Bioavailability of Polyphenol Liposomes: A Challenge Ahead
Abstract
:1. Introduction
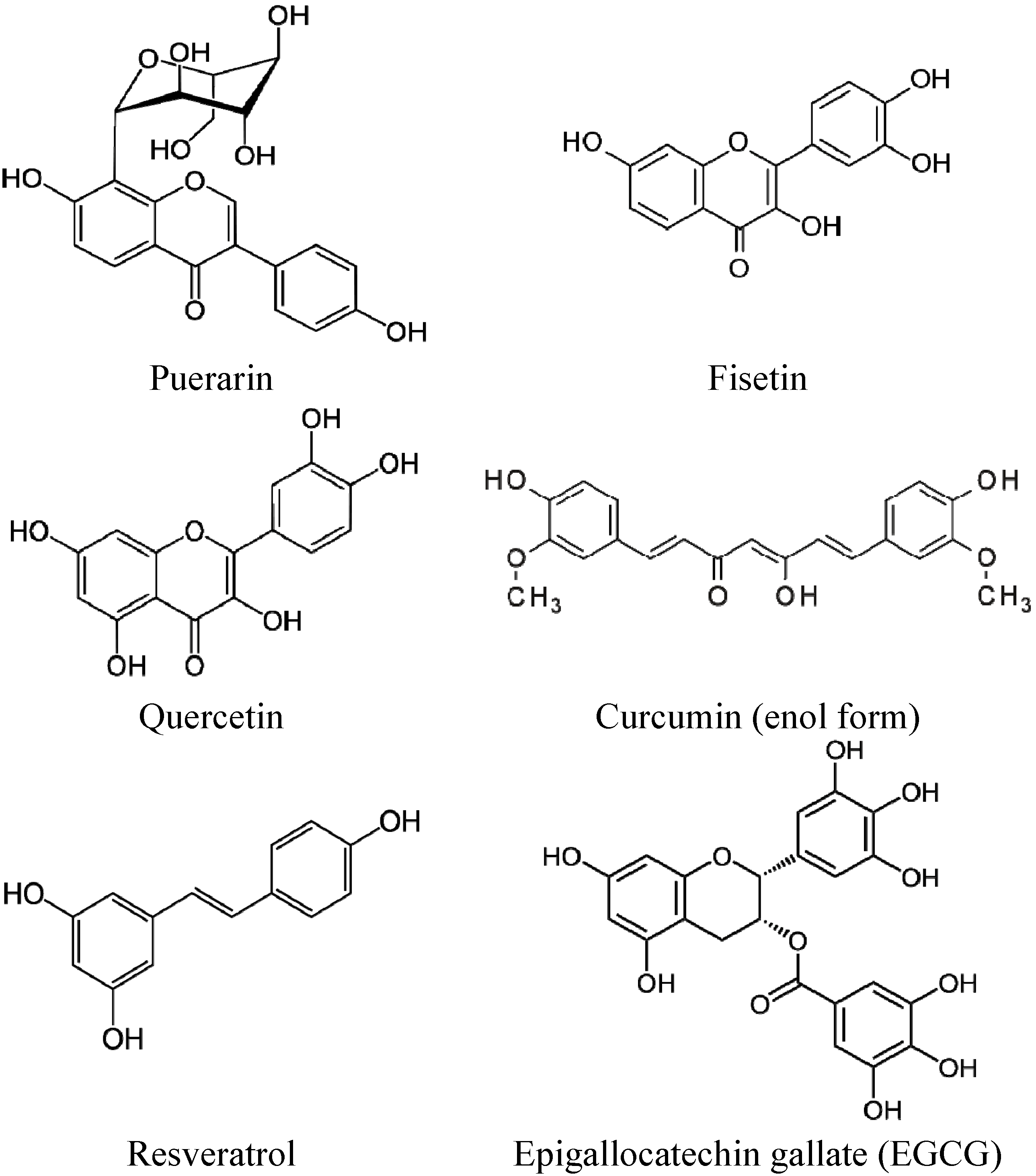
2. Liposomal Formulations of Polyphenols
2.1. Lipid-Polyphenol Molecular Interactions
2.2. Influence of the Lipid Charge in the Encapsulation and the Release of Polyphenol
3. Biological Effects of Liposomal Polyphenols
| Polyphenol | Formulation | % (w/w) 1 | Effect of the formulation 2 | References |
|---|---|---|---|---|
| Catechin | Epikuron 200/Chol/Tween 80/ethanol 6/1/80/13, film hydration | 0.3 | Increased bioavailability and cerebral distribution | [54] |
| Curcumin | SPC; film Hydration, extrusion, MLV | 3 | Prolonged antioxidant protective effect | [52] |
| Curcumin | DMPC or DMPC/DMPG lyophilisate | 10 to 25 | Similar efficacy in vitro. Antiangiogenic effect and tumor growth reduction in vivo | [47,55] |
| Curcumin/resveratrol | DMPC, lyophilisate | 20 | Improved bioavailability and reduction of prostate cancer incidence | [56] |
| Dehydro-silymarin | SPC/Chol/IPM/sodium cholate 1.5/0.3/1/1 film + freeze-drying | 25 | Increased oral bioavailability | [48] |
| EGCG Catechin | EPC/Chol/DA 4/1/0.25 film hydration + sonication or extrusion | 20 | Protection from degradation; Increased carcinoma cell death at lower concentrations | [53] |
| Fisetin | DOPC/DOPC/DODA-PEG2000/Fis 8/1.3/0.4/0.3 film hydration + extrusion, MLV | 18 | Increased bioavailability and antitumor efficacy | [25,37] |
| Quercetin | PE/Chol/DPC/QC 7/1/1/1 film hydration + sonication | 10 | Antioxidative effect with the formulation (i.v.) | [57] |
| Quercetin | Lecithin/Chol/ PEG 4000film hydration + lyophilization, SUV | 30 | Increased solubility, bioavailability and antitumor efficacy in vivo (i.v.) | [24] |
| Resveratrol | DPPC/DSPE PEG2000/Chol 1.85/0.15/1 film Hydration + extrusion | 0.1–5 | Improved solubility and chemical stability | [51] |
| Resveratrol | P90G/DCP/Chol sonication + extrusion | 1.5 | Prolonged efficacy and improved protection from UV B | [58] |
| Silymarin | Lecithin/Chol/stearyl amine/Tween 20 9/1/1/0.5 film hydration | Increased stability, bioavailability and liver protection | [59,60] | |
| Silymarin | Mannitol, phospholipids proliposomes | 20 | Improved oral bioavailability | [50] |
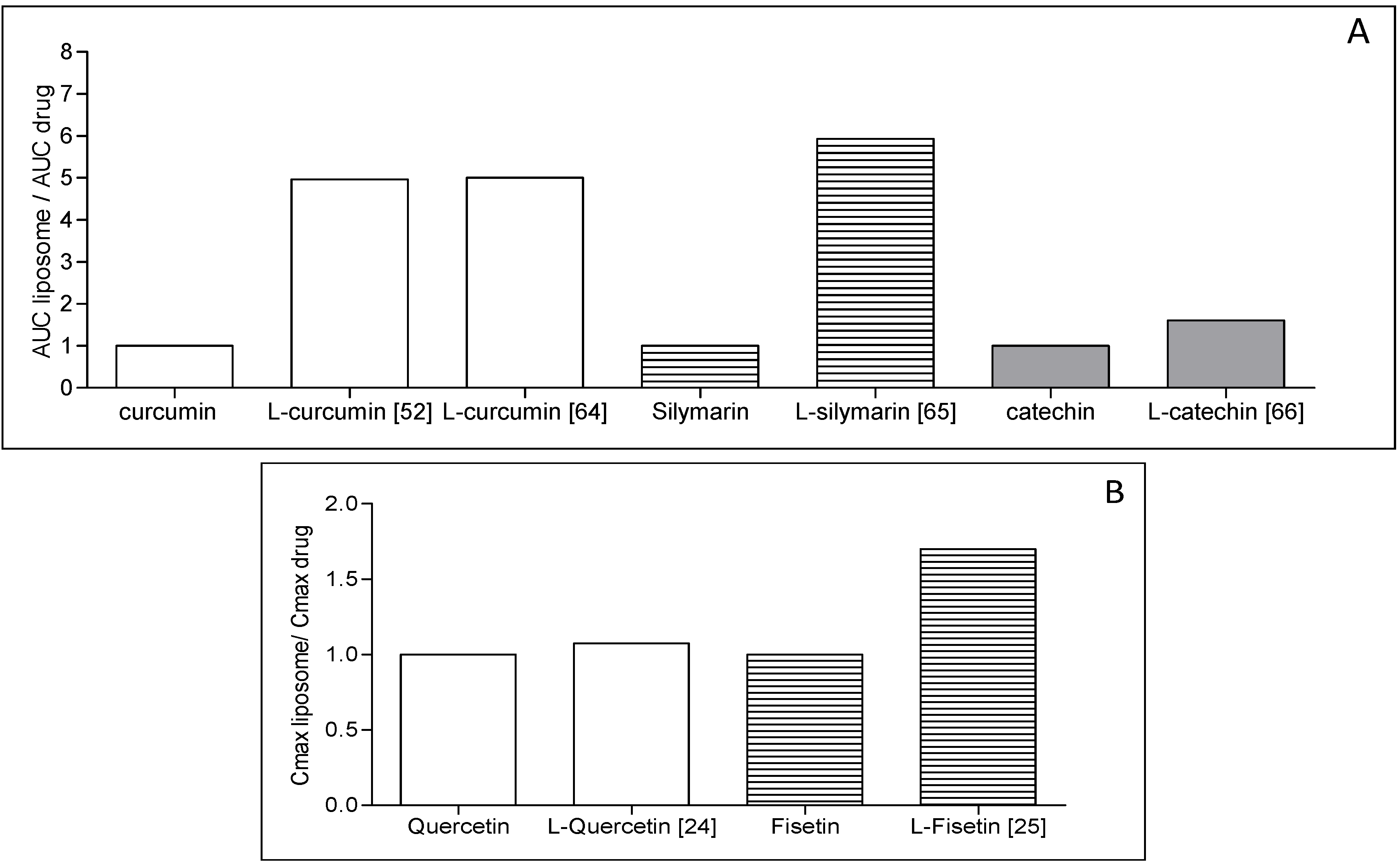
4. Liposome Development Issues
5. Concluding Remarks
Abbreviations
| Chol | cholesterol |
| DA | deoxycholic acid |
| DC-Chol | dicarbamate-cholesterol |
| DMPC | dimyristoyl-phosphatidylcholine |
