Nano Delivers Big: Designing Molecular Missiles for Cancer Therapeutics
Abstract
: Current first-line treatments for most cancers feature a short-list of highly potent and often target-blind interventions, including chemotherapy, radiation, and surgical excision. These treatments wreak considerable havoc upon non-cancerous tissue and organs, resulting in deleterious and sometimes fatal side effects for the patient. In response, this past decade has witnessed the robust emergence of nanoparticles and, more relevantly, nanoparticle drug delivery systems (DDS), widely touted as the panacea of cancer therapeutics. While not a cure, nanoparticle DDS can successfully negotiate the clinical payoff between drug dosage and side effects by encompassing target-specific drug delivery strategies. The expanding library of nanoparticles includes lipoproteins, liposomes, dendrimers, polymers, metal and metal oxide nano-spheres and -rods, and carbon nanotubes, so do the modes of delivery. Importantly, however, the pharmaco-dynamics and –kinetics of these nano-complexes remain an urgent issue and a serious bottleneck in the transition from bench to bedside. This review addresses the rise of nanoparticle DDS platforms for cancer and explores concepts of gene/drug delivery and cytotoxicity in pre-clinical and clinical contexts.1. Introduction
Nanotechnology is, in part, a science of synthesizing molecular sized materials that can range from a few nanometers to micrometers that are invisible to the human eye [1-3]. At this nanoscale, the tensile strength, opto-electrical properties, and surface chemistry of materials become radically changed. These features, if successfully exploited, could revolutionize medicine [4,5]. The application of nanotechnology in medicine is now known as nanomedicine, and the drug formulations using the nanomaterials are referred to as nanoformulations [6-8].
Despite recent progress in decreasing mortality, cancer still ranks as the second most frequent cause of death in the United States [9]. In this regard, advances in nanoformulated drug delivery systems (DDS) hold great promise for improved cancer patient outcomes. These DDS have been designed with formulations that were initially based upon drugs loaded into liposomes or polymer nanoparticles to improve their pharmacological properties and therapeutic outcome for parenterally administered drugs [10]. Using this approach, several anti-cancer DDS have now been approved for clinical use and have already greatly impacted oncological therapeutics [11]. More recently, research has moved toward development of related systems based on metal, metal oxide, and carbon nanoparticles which promise to solve some of the stability and toxicity problems of liposomal and polymer DDS [12-14]. These new drug delivery systems for cancer treatment with potentially reduced toxic side effects have enabled the development of improved therapeutic regimens with existing drugs that are currently standard-of-care. Examples of these delivery systems feature passive and active targeting, which essentially takes advantage of characteristic tumor features that allow nano-sized drug delivery systems to accumulate in the target area of the cancer; allow for superior cancer therapeutics by virtue of efficient cell entry; and lower toxicity. In this review, we describe examples of key developments in the area of cancer drug delivery systems using nanomaterials.
2. The Need for Nano
The arsenal of chemotherapeutic drugs used against most cancers is sufficiently potent to diminish their growth – that is, provided the drugs reach their desired location with high fidelity and efficiency. Owing to the non-specific and cytotoxic nature of first-line anti-cancer drugs, physicians are often forced to balance dosage with painful patient side effects. This war of attrition—a question of which cells are last to die, normal or cancerous—is further complicated by the high mutation frequency of certain cancers. As a result of a weakened drug regimen, multi-drug resistant cancer cell populations are more likely to evolve [15-17].
These shortcomings, among others, have led to a call for targeted drug delivery strategies that deliver a greater payload of drugs to the tumor site and limit the damage to normal, non-cancerous tissue [18]. The rapidly advancing field of nanomedicine has produced nanoparticle platforms that can ultimately function as molecular missiles, capable of delivering not only drugs but also genetic material, and acting as cancer-killing and imaging-contrast agents. The once small class of drug delivery vehicles that included only liposomes and polymers now comprises dendrimers, metallic and metal oxide nano-beads/shells, carbon nanotubes, and lipoproteins (Table 1) [19,20]. In a similar way, seek-and-destroy methods utilized are as variegated as their apparatuses and include receptor-mediated endocytosis, magnetically-directed localization, pH and thermal sensitive drug release, and external laser ablation, among others [21].
