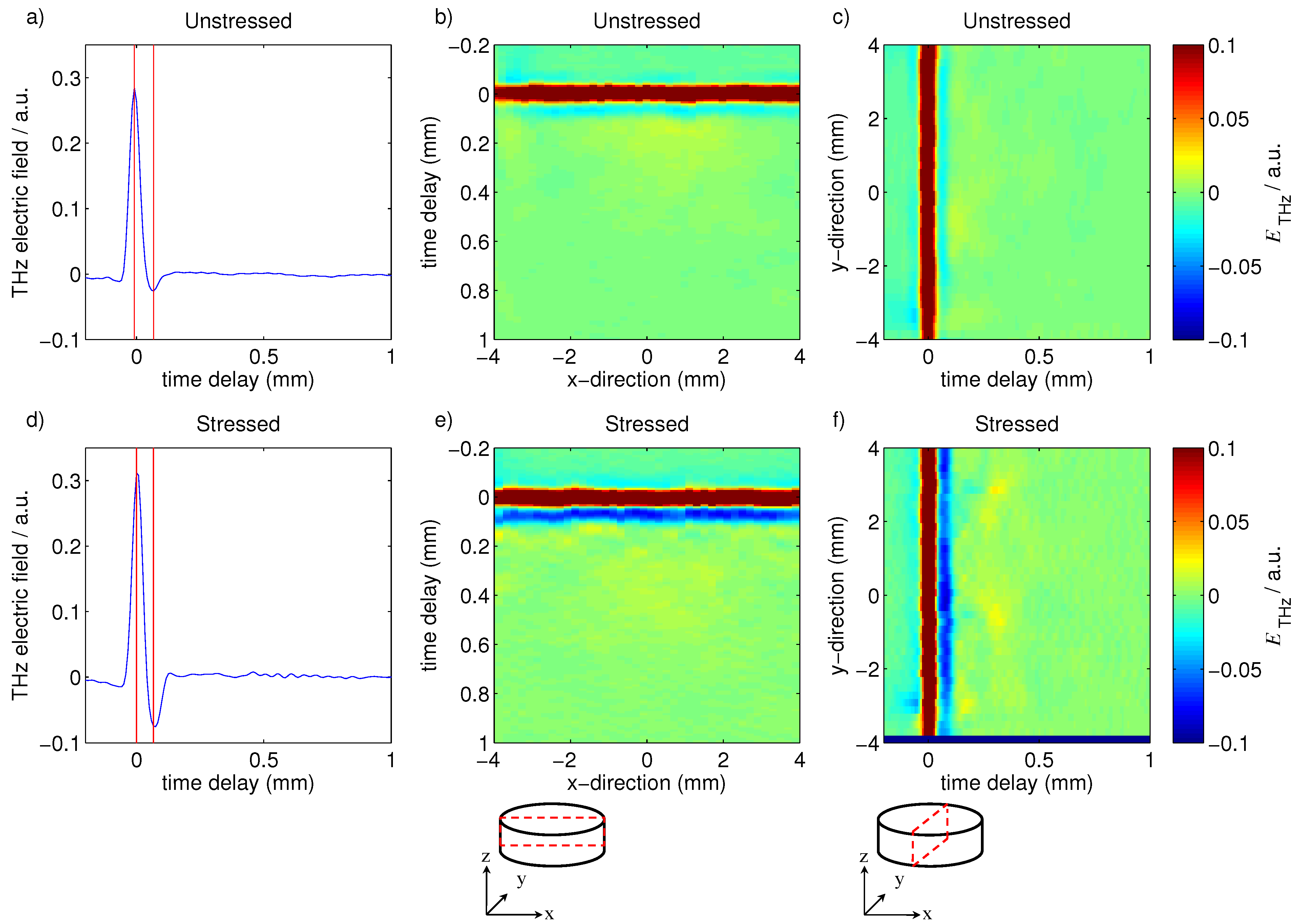
3.1.2. Tablet Dissolution Testing
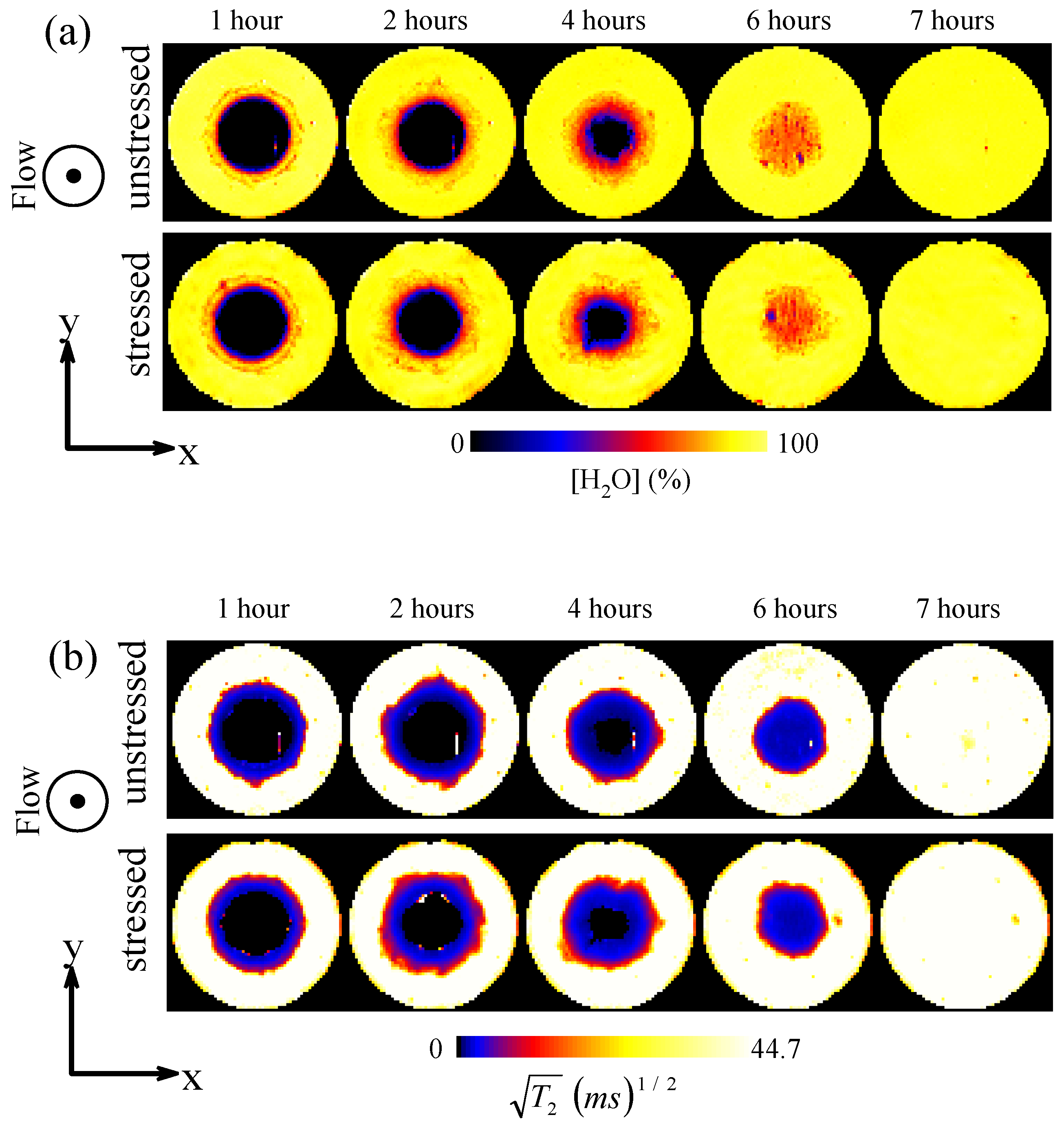
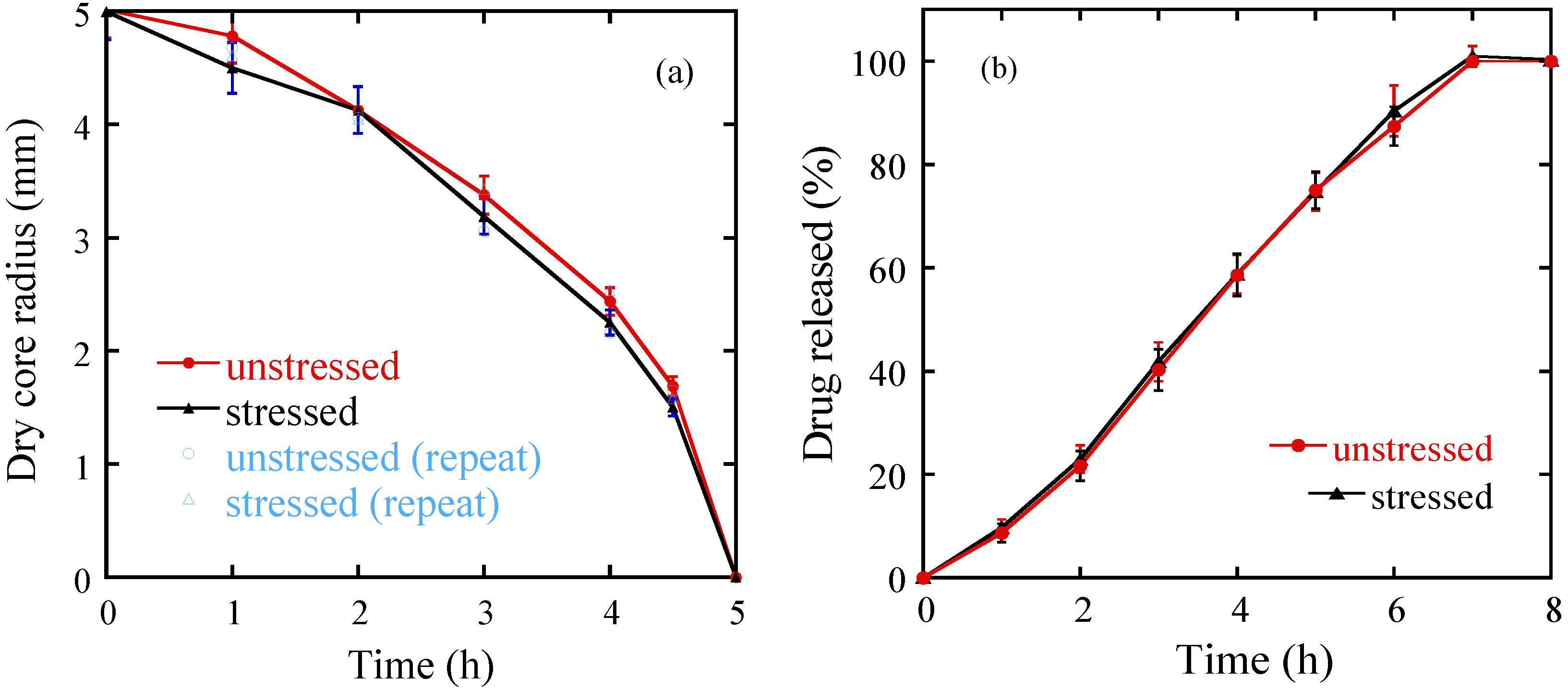
3.2. Discussion
3.2.1. The Effect of Storage on Tablet Coating in Lescol® XL
3.2.2. The Effect of Storage on Dissolution Processes
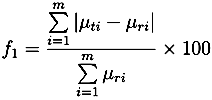

| Lescol® XL | |
|---|---|
| f1 | 2.1 |
| f2 | 99.9 |
3.2.3. The Effect of the Coating Layer on Drug Release
4. Conclusions
Acknowledgments
References
- Melveger, A.J.; Huynh-Ba, K. Critical Regulatory Requirements for a Stability Program. In Handbook of Stability Testing in Pharmaceutical Development; Springer: New York, NY, USA, 2009; pp. 9–19. [Google Scholar]
- Risha, P.G.; Vervaet, C.; Vergote, G.; van Bortel, L.; Remon, J.P. Drug formulations intended for the global market should be tested for stability under tropical climatic conditions. Eur. J. Clin. Pharmacol. 2003, 59, 135–141. [Google Scholar]
- Papandreou, G.; Zorpas, K.; Archontaki, H. Development and validation of a liquid chromatographic method for the simultaneous determination of aniracetam and its related substances in the bulk drug and a tablet formulation. Chem. Phys. Lett. 2011, 56, 615–622. [Google Scholar]
- Süvegh, K.; Zelkó, R. Physical aging of poly(vinylpyrrolidone) under different humidity conditions. Macromolecules 2002, 35, 795–800. [Google Scholar] [CrossRef]
- Goskonda, V.R.; Reddy, I.K.; Durrani, M.J.; Wilber, W.; Khan, M.A. Solid-state stability assessment of controlled release tablets containing Carbopol® 971P. J. Control. Release 1998, 54, 87–93. [Google Scholar] [CrossRef]