| DODA-PEG | dioctadecylcarbamoylmethoxyacetylamino)acetic acid(methoxy)-polyethylene glycol |
| DOPC | dioleoyl-phosphatidylcholine |
| DPPC | dipalmitoyl-phosphatidylcholine |
| EGCG | epigallocatechin gallate |
| EPC | egg phosphatidylcholine |
| HLB | hydrophilic-lipophilic balance |
| QC | quercetin, NMR, Nuclear Magnetic Resonance |
| SUV | Small Unilamellar Vesicle |
| MLV | Multilamellar Vesicle |
| DMSO | dimethylsulfoxide |
| CD-31 | Cluster of Differentiation 31 |
| VEGF | Anti-vascular endothelial growth factor |
| IL-8 | Interleukin-8 |
Acknowledgments
Conflicts of Interest
References
- Harborne, J.B. Nature, Distribution, and Function of Plant Flavonoids. In Plant Flavonoids in Biology and Medicine—Biochemical, Pharmacological, and Structure-Activity Relationships; Cody, V., Middleton, E., Jr., Harborne, J.B., Eds.; Alan R. Liss, Inc.: New York, NY, USA, 1986; pp. 15–24. [Google Scholar]
- Havsteen, B.H. The biochemistry and medical significance of the flavonoids. Pharmacol. Ther. 2002, 96, 67–202. [Google Scholar] [CrossRef]
- Winkel-Shirley, B. Biosynthesis of flavonoids and effects of stress. Curr. Opin. Plant Biol. 2002, 5, 218–223. [Google Scholar] [CrossRef]
- Di Carlo, G.; Mascolo, N.; Izzo, A.A.; Capasso, F. Flavonoids: Old and new aspects of a class of natural therapeutic drugs. Life Sci. 1999, 65, 337–353. [Google Scholar] [CrossRef]
- Nijveldt, R.J.; van Nood, E.; van Hoorn, D.E.; Boelens, P.G.; van Norren, K.; van Leeuwen, P.A. Flavonoids: A review of probable mechanisms of action and potential applications. Am. J. Clin. Nutr. 2001, 74, 418–425. [Google Scholar]
- Arts, I.C. A review of the epidemiological evidence on tea, flavonoids, and lung cancer. J. Nutr. 2008, 138, 1561S–1566S. [Google Scholar]
- Gates, M.A.; Tworoger, S.S.; Hecht, J.L.; de Vivo, I.; Rosner, B.; Hankinson, S.E. A prospective study of dietary flavonoid intake and incidence of epithelial ovarian cancer. Int. J. Cancer 2007, 121, 2225–2232. [Google Scholar] [CrossRef]
- Peterson, J.; Lagiou, P.; Samoli, E.; Lagiou, A.; Katsouyanni, K.; La Vecchia, C.; Dwyer, J.; Trichopoulos, D. Flavonoid intake and breast cancer risk: A case-control study in Greece. Br. J. Cancer 2003, 89, 1255–1259. [Google Scholar] [CrossRef]
- Asensi, M.; Ortega, A.; Mena, S.; Feddi, F.; Estrela, J.M. Natural polyphenols in cancer therapy. Crit. Rev. Clin. Lab. Sci. 2011, 48, 197–216. [Google Scholar] [CrossRef]
- Manach, C.; Scalbert, A.; Morand, C.; Remesy, C.; Jimenez, L. Polyphenols: Food sources and bioavailability. Am. J. Clin. Nutr. 2004, 79, 727–747. [Google Scholar]