Though their versatility is unarguable, nanoparticle DDS toxicity still remains an impasse that researchers are attempting to navigate. Single-wall carbon nanotubes are hydrophobic in their natural pristine state, and unless oxidized they generally remain uncharged and neutral and susceptible to aggregating that can lead to arterial blockage in the lungs and kidneys of mice [37]. To address questions of biodistribution and toxicity, recent studies have demonstrated that when carbon nanotubes are oxidized to shorten them and to add negatively charged groups, and if these are then coated with solubilizing polymers [38] or various proteins [39], the nanotubes are much less toxic [40]. These treatments effectively eliminate aggregation, increase blood circulation time and eliminate induced interferon responses. Notwithstanding problems that still need to be solved, nanoparticles are versatile, multifunctional platforms with far-reaching and quickly materializing biological potential [41-43].
3. Nucleic Acid Delivery
The lipid bilayer of the cell membrane acts as a biological wall against foreign, pathogenic nucleic acids, and prevents therapeutic delivery of small interfering RNA (siRNA) or plasmid DNA [44]. Several viral and polymeric nanocapsules, cationic liposomes and, non-viral vectors (lipoplexes, polyplexes and inorganic nanoparticles) have been developed that can actively cross the lipid membrane and deliver nucleic acid cargos with relative ease and efficiency and with limited toxicity in vitro. While the library and efficiency of in vitro transfection and infection methods expands and improves, there is mounting clinical uncertainty with regards to gene transfer and therapy methods in living systems. Such in vivo therapies must not only selectively infiltrate the target cells but also do so without inducing inflammatory responses [45-47]. Several gene therapy alternatives exist: most seek to incorporate synthetic DNA into the host genome, a process that requires additional penetration of the nuclear envelope; others engage RNA-interference, the mechanism of action by which siRNA sequences binds to target messenger RNA and initiates its degradation. Historically, viral vectors were the preferred delivery mode, but preliminary clinical studies triggered fatal inflammatory reactions in one patient and leukemogenesis in another, diverting efforts into synthetic, less immunogenic vector systems able to be systemically administered [48]. Examples include cyclodextrins with transferrin used as a targeting ligand [49]; atelocollagen, a highly purified type I collagen that is free of immunogenic telopeptides and, when complexed with siRNA to form nanoparticles, these have been used to deliver with efficiency and little immunogenicity to bone metastatic tumors in vivo [50]; logic-embedded vectors [51] and microparticle carriers consisting of mesoporous silicon, allowing for a sustained release of second-stage neutral nanoliposomes (1,2-dioleoyl-sn-glycero-3-phosphatidylcholine (DOPC)), and used for delivering siRNA to the target, a cancer related receptor (EphA2 encoding ephrin type A receptor 2) and, resulting in a robust antitumor effect, without toxicity, in orthotopic models of ovarian cancer [52].
Like their viral predecessors, synthetic nano-vectors must fulfill two obligations: They must (1) efficiently and systematically traverse the cellular membrane, and (2) remain in circulation for a long enough time to do so. Moreover, the need to minimize nanoparticle toxicity must be balanced with the equally important need for vectors to maximize their interactions with cell-surface receptors. Following intracellular localization, the nucleic acid cargo must be efficiently cleaved and safely transported to avoid endosomal or nuclease degradation. If the target is the nucleus, as in the case of genetic introduction, the foreign DNA must traverse another barrier, the nuclear membrane [53]. Conversely, a siRNA mechanism of action, though difficult to deliver, represents a more facile and clinically effective approach to gene therapy and transfer. Because siRNA acts upon mRNA transcripts released into the cytoplasm, the additional rate-limiting step of nuclear localization is avoided [6,7].