- Waterman, K.C.; MacDonald, B.C. Package selection for moisture protection for solid, oral drug products. J. Pharm. Sci. 2010, 99, 4437–4452. [Google Scholar] [CrossRef]
- Murthy, K.S.; Ghebre-Sellassie, I. Current perspectives on the dissolution stability of solid oral dosage forms. J. Pharm. Sci. 1993, 82, 113–126. [Google Scholar] [CrossRef]
- Krishnaiah, Y.S.R.; Karthikeyan, R.S.; Gouri Sankar, V.; Satyanarayana, V. Three-layer guar gum matrix tablet formulations for oral controlled delivery of highly soluble trimetazidine dihydrochloride. J. Control. Release 2002, 81, 45–56. [Google Scholar] [CrossRef]
- Nafee, N.A.; Ismail, F.A.; Boraie, N.A.; Mortada, L.M. Mucoadhesive buccal patches of miconazole nitrate: In vitro/in vivo performance and effect of ageing. Int. J. Pharm. 2003, 264, 1–14. [Google Scholar] [CrossRef]
- Rohrs, B.; Thamann, T.; Gao, P.; Stelzer, D.; Bergren, M.; Chao, R. Tablet dissolution affected by a moisture mediated solid-state interaction between drug and disintegrant. Pharm. Res. 1999, 16, 1850–1856. [Google Scholar] [CrossRef]
- Wang, J.T.; Shiu, G.K.; Ong-Chen, T.; Viswanathan, C.T.; Skelly, J.P. Effects of humidity and temperature on in vitro dissolution of carbamazepine tablets. J. Pharm. Sci. 1993, 82, 1002–1005. [Google Scholar] [CrossRef]
- Hirasawa, N.; Ishise, S.; Miyata, H.; Danjo, K. An attempt to stabilize nilvadipine solid dispersion by the use of ternary systems. Drug Dev. Ind. Pharm. 2003, 29, 997–1004. [Google Scholar] [CrossRef]
- Engineer, S.; Shao, Z.J.; Khagani, N.A. Temperature/humidity sensitivity of sustained—Release formulations containing Kollidon® SR. Drug Dev. Ind. Pharm. 2004, 30, 1089–1094. [Google Scholar] [CrossRef]