- US Department of Agriculture. Database for the Flavonoid Content of Selected Foods—Release 2.1. Beltsville, Maryland, USA, 2007. Available online: http://www.ars.usda.gov/nutrientdata (accessed on 7 June 2013).
- US Department of Agriculture. Database for the Isoflavone Content of Selected Foods—Release 2.0. Beltsville, Maryland, USA, 2008. Available online: http://www.ars.usda.gov/nutrientdata (accessed on 7 June 2013).
- Aherne, S.A.; O’Brien, N.M. Dietary flavonols: Chemistry, food content, and metabolis. Nutrition 2002, 18, 75–81. [Google Scholar] [CrossRef]
- Scalbert, A.; Williamson, G. Dietary intake and bioavailability of polyphenols. J. Nutr. 2000, 130, 2073S–2085S. [Google Scholar]
- Brat, P.; George, S.; Bellamy, A.; Du, C.L.; Scalbert, A.; Mennen, L.; Arnault, N.; Amiot, M.J. Daily polyphenol intake in France from fruit and vegetables. J. Nutr. 2006, 136, 2368–2373. [Google Scholar]
- Tsao, R. Chemistry and biochemistry of dietary polyphenols. Nutrients 2010, 2, 1231–1246. [Google Scholar] [CrossRef]
- D’Archivio, M.; Filesi, C.; Vari, R.; Scazzocchio, B.; Masella, R. Bioavailability of the polyphenols: Status and controversies. Int. J. Mol. Sci. 2010, 11, 1321–1342. [Google Scholar] [CrossRef]
- Haslam, E.; Cai, Y. Plant polyphenols (vegetable tannins): Gallic acid metabolism. Nat. Prod. Rep. 1994, 11, 41–66. [Google Scholar] [CrossRef]
- Chen, J.; Lin, H.; Hu, M. Metabolism of flavonoids via enteric recycling: Role of intestinal disposition. J. Pharmacol. Exp. Ther. 2003, 304, 1228–1235. [Google Scholar] [CrossRef]
- Mulholland, P.J.; Ferry, D.R.; Anderson, D.; Hussain, S.A.; Young, A.M.; Cook, J.E.; Hodgkin, E.; Seymour, L.W.; Kerr, D.J. Pre-clinical and clinical study of QC12, a water-soluble, pro-drug of quercetin. Ann. Oncol. 2001, 12, 245–248. [Google Scholar] [CrossRef]
- Pralhad, T.; Rajendrakumar, K. Study of freeze-dried quercetin-cyclodextrin binary systems by DSC, FT-IR, X-ray diffraction and SEM analysis. J. Pharm. Biomed. Anal. 2004, 34, 333–339. [Google Scholar] [CrossRef]
- Barras, A.; Mezzetti, A.; Richard, A.; Lazzaroni, S.; Roux, S.; Melnyk, P.; Betbeder, D.; Monfilliette-Dupont, N. Formulation and characterization of polyphenol-loaded lipid nanocapsules. Int. J. Pharm. 2009, 379, 270–277. [Google Scholar]
- Ragelle, H.; Crauste-Manciet, S.; Seguin, J.; Brossard, D.; Scherman, D.; Arnaud, P.; Chabot, G.G. Nanoemulsion formulation of fisetin improves bioavailability and antitumour activity in mice. Int. J. Pharm. 2012, 427, 452–459. [Google Scholar] [CrossRef] [Green Version]