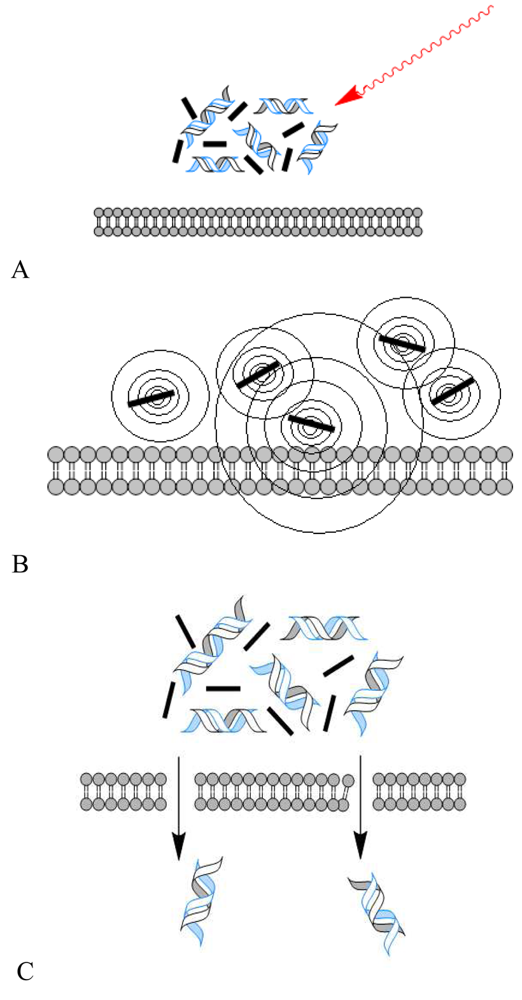
To bypass some of the limitations of current delivery methods, most notably host-immune rejection, and a need for greater efficiency of intracellular delivery of therapeutic agents, a recent study by Chakravarty et al. demonstrated a novel approach of membrane traversal, using carbon black nanoparticles (25–200 nm aggregates) activated by femtosecond laser pulses [53]. Drawing upon previously published data that such laser excitation results in carbon-steam reaction leading to consumption of carbon black and, generating local, highly controlled acoustic shock waves that create nanoscale holes in the lipid bilayer, the authors were able to deliver small molecules, proteins such as bovine serum albumin, and DNA into different model cell types. Contrary to the more physical, direct methods of electroporation, ultrasound, and laser irradiation, laser-activated carbon black nanoparticles are able to deliver small and potentially therapeutic molecules efficiently without greatly compromising cell viability (Figure 1 A-C).
A major roadblock to gene delivery occurs in the transition from in vitro conditions to in vivo models. Because siRNA-delivery relies principally upon the identification of a target cell population, it is nearly impossible to recapitulate systemic administration of the siRNA-nanoparticle vector in vitro as well as quantitatively evaluate clinical efficacy. Transgenic mice are one solution to the dilemma, having already been used to assess whole-body siRNA pharmacodynamics [54,55]. The luciferase transgenic mouse model is already established, and has allowed characterization of siRNA delivery dynamics via high-throughput, bioluminescent imaging. In this context, Tao et al. elegantly devised a novel process that enables evaluation of target-specific delivery of siRNA through the generation of a mouse model with liver specific expression of firefly luciferase [56]. They employed a group of lipid nanoparticles (LNPs) with different consistencies of cationic lipid, polyethylene glycol (PEG) lipid, and cholesterol as delivery vehicles, with luciferase siRNA (lucR) as the “drug payload.” Following systemic administration of 3 mg/kg of siRNA, ex vivo analyses (collection of mRNA and analysis using TaqMan qRT-PCR assay) was collated with the noninvasive, bioluminescence data. Both forms of analyses showed a dose-dependent repression of luciferase at 24, 72, and 96 hours after tail vein injection [56].
Additionally, recently published data by Davis et al. revealed the efficacy of drug delivery platforms in ferrying siRNA through the blood-waterways of the body [57]. As the first siRNA clinical trial to use a targeted nanoparticle-delivery system, the study confirmed the uptake of the siRNA and subsequent downregulation of the target protein (RRM2, a protein that forms the second subunit of the ribosome). The molecular schematic consisted of four elements: (1) a linear, cyclodextrin-based polymer (CDP), (2) the ligand of human transferrin protein (hTf) decorating the polymer's surface, (3) and (4) siRNA specific to the RRM2. Each patient (a total of three), who all presented with late stage metastatic melanoma and were refractory to standard-of-care therapies, received 18, 25, and 30 mg/m2, respectively, of siRNA. Notably, ex vivo analysis of the biopsies revealed a dose-dependent reduction of RRM2 mRNA and protein product (confirmed through qRT-PCR and Western blot analysis) in the cancerous tissue [57,58]. Altogether, the increased efficacy and translational relevance of targeted gene therapy is becoming more and more apparent. Facilitated by nanoparticles—ranging from lipid structures to polymer-based compounds to carbon nanotubes—siRNA delivery is becoming an increasingly viable gene-specific, tissue-specific therapeutic choice.