- Zeitler, J.A.; Gladden, L.F. In vitro tomography and non-destructive imaging at depth of pharmaceutical solid dosage forms. Eur. J. Pharm. Biopharm. 2009, 71, 2–22. [Google Scholar] [CrossRef]
- Ho, L.; Mueller, R.; Gordon, K.C.; Kleinebudde, P.; Pepper, M.; Rades, T.; Shen, Y.; Taday, P.F.; Zeitler, J.A. Applications of terahertz pulsed imaging to sustained-release tablet film coating quality assessment and dissolution performance. J. Control. Release 2008, 127, 79–87. [Google Scholar] [CrossRef]
- Shen, Y.; Taday, P.F. Development and application of terahertz pulsed imaging for nondestructive inspection of pharmaceutical tablet. IEEE J. Sel Top. Quantum Electron. 2008, 14, 407–415. [Google Scholar] [CrossRef]
- Zhang, Q.; Gladden, L.F.; Avalle, P.; Mantle, M. In vitro quantitative 1H and 19F nuclear magnetic resonance spectroscopy and imaging studies of fluvastatin™ in Lescol® XL tablets in a USP-IV dissolution cell. J. Control. Release 2011, 156, 345–354. [Google Scholar] [CrossRef]
- Chen, Y.Y.; Hughes, L.P.; Gladden, L.F.; Mantle, M.D. Quantitative ultra-fast MRI of HPMC swelling and dissolution. J. Pharm. Sci. 2010, 99, 3462–3472. [Google Scholar]
- Ho, L.; Mueller, R.; Krueger, C.; Gordon, K.C.; Kleinebudde, P.; Pepper, M.; Rades, T.; Shen, Y.; Taday, P.F.; Zeitler, J.A. Investigating dissolution performance critical areas on coated tablets: A case study using terahertz pulsed imaging. J. Pharm. Sci. 2010, 99, 392–402. [Google Scholar] [CrossRef]