- Yuan, Z.P.; Chen, L.J.; Fan, L.Y.; Tang, M.H.; Yang, G.L.; Yang, H.S.; Du, X.B.; Wang, G.Q.; Yao, W.X.; Zhao, Q.M.; et al. Liposomal quercetin efficiently suppresses growth of solid tumors in murine models. Clin. Cancer Res. 2006, 12, 3193–3199. [Google Scholar] [CrossRef]
- Seguin, J.; Brulle, L.; Boyer, R.; Lu, Y.M.; Ramos, R.M.; Touil, Y.S.; Scherman, D.; Bessodes, M.; Mignet, N.; Chabot, G.G. Liposomal encapsulation of the natural flavonoid fisetin improves bioavailability and antitumor efficacy. Int. J. Pharm. 2013, 444, 146–154. [Google Scholar] [CrossRef]
- Khushnud, T.; Mousa, S.A. Potential role of naturally derived polyphenols and their nanotechnology delivery in cancer. Mol. Biotechnol. 2013, 55, 78–86. [Google Scholar] [CrossRef]
- Gregoriadis, G.; Florence, A.T. Liposomes in drug delivery. Clinical, diagnostic and ophthalmic potential. Drugs 1993, 45, 15–28. [Google Scholar] [CrossRef]
- Gregoriadis, G.; Florence, A.T.; Patel, H.M. Liposomes in Drug Delivery; Taylor and Francis Ltd.: London, UK, 1993. [Google Scholar]
- Chang, H.I.; Yeh, M.K. Clinical development of liposome-based drugs: Formulation, characterization, and therapeutic efficacy. Int. J. Nanomed. 2012, 7, 49–60. [Google Scholar]
- Immordino, M.L.; Dosio, F.; Cattel, L. Stealth liposomes: Review of the basic science, rationale, and clinical applications, existing and potential. Int. J. Nanomed. 2006, 1, 297–315. [Google Scholar] [CrossRef]
- Wagner, A.; Vorauer-Uhl, K. Liposome technology for industrial purposes. J. Drug Deliv. 2011, 2011, 591325. [Google Scholar]
- Maurer, N.; Fenske, D.B.; Cullis, P.R. Developments in liposomal drug delivery systems. Expert Opin. Biol. Ther. 2001, 1, 923–947. [Google Scholar] [CrossRef]
- Fahr, A.; van Hoogevest, P.; May, S.; Bergstrand, N.; Leigh, M.L.S. Transfer of lipophilic drugs between liposomal membranes and biological interfaces: Consequences for drug delivery. Eur. J. Pharm. Sci. 2005, 26, 251–265. [Google Scholar] [CrossRef]
- Rice-Evans, C.A.; Miller, N.J.; Paganga, G. Structure-antioxidant activity relationships of flavonoids and phenolic acids. Free Radic. Biol. Med. 1996, 20, 933–956. [Google Scholar] [CrossRef]
- Kumazawa, S.; Kajiya, K.; Naito, A.; Saito, H.; Tuzi, S.; Tanio, M.; Suzuki, M.; Nanjo, F.; Suzuki, E.; Nakayama, T. Direct evidence of interaction of a green tea polyphenol, epigallocatechin gallate, with lipid bilayers by solid-state Nuclear Magnetic Resonance. Biosci. Biotechnol. Biochem. 2004, 68, 1743–1747. [Google Scholar] [CrossRef]