Although synthetic DNA delivery systems are safe and versatile, they nonetheless remain inefficient. To address this, several studies have reported the potential use of nanoparticles with plasmid DNA delivery. Singh et al. developed nanotube-based gene delivery vectors, where several forms of functionalized nanotubes were able to condense plasmid DNA on to their surfaces. The study found that the charge density and the large surface area were critical parameters for this interaction, and these complexes were successfully used to deliver to the target cells, and the DNA facilitated gene expression [59]. Additionally, biodegradable polymers that include water-soluble cationic polymers and micro- and nanoparticles, have also been developed as efficient carriers of plasmid DNA, and once inside the cells, the carriers undergo degradation and the plasmid DNA is released into the cytosol [29].
4. Drug Delivery and Target Specificity
Cancer stands out among human diseases for its unique transformative abilities, rendering protein expression, and to some extent, entire cell behavior radically different from the normal counterpart. Whereas normal, non-cancerous cells may display a small numbers of growth factor receptors, cancer cells usually are decorated with large surface concentrations of receptors designed to maximize ligand-binding and intracellular signal initiation, providing an increased growth advantage [60]. For this reason, nanoparticles conjugated with the ligand partners to their cognate receptors are drawn towards the cancer cells with greatly enhanced affinity.
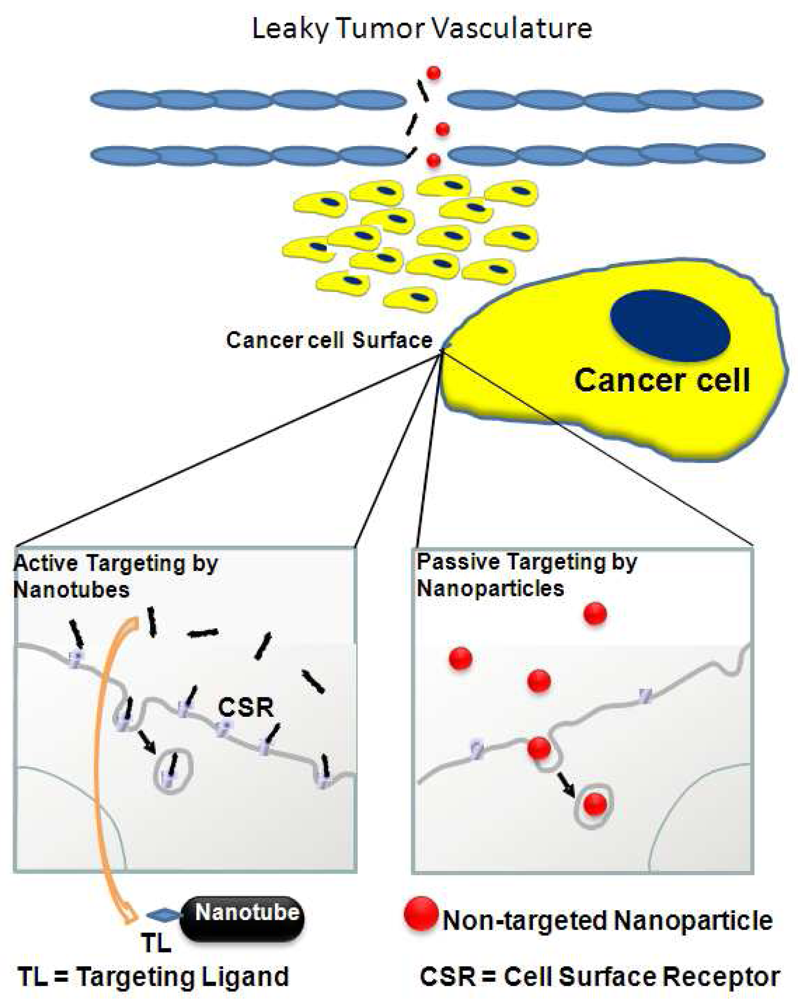
Two main modes currently prevail for nanoparticle administration to the cancer site, one of which does not require surface identification (Figure 2, right box). This mode of delivery, termed passive targeting, relies on abnormal large gap junctions in the endothelium of tumor blood vessels. This so-called enhanced permeability and retention (EPR) effect depends upon particle size, shape, and composition. Through this feature, nanoparticles gradually accumulate at the target site. Passive targeting, though, is limited in its usefulness for several reasons [18,21]. First, the EPR effect is highly dependent upon the location of the tumor and is strengthened or weakened by the presence or absence of adjacent blood vasculature networks. EPR's greatest deficiency is exposed when looking at the vasculature signature of solid tumors. In such cases, tumor vessels are poorly perfused with blood and are rendered dysfunctional, leading to poor penetration. Coupled with the high interstitial pressure of the vessels, which limits nanoparticle extravasation, passive diffusion is nearly impossible [21]. However, active targeting (Figure 2, left box), most often with a homing ligand that recognizes a surface receptor protein, compensates for these limiting factors. Among the tumor targeting moieties, include ligands (essentially defined as small molecule that binds specifically to a larger molecule), such as antibodies, and peptides that have strong affinity to their cognate, cellular binding partners, for example, tumor antigens, cell surface receptors, and tumor vasculature [18].
