Application and Characterization of Gum from Bombax buonopozense Calyxes as an Excipient in Tablet Formulation
Abstract
:1. Introduction
2. Results and Discussion

2.1. Viscosity Analysis of Gum from Dried Calyx of Bombax buonopozense

2.2. Characterization of Granules
| Test | Binder | ||
|---|---|---|---|
| Bombax gum | Acacia gum | Tragacanth gum | |
| Flow rate (g/sec) | 6.15 ± 0.05 | 9.80 ± 0.10 | 7.75 ± 0.05 |
| Angle of repose (°) | 28.60 ± 0.10 | 26.50 ± 0.15 | 25.20 ± 0.10 |
| Bulk density (g/mL) | 0.487 ± 0.01 | 0.476 ± 0.01 | 0.465 ± 0.01 |
| Tapped density (g/mL) | 0.6200 ± 0.01 | 0.6250 ± 0.005 | 0.6061 ± 0.01 |
| % compressibility | 21.30 ± 0.35 | 23.80 ± 0.36 | 23.30 ± 0.4 |
| Hausner’s quotient | 1.27 ± 0.03 | 1.31 ± 0.03 | 1.30 ± 0.03 |
2.3. Evaluation of Tablets Properties
| Test | Binder | ||
|---|---|---|---|
| Bombax gum | Acacia gum | Tragacanth gum | |
| Friability (%) | 3.10 ± 0.03 | 7.54 ± 0.03 | 9.98 ± 0.03 |
| Mean Hardness (kp) 1 | 6.3 ± 0.27 | 5.5 ± 0.20 | 5.3 ± 0.1 |
| Disintegration time (Minutes) | >60.00 | 4.00 ± 0.17 | 2.00 ± 0.1 |
| Uniformity of weight | 660 ± 0.21 | 660 ± 1.1 | 634 ± 1.8 |
| Mean Diameter (mm) | 11.20 ± 0.1 | 11.20 ± 0.1 | 11.20 ± 0.1 |
| Mean Thickness (mm) | 3.5 ± 0.05 | 3.5 ± 0.1 | 3.5 ± 0.06 |
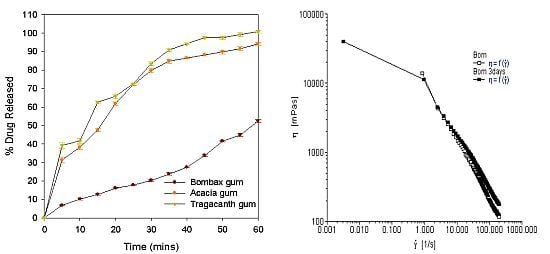
| % drug release at T45 | 33.90 ± 0.63 | 88.14 ± 0.60 | 97.5 ± 0.61 |
| % drug release at T60 | 52.50 ± 0.7 | 94.0 ± 0.75 | 100.8 ± 0.8 |
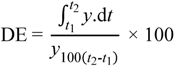
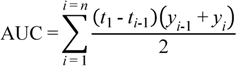
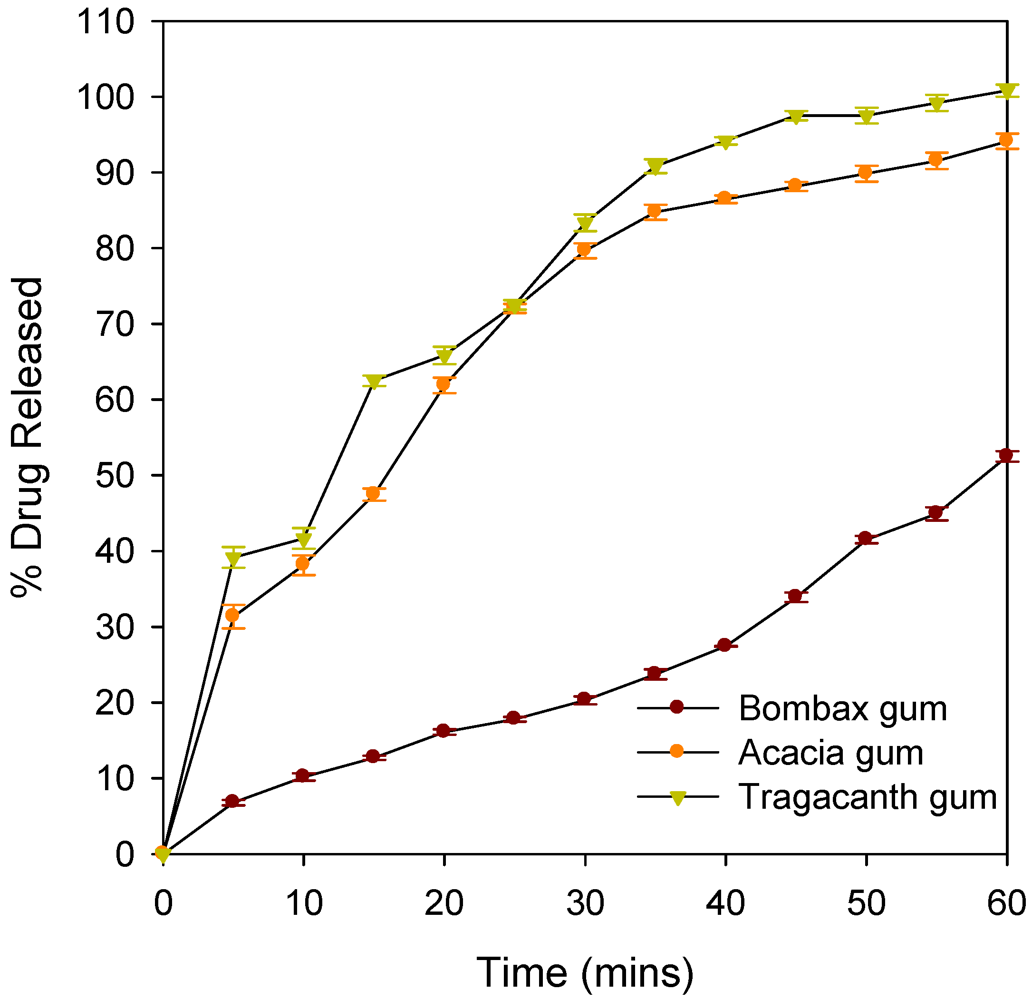
| Binder | Zero Order | First Order | DE (%) | ||||
|---|---|---|---|---|---|---|---|
| r | r2 | K0 | r | r2 | K1 | ||
| Bombax Gum | 0.9835 | 0.9673 | 0.7947 | 0.9588 | 0.9192 | −0.0049 | 44.65 |
| Acacia Gum | 0.9228 | 0.8516 | 1.3804 | 0.9923 | 0.9846 | −0.0196 | 72.48 |
| Tragacanth Gum | 0.9184 | 0.8435 | 1.4375 | 0.9803 | 0.9609 | −0.0367 | 73.95 |
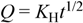
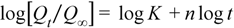
| Binder | Higuchi | Korsmeyer-Peppas | n | ||||
|---|---|---|---|---|---|---|---|
| r | r2 | KH | r | r2 | KKP | ||
| Bombax Gum | 0.9271 | 0.8056 | 4.983 | 0.9877 | 0.9727 | 0.471 | 1.136 |