- Bonechi, C.; Martini, S.; Ciani, L.; Lamponi, S.; Rebmann, H.; Rossi, C.; Ristori, S. Using liposomes as carriers for polyphenolic compounds: The case of trans-resveratrol. PLoS One 2012, 7, e41438. [Google Scholar]
- Mignet, N.; Seguin, J.; Ramos Romano, M.; Brullé, L.; Touil, Y.S.; Scherman, D.; Bessodes, M.; Chabot, G.G. Development of a liposomal formulation of the natural flavonoid fisetin. Int. J. Pharm. 2012, 423, 69–76. [Google Scholar] [CrossRef]
- Quan, D.Q.; Xu, G.X.; Wu, X.G. Studies on preparation and absolute bioavailability of a self-emulsifying system containing puerarin. Chem. Pharm. Bull. 2007, 55, 800–803. [Google Scholar] [CrossRef]
- Quan, D.Q.; Xu, G.X. Formulation optimization of self-emulsifying preparations of puerarin through self-emulsifying performances evaluation in vitro and pharmacokinetic studies in vivo. Yao Xue Xue Bao 2007, 42, 886–891. [Google Scholar]
- Hung, C.F.; Chen, J.K.; Liao, M.H.; Lo, H.M.; Fang, J.Y. Development and evaluation of emulsion-liposome blends for resveratrol delivery. J. Nanosci. Nanotechnol. 2006, 6, 2950–2958. [Google Scholar] [CrossRef]
- Uekusa, Y.; Takeshita, Y.; Ishii, T.; Nakayama, T. Partition coefficients of polyphenols for phosphatidylcholine investigated by HPLC with an immobilized artificial membrane column. Biosci. Biotechnol. Biochem. 2008, 72, 3289–3292. [Google Scholar] [CrossRef]
- Patra, D.; Ahmadieh, D.; Aridi, R. Study on interaction of bile salts with curcumin and curcumin embedded in dipalmitoyl-sn-glycero-3-phosphocholine liposome. Colloids Surf. B Biointerfaces 2013, 110, 296–304. [Google Scholar] [CrossRef]
- Oteiza, P.I.; Erlejman, A.G.; Verstraeten, S.V.; Keen, C.L.; Fraga, C.G. Flavonoid-membrane interactions: A protective role of flavonoids at the membrane surface? Clin. Dev. Immunol. 2005, 12, 19–25. [Google Scholar] [CrossRef]
- Fang, J.Y.; Hung, C.F.; Hwang, T.L.; Huang, Y.L. Physicochemical characteristics and in vivo deposition of liposome-encapsulated tea catechins by topical and intratumor administrations. J. Drug Target. 2005, 13, 19–27. [Google Scholar] [CrossRef]
- Uekusa, Y.; Kamihira, M.; Nakayama, T. Dynamic behavior of tea catechins interacting with lipid membranes as determined by NMR spectroscopy. J. Agric. Food Chem. 2007, 55, 9986–9992. [Google Scholar] [CrossRef]
- Ke, D.; Wang, X.; Yang, Q.; Niu, Y.; Chai, S.; Chen, Z.; An, X.; Shen, W. Spectrometric study on the interaction of dodecyltrimethylammonium bromide with curcumin. Langmuir 2011, 27, 14112–14117. [Google Scholar] [CrossRef]