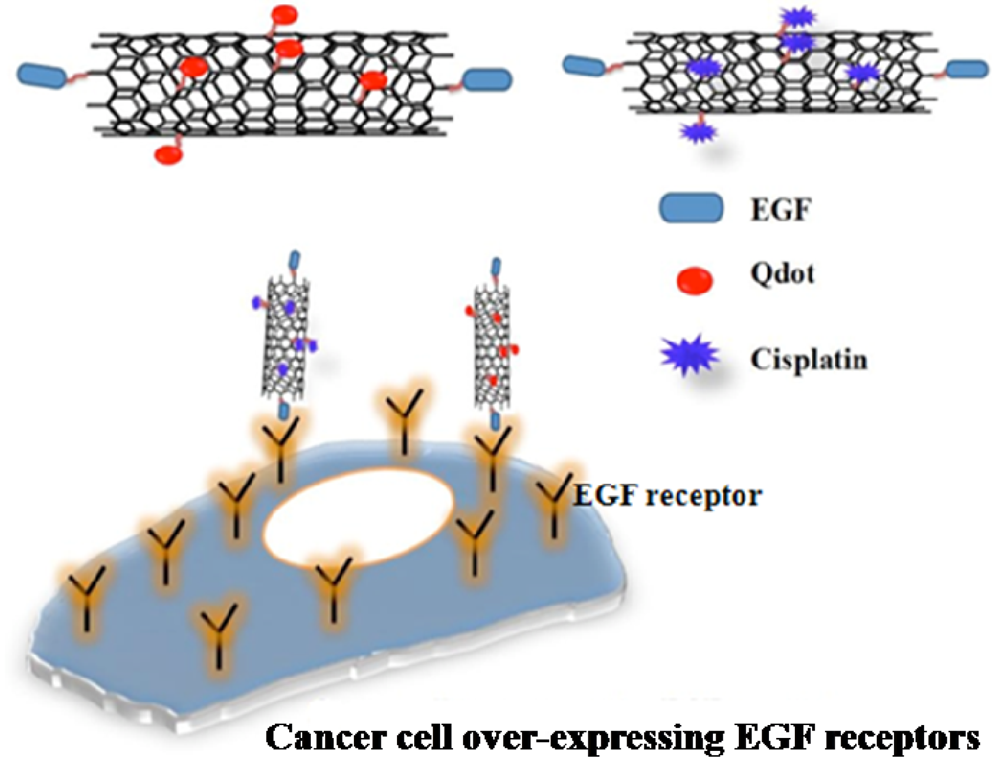
Our research team recently demonstrated [61] the efficacy of EGF-cisplatin-conjugated single walled carbon nanotube (SWCNT) complexes in reducing tumor growth in mice (Figure 3). Briefly, EGF and cisplatin molecules were covalently linked to the surface of oxidized, shortened single-walled carbon nanotubes, creating molecular missiles that targeted an EGFR-overexpressing human head and neck cancer cell line. Strikingly, subsequent in vivo systemic administration of the bioconjugates (via tail vein injection) was found to retard growth of flank xenografts on athymic nude mice. Complexes lacking the EGF ligand showed little to no effect in the same set of in vivo experiments [61,62]. Luminescent quantum dot nanoparticles (Qdots) were used to track the fate of these nanotubes with optical imaging.