| Acacia Gum | 0.9858 | 0.9705 | 13.154 | 0.9875 | 0.9750 | 15.824 | 0.449 |
| Tragacanth Gum | 0.9866 | 0.9678 | 14.275 | 0.9905 | 0.9811 | 19.437 | 0.415 |
3. Experimental Section
3.1. Materials
3.2. Extraction of Gum from Dried Calyx of Bombax buonopozense
3.3. Viscosity Determination of Gum from Dried Calyx of Bombax buonopozense
3.4. Preparation and Characterization of Granules
| Components | Percentage |
|---|---|
| Maize starch | 15% |
| Binder | 3.5% |
| Talc | 2% |
| Magnesium stearate | 0.5% |
| Paracetamol | 79% |
3.5. Granules Compression and Tablet Evaluation
3.6. In vitro Drug Release Studies
3.7. Data Analysis
4. Conclusions
References
- Ursino, M.G.; Poluzzi, E.; Caramella, C.; de Ponti, F. Excipients in medicinal products used in gastroenterology as a possible cause of side effects. Regul. Toxicol. Pharmacol. 2011, 60, 93–105. [Google Scholar] [CrossRef]
- Manjanna, K.; Kumar, T.; Shivakumar, B. Natural polysaccharide hydrogels as novel excipients for modified drug delivery systems: A review. Int. J. Chem. Tech. Res. 2010, 2, 509–525. [Google Scholar]
- Ejikeme, P. Investigation of the physicochemical properties of microcrystalline cellulose from agricultural wastes I: Orange mesocarp. Cellulose 2008, 15, 1411–1447. [Google Scholar]
- Beneke, C.E.; Viljoen, A.M.; Hamman, J.H. Polymeric plant-derived excipients in drug delivery. Molecules 2009, 14, 2602–2620. [Google Scholar] [CrossRef]
- Addo-Fordjour, P.; Obeng, S.; Addo, M.G.; Akyeampong, S. Effects of human disturbances and plant invasion on Liana community structure and relationship with trees in the Tinte Bepo Forest Reserve, Ghana. For. Ecol. Manag. 2009, 258, 728–734. [Google Scholar] [CrossRef]
- Gyasi, E.; Agyepong, G.; Ardayfio-Schandorf, E.; Enu-Kwesi, L.; Nabila, J.; Owusu-Bennoah, E. Production pressure and environmental change in the forest-savanna zone of Southern Ghana. Glob. Environ. Chang. 1995, 5, 355–366. [Google Scholar] [CrossRef]
- Madge, C. Therapeutic landscapes of the Jola, the Gambia, West Afric. Health Place 1998, 4, 293–311. [Google Scholar] [CrossRef]
- Kamanzi Atindehou, K.; Schmid, C.; Brun, R.; Koné, M.W.; Traore, D. Antitrypanosomal and antiplasmodial activity of medicinal plants from Côte d’ivoire. J. Ethnopharmacol. 2004, 90, 221–227. [Google Scholar] [CrossRef]