- Li, L.; Braiteh, F.S.; Kurzrock, R. Liposome-encapsulated curcumin: In vitro and in vivo effects on proliferation, apoptosis, signaling, and angiogenesis. Cancer 2005, 104, 1322–1331. [Google Scholar] [CrossRef]
- Chu, C.; Tong, S.S.; Xu, Y.; Wang, L.; Fu, M.; Ge, Y.R.; Yu, J.N.; Xu, X.M. Proliposomes for oral delivery of dehydrosilymarin: Preparation and evaluation in vitro and in vivo. Acta Pharmacol. Sin. 2011, 32, 973–980. [Google Scholar] [CrossRef]
- Song, K.H.; Chung, S.J.; Shim, C.K. Preparation and evaluation of proliposomes containing salmon calcitonin. J. Control. Release 2002, 84, 27–37. [Google Scholar] [CrossRef]
- Xiao, Y.-Y.; Song, Y.-M.; Chen, Z.-P.; Ping, Q.-N. Preparation of silymarin proliposome: A new way to increase oral bioavailability of silymarin in beagle dogs. Int. J. Pharm. 2006, 319, 162–168. [Google Scholar] [CrossRef]
- Coimbra, M.; Isacchi, B.; van, B.L.; Torano, J.S.; Ket, A.; Wu, X.; Broere, F.; Metselaar, J.M.; Rijcken, C.J.; Storm, G.; et al. Improving solubility and chemical stability of natural compounds for medicinal use by incorporation into liposomes. Int. J. Pharm. 2011, 416, 433–442. [Google Scholar] [CrossRef]
- Tonnesen, H.H.; Smistad, G.; Agren, T.; Karlsen, J. Studies on curcumin and curcuminoids. XXIII: Effects of curcumin on liposomal lipid peroxidation. Int. J. Pharm. 1993, 90, 221–228. [Google Scholar] [CrossRef]
- Fang, J.Y.; Lee, W.R.; Shen, S.C.; Huang, Y.L. Effect of liposome encapsulation of tea catechins on their accumulation in basal cell carcinomas. J. Dermatol. Sci. 2006, 42, 101–109. [Google Scholar] [CrossRef]
- Huang, Y.B.; Tsai, M.J.; Wu, P.C.; Tsai, Y.H.; Wu, Y.H.; Fang, J.Y. Elastic liposomes as carriers for oral delivery and the brain distribution of (+)-catechin. J. Drug Target. 2011, 19, 709–718. [Google Scholar] [CrossRef]
- Li, L.; Ahmed, B.; Mehta, K.; Kurzrock, R. Liposomal curcumin with and without oxaliplatin: Effects on cell growth, apoptosis, and angiogenesis in colorectal cancer. Mol. Cancer Ther. 2007, 6, 1276–1282. [Google Scholar] [CrossRef]
- Narayanan, N.K.; Nargi, D.; Randolph, C.; Narayanan, B.A. Liposome encapsulation of curcumin and resveratrol in combination reduces prostate cancer incidence in PTEN knockout mice. Int. J. Cancer 2009, 125, 1–8. [Google Scholar] [CrossRef]
- Mandal, A.K.; Sinha, J.; Mandal, S.; Mukhopadhyay, S.; Das, N. Targeting of liposomal flavonoid to liver in combating hepatocellular oxidative damage. Drug Deliv. 2002, 9, 181–185. [Google Scholar] [CrossRef]