Targeted therapies involving nanoparticle vehicles, most notably liposomes have been in clinical use for some time [63]. Importantly, these liposomes with surfaces particularly amendable to surface conjugation and biological targeting have not only enhanced specificity, but also reduced toxicity, in stark contrast to free, labile chemotherapeutic drugs (e.g., cisplatin). A shift in vehicle (polymers, dendrimers, nanotubes) has also seen a shift in mode of action from the conventional receptor-mediated endocytotic mechanism. pH, thermal and enzyme sensitive release and, photothermal excitation of nanoparticles are but a few novel therapeutic mechanisms. With particular focus on the latter, the chemical and physical properties of nanoparticles themselves can be exploited in cancer therapeutics [21]. Notable examples of these include micelles that form from self-assembled cyclodextrin dimers containing the Arg-Gly-Asp (RGD) sequence for target specificity, and doxorubicin as a therapeutic drug load within the inner core, which could be exploited for release by PEG removal only when exposed to a pH and temperature sensitive tumor environment [64]. Enzyme sensitive targeting has also been developed. A study by Medina et al. took advantage of the fact that gelatinases are highly expressed in several cancers, an indicator of poor prognosis, and modifying one of the peptide inhibitors identified by random phage display (CTT2) resulted in the formation of PEG-PE-CTT2 peptide-bound micelles, which than could be attached to PEGylated liposomes loaded with doxorubicin, creating a targeted drug delivery vehicle [65,66]. Also included is photothermal therapy, which essentially makes use of nanoshells, which have high absorption in the near infrared region. Systemic administration of the nanoparticles, as demonstrated by O’Neal et al., coupled with high frequency laser excitation led to extensive thermal ablation of tumors in a mouse model [67]. Rather like precision guided ‘smart bombs’, the nanoshells, usually coated with gold, generate significant heat upon laser activation and in the process destroy the surrounding tumor area. For this reason and others, including a high functionality in biological circulation, targeted gold nanoparticles have been widely evaluated as nanoscale precision guided vehicles [68]. Intriguingly, the physical properties that aid in therapy also are being exploited for disease diagnostics. Nanoparticles can be conceivably designed so as to be detectable by microPET scanners. The simultaneous diagnosis and treatment of a disease, with easy monitoring of drug uptake and circulation would be viewed as a technological breakthrough for both patients and doctors [69].
5. Nanoparticle Toxicity
An ideal nanoformulation should address two main aspects of targeted delivery: high specificity to the target site and low cytotoxicity to non-diseased cells. While in principle, target specificity has been achieved by a number of nanoformulations, cytotoxicity needs to be addressed in greater depth. Both acute (short term or immediate) and chronic (long term or prolonged) toxicity of the nanomaterials has to be looked at carefully. The most common problem being faced by the research community is the in vitro and in vivo aggregation behavior of the various nanomaterials eventually leading to pathological and, often life threatening responses from the host. Apart from nano-aggregation-related toxicity of the nanomaterials, side effects are mainly dependent on the dosage level, surface chemistry and route of administration. Reports of toxicity of metallic nanoparticles (NP), organic NPs, and carbon nanomaterials have been published based on the above mentioned nanotoxicity criteria. However, many of these studies do not replicate the conditions in which nanoparticle DDS are employed in vivo, and this has led to confusion regarding the practical use of these materials in medicine [70-73]. For example, while needle-like multi-wall carbon-nanotubes longer than 20 mm show toxic effects in animals when administered by inhalation and injection, [74] shortened nanotubes (e.g., <1 mm) that are functionalized with biomolecules or PEG to provide good aqueous solubility or dispersibility show negligible cytotoxicity even at high concentrations [38,75-77]. These later studies document few short term toxicity issues in animals; however, chronic toxicity has been insufficiently studied for a range of animal models. Results suggest that shortening and adding chemical surface functionality to nanotubes can mediate toxic response [78]. These studies illustrate a strong need for comparative toxicity testing of actual nanoparticle DDS themselves, as well as for uncovering the dependence of toxicity on the actual sizes and surface functionalities of the DDS. Encouragingly, there has been considerable progress in lowering nanoparticle toxicity for imminent clinical use. As suggested above, increased dispersibility or solubility of any nanoformulation improves performance and lowers toxicity [79]. PEGylation of nanomaterials aids in reducing toxicity, helps avoid opsonization by making nanoformulations less visible to phagocytic cells, and lengthens blood circulation times [80-83]. Related risk factors regarding nanoparticle toxicity include the potential to produce CD8 and CD4 type 1 T cell responses, and it is suggested that those particles between 40 and 50 nm give rise to the maximum effect [84]. Finally, a note should be made on the need for a decision support system, for classifying nanomaterials for potential biomedical use into different risk categories, with the single goal of improving our understanding of the toxicological and potential side effects, including those parameters associated with ecotoxicity and environmental risks, and a need to identify manufacturing technology that maximizes manufacturing efficiencies and minimizes life cycle environmental risks [85,86].