- Asase, A.; Oppong-Mensah, G. Traditional antimalarial phytotherapy remedies in herbal markets in Southern Ghana. J. Ethnopharmacol. 2009, 126, 492–499. [Google Scholar] [CrossRef]
- Agyare, C.; Asase, A.; Lechtenberg, M.; Niehues, M.; Deters, A.; Hensel, A. An ethnopharmacological survey and in vitro confirmation of ethnopharmacological use of medicinal plants used for wound healing in Bosomtwi-Atwima-Kwanwoma area, Ghana. J. Ethnopharmacol. 2009, 125, 393–403. [Google Scholar] [CrossRef]
- Mann, A.; Salawu, F.B.; Abdulrauf, I. Antimicrobial activity of Bombax buonopozense p. beauv. (Bombacaceae) edible floral extracts. Eur. J. Sci. Res. 2011, 48, 627–630. [Google Scholar]
- Oliver-Bever, B. Medicinal plants in tropical West Africa III. Anti-infection therapy with higher plants. J. Ethnopharmacol. 1983, 9, 1–83. [Google Scholar] [CrossRef]
- Insoll, T. Natural’ or ‘human’ spaces? Tallensi sacred groves and shrines and their potential implications for aspects of northern European prehistory and phenomenological interpretation. Nor. Archaeol. Rev. 2007, 40, 138. [Google Scholar] [CrossRef]
- Ngwuluka, N.; Idiakhoa, B.; Nep, E.; Ogaji, I.; Okafor, I. Formulation and evaluation of paracetamol tablets manufactured using the dried fruit of Phoenix dactylifera linn as an excipient. Res. Pharm. Biotechnol. 2010, 2, 25–32. [Google Scholar]
- Virtanen, S.; Antikainen, O.; Räikkönen, H.; Yliruusi, J. Granule size distribution of tablets. J. Pharm. Sci. 2010, 99, 2061–2069. [Google Scholar]
- Reddy, K.; Mutalik, S.; Reddy, S. Once-daily sustained-release matrix tablets of nicorandil: Formulation and in vitro evaluation. AAPS PharmSciTech 2003, 4, 480–488. [Google Scholar] [CrossRef]
- Panda, D.; Choudhury, N.S.K.; Yedukondalu, M.; Si, S.; Gupta, R. Evaluation of gum of Moringa oleifera as a binder and release retardant in tablet formulation. Ind. J. Pharm. Sci. 2008, 70, 614–618. [Google Scholar] [CrossRef]
- Bacher, C.; Olsen, P.M.; Bertelsen, P.; Sonnergaard, J.M. Compressibility and compactibility of granules produced by wet and dry granulation. Int. J. Pharm. 2008, 358, 69–74. [Google Scholar] [CrossRef]