- Caddeo, C.; Teskac, K.; Sinico, C.; Kristl, J. Effect of resveratrol incorporated in liposomes on proliferation and UV-B protection of cells. Int. J. Pharm. 2008, 363, 183–191. [Google Scholar] [CrossRef]
- El-Samaligy, M.S.; Afifi, N.N.; Mahmoud, E.A. Evaluation of hybrid liposomes-encapsulated silymarin regarding physical stability and in vivo performance. Int. J. Pharm. 2006, 319, 121–129. [Google Scholar] [CrossRef]
- El-Samaligy, M.S.; Afifi, N.N.; Mahmoud, E.A. Increasing bioavailability of silymarin using a buccal liposomal delivery system: Preparation and experimental design investigation. Int. J. Pharm. 2006, 308, 140–148. [Google Scholar] [CrossRef]
- Wang, G.; Wang, J.J.; Yang, G.Y.; Du, S.M.; Zeng, N.; Li, D.S.; Li, R.M.; Chen, J.Y.; Feng, J.B.; Yuan, S.H.; et al. Effects of quercetin nanoliposomes on C6 glioma cells through induction of type III programmed cell death. Int. J. Nanomed. 2012, 7, 271–280. [Google Scholar] [CrossRef]
- Takahashi, M.; Uechi, S.; Takara, K.; Asikin, Y.; Wada, K. Evaluation of an oral carrier system in rats: Bioavailability and antioxidant properties of liposome-encapsulated curcumin. J. Agric. Food Chem. 2009, 57, 9141–9146. [Google Scholar] [CrossRef]
- Marczylo, T.H.; Verschoyle, R.D.; Cooke, D.N.; Morazzoni, P.; Steward, W.P.; Gescher, A.J. Comparison of systemic availability of curcumin with that of curcumin formulated with phosphatidylcholine. Cancer Chemother. Pharmacol. 2007, 60, 171–177. [Google Scholar] [CrossRef]
- Atrooz, O.M. The incorporation effects of methanolic extracts of some plant seeds on the stability of phosphatidylcholine liposomes. Pak. J. Biol. Sci. 2007, 10, 1643–1648. [Google Scholar] [CrossRef]
- Semple, S.C.; Chonn, A.; Cullis, P.R. Influence of cholesterol on the association of plasma proteins with liposomes. Biochemistry 1996, 35, 2521–2525. [Google Scholar] [CrossRef]
- Mu, X.; Zhong, Z. Preparation and properties of poly(vinyl alcohol)-stabilized liposomes. Int. J. Pharm. 2006, 318, 55–61. [Google Scholar] [CrossRef]
- Oja, C.D.; Semple, S.C.; Chonn, A.; Cullis, P.R. Influence of dose on liposome clearance: Critical role of blood proteins. Biochim. Biophys. Acta 1996, 1281, 31–37. [Google Scholar] [CrossRef]
- Khan, R.; Rezler, E.; Lauer-Fields, J.; Fields, G. Effects of drug hydrophobicity on liposomal stability. Chem. Biol. Drug Des. 2008, 71, 3–7. [Google Scholar]