- Russe, I.S.; Brock, D.; Knop, K.; Kleinebudde, P.; Zeitler, J.A. Validation of terahertz coating thickness measurements using X-ray microtomography. Mol. Pharm. 2012, 9, 3551–3559. [Google Scholar] [CrossRef]
- May, R.K.; Evans, M.J.; Zhong, S.; Warr, I.; Gladden, L.F.; Shen, Y.; Zeitler, J.A. Terahertz in-line sensor for direct coating thickness measurement of individual tablets during film coating in real-time. J. Pharm. Sci. 2011, 100, 1535–1544. [Google Scholar] [CrossRef]
- Zeitler, J.A.; Shen, Y.; Baker, C.; Taday, P.F.; Pepper, M.; Rades, T. Analysis of coating structures and interfaces in solid oral dosage forms by three dimensional terahertz pulsed imaging. J. Pharm. Sci. 2007, 96, 330–340. [Google Scholar] [CrossRef]
- McGinity, J.; Felton, L. Aqueous Polymeric Coatings for Pharmaceutical Dosage Forms; Informa Healthcare: London, UK, 2008. [Google Scholar]
- Gendre, C.; Genty, M.; Silva, J.C.D.; Tfayli, A.; Boiret, M.; Lecoq, O.; Baron, M.; Chaminade, P.; Péan, J.M. Comprehensive study of dynamic curing effect on tablet coating structure. Eur. J. Pharm. Biopharm. 2012, 81, 657–665. [Google Scholar] [CrossRef]
- Palermo, R.; Cogdill, R.; Short, S.; Drennen Iii, J.; Taday, P.F. Density mapping and chemical component calibration development of four-component compacts via terahertz pulsed imaging. J. Pharm. Biomed. Anal. 2008, 46, 36–44. [Google Scholar] [CrossRef]
- May, R.K.; Su, K.; Han, L.; Zhong, S.; Elliott, J.A.; Gladden, L.F.; Evans, M.; Shen, Y.; Zeitler, J.A. Hardness and density distributions of pharmaceutical tablets measured by terahertz pulsed imaging. J. Pharm. Sci. 2013, 102, 2179–2186. [Google Scholar] [CrossRef]
- Moore, J.W.; Flanner, H.H. Mathematical comparison of dissolution profiles. Pharm. Technol. 1996, 20, 64–74. [Google Scholar]