- Mehrotra, A.; Chaudhuri, B.; Faqih, A.; Tomassone, M.S.; Muzzio, F.J. A modeling approach for understanding effects of powder flow properties on tablet weight variability. Powder Technol. 2009, 188, 295–300. [Google Scholar] [CrossRef]
- United States Pharmacopeial Convention, The United States Pharmacopeia, USP 23. The National Formulary, NF 18: Official from January 1, 1995, 23rd ed; United States Pharmacopeial Convention, Inc.: Chicago, IL, USA, 1994; Volume 2.
- Allen, L.V.; Popovich, N.G.; Ansel, H.C. Ansel’s Pharmaceutical Dosage Forms and Drug Delivery Systems, 8th ed; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2004; p. 236. [Google Scholar]
- Riippi, M.; Antikainen, O.; Niskanen, T.; Yliruusi, J. The effect of compression force on surface structure, crushing strength, friability and disintegration time of erythromycin acistrate tablet. Eur. J. Pharm. Biopharm. 1998, 46, 339–345. [Google Scholar] [CrossRef]
- Anderson, N.H.; Bauer, M.; Boussac, N.; Khan-Malek, R.; Munden, P.; Sardaro, M. An evaluation of fit factors and dissolution efficiency for the comparison of in vitro dissolution profiles. J. Pharm. Biomed. Anal. 1998, 17, 811–822. [Google Scholar] [CrossRef]
- Merchant, H.; Shoaib, H.; Tazeen, J.; Yousuf, R. Once-daily tablet formulation and in vitro release evaluation of cefpodoxime using hydroxypropyl methylcellulose: A technical note. AAPS PharmSciTech 2006, 7, E178–E183. [Google Scholar] [CrossRef]
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Ngwuluka, N.C.; Kyari, J.; Taplong, J.; Uwaezuoke, O.J. Application and Characterization of Gum from Bombax buonopozense Calyxes as an Excipient in Tablet Formulation. Pharmaceutics 2012, 4, 354-365. https://doi.org/10.3390/pharmaceutics4030354
Ngwuluka NC, Kyari J, Taplong J, Uwaezuoke OJ. Application and Characterization of Gum from Bombax buonopozense Calyxes as an Excipient in Tablet Formulation. Pharmaceutics. 2012; 4(3):354-365. https://doi.org/10.3390/pharmaceutics4030354
Chicago/Turabian StyleNgwuluka, Ndidi C., Jehu Kyari, John Taplong, and Onyinye J. Uwaezuoke. 2012. "Application and Characterization of Gum from Bombax buonopozense Calyxes as an Excipient in Tablet Formulation" Pharmaceutics 4, no. 3: 354-365. https://doi.org/10.3390/pharmaceutics4030354