- Ali, M.H.; Moghaddam, B.; Kirby, D.J.; Mohammed, A.R.; Perrie, Y. The role of lipid geometry in designing liposomes for the solubilisation of poorly water soluble drugs. Int. J. Pharm. 2013, 453, 225–232. [Google Scholar] [CrossRef]
- Bansal, S.S.; Goel, M.; Aqil, F.; Vadhanam, M.V.; Gupta, R.C. Advanced drug delivery systems of curcumin for cancer chemoprevention. Cancer Prev. Res. 2011, 4, 1158–1171. [Google Scholar] [CrossRef]
- Howells, L.M.; Berry, D.P.; Elliott, P.J.; Jacobson, E.W.; Hoffmann, E.; Hegarty, B.; Brown, K.; Steward, W.P.; Gescher, A.J. Phase I randomized, double-blind pilot study of micronized resveratrol (SRT501) in patients with hepatic metastases--safety, pharmacokinetics, and pharmacodynamics. Cancer Prev. Res. 2011, 4, 1419–1425. [Google Scholar] [CrossRef]
- Teiten, M.H.; Eifes, S.; Dicato, M.; Diederich, M. Curcumin-the paradigm of a multi-target natural compound with applications in cancer prevention and treatment. Toxins 2010, 2, 128–162. [Google Scholar] [CrossRef]
- Hirpara, K.V.; Aggarwal, P.; Mukherjee, A.J.; Joshi, N.; Burman, A.C. Quercetin and its derivatives: Synthesis, pharmacological uses with special emphasis on anti-tumor properties and prodrug with enhanced bio-availability. Anticancer Agents Med. Chem. 2009, 9, 138–161. [Google Scholar] [CrossRef]
- Walle, T. Methylation of dietary flavones greatly improves their hepatic metabolic stability and intestinal absorption. Mol. Pharm. 2007, 4, 826–832. [Google Scholar] [CrossRef]
- Rimando, A.M.; Cuendet, M.; Desmarchelier, C.; Mehta, R.G.; Pezzuto, J.M.; Duke, S.O. Cancer chemopreventive and antioxidant activities of pterostilbene, a naturally occurring analogue of resveratrol. J. Agric. Food Chem. 2002, 50, 3453–3457. [Google Scholar] [CrossRef]
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Mignet, N.; Seguin, J.; Chabot, G.G. Bioavailability of Polyphenol Liposomes: A Challenge Ahead. Pharmaceutics 2013, 5, 457-471. https://doi.org/10.3390/pharmaceutics5030457
Mignet N, Seguin J, Chabot GG. Bioavailability of Polyphenol Liposomes: A Challenge Ahead. Pharmaceutics. 2013; 5(3):457-471. https://doi.org/10.3390/pharmaceutics5030457
Chicago/Turabian StyleMignet, Nathalie, Johanne Seguin, and Guy G. Chabot. 2013. "Bioavailability of Polyphenol Liposomes: A Challenge Ahead" Pharmaceutics 5, no. 3: 457-471. https://doi.org/10.3390/pharmaceutics5030457
APA StyleMignet, N., Seguin, J., & Chabot, G. G. (2013). Bioavailability of Polyphenol Liposomes: A Challenge Ahead. Pharmaceutics, 5(3), 457-471. https://doi.org/10.3390/pharmaceutics5030457