- Nazzal, S.; Khan, M.A. Controlled release of a self-emulsifying formulation from a tablet dosage form: Stability assessment and optimization of some processing parameters. Int. J. Pharm. 2006, 315, 110–121. [Google Scholar] [CrossRef]
- Castellanos Gil, E.; Iraizoz Colarte, A.; Lara Sampedro, J.L.; Bataille, B. Subcoating with Kollidon VA 64 as water barrier in a new combined native dextran/HPMC–cetyl alcohol controlled release tablet. Eur. J. Pharm. Biopharm. 2008, 69, 303–311. [Google Scholar]
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Zhang, Q.; Gladden, L.F.; Avalle, P.; Zeitler, J.A.; Mantle, M.D. Terahertz Pulsed Imaging and Magnetic Resonance Imaging as Tools to Probe Formulation Stability. Pharmaceutics 2013, 5, 591-608. https://doi.org/10.3390/pharmaceutics5040591
Zhang Q, Gladden LF, Avalle P, Zeitler JA, Mantle MD. Terahertz Pulsed Imaging and Magnetic Resonance Imaging as Tools to Probe Formulation Stability. Pharmaceutics. 2013; 5(4):591-608. https://doi.org/10.3390/pharmaceutics5040591
Chicago/Turabian StyleZhang, Qilei, Lynn F. Gladden, Paolo Avalle, J. Axel Zeitler, and Michael D. Mantle. 2013. "Terahertz Pulsed Imaging and Magnetic Resonance Imaging as Tools to Probe Formulation Stability" Pharmaceutics 5, no. 4: 591-608. https://doi.org/10.3390/pharmaceutics5040591
APA StyleZhang, Q., Gladden, L. F., Avalle, P., Zeitler, J. A., & Mantle, M. D. (2013). Terahertz Pulsed Imaging and Magnetic Resonance Imaging as Tools to Probe Formulation Stability. Pharmaceutics, 5(4), 591-608. https://doi.org/10.3390/pharmaceutics5040591