6. Clinical Use
So far, only a handful of FDA-approved nanoparticle drugs exist. Only two—Doxil® (Centocor Ortho Biotech Products L.P) and Abraxane (Abraxis Bioscience) —address cancer therapeutics. Doxil, [87] FDA approved in 1995, was originally developed to treat HIV-related Kaposi's sarcoma, and has evolved as a second-line treatment for ovarian cancer and multiple myeloma. Doxil®, a reformulation of doxorubicin, with the drug encased in a PEGylated liposome (∼100 nM), increases its functionality and specificity while decreasing its cardiotoxicity [88]. However, the drug's tendency to concentrate in the skin induces hand-foot syndrome in over half of all patients; the redness and acutely painful peeling of the skin associated with this side-effect inhibits the complete clinical substitution of the liposomal complex for free doxorubicin [23,89]. In a similar way, Abraxane® [90] developed for treatment of breast cancer, comprises the drug paclitaxel encased in an albumin shell (∼130 nm diameter). With the secretion of SPARC (secreted protein acidic and rich in cytosine) from the tumor microenvironment, to which albumin has an affinity, the complexes become localized at the tumor site and by entering the cell through caveolae-mediated endocytosis, cancer cells undergo drug-induced destruction, resulting in a reduced tumor burden. Notable examples of nanoformulations under clinical evaluation are summarized in Table 2.
7. Preclinical Evaluation and Theranostics
Beyond the trials reported by Davis et al. in siRNA systemic delivery [57], there are several nanotechnology-enabled diagnostic and therapeutic agents currently being developed and under preclinical evaluation under the NCI Alliance for Nanotechnology in Cancer initiative ( http://nano.cancer.gov/learn/now/clinical-trials.asp; [91]). These include a novel PET contrast agent family, [18F]-FAC, and can be used to illuminate cancerous regions of the body and help determine if patients are responding to either therapeutic drugs such as gemcitabine, cytarabine and fludarabine [95], or those in nanoformulation. Another example includes a trial led by Mirkin et al. at Northwestern University/ International Institute for Nanotechnology that has already received FDA approval comprises a nanosensor test for Coumadin, a drug used to prevent heart attacks, strokes, and blood clots in veins and arteries [91]. It follows that the same platform can be used to detect important cancer biomarkers, as well as measure blood levels of anticancer agents. Similarly, Ross et al., (MIT-Harvard Center for Cancer Nanotechnology Excellence [CCNE]), reported findings from a clinical trial determining if lymphotrophic superparamagnetic nanoparticles can be used for early detection of lymph node metastases [96]. On another front, work by Heath et al. (Caltech) is aimed at conducting a validation study measuring the levels of roughly 800 miRNAs from melanoma patients before and after therapy [91]. As drug companies look to develop more refined, intricate drug formulations combined with targeted delivery, current research is now moving in the direction of incorporating conventional drugs, currently freely administered, into nanocarriers. A recent representative example is the engineering of cisplatin nanoparticles that have enhanced antitumor effects and lower cytotoxicity. Paraskar et al. [97] developed a unique, self-assembling cisplatin nanoparticle that releases the drug in a pH-sensitive manner. The polymer to which the drug is complexed, glucosamine-functionalized polyisobutylene-maleic acid, also delivers a palliative improvement. When tested in mice, the specialized delivery noticeably reduced systemic- and nephrotoxicity, as compared with free, labile cisplatin. Dai's research group at Stanford University have carried out extensive animal studies involving carbon nanotubes where chemotherapeutic drugs were loaded onto aqueous solubilized nanotubes to efficiently target the cancer in model systems [98-103]. Our research team obtained similar results in earlier studies on cisplatin derivatized carbon nanoparticles targeted to EGF receptors [61]. Such results can potentially have global ramifications in the clinic. Whereas with free cisplatin doctors are now concerned with dosage-monitoring and buildup of platinum in the kidneys, engineered cisplatin nanoparticles herald a future where dosages can be kept minimal and toxicity greatly reduced. Notable preclinical studies on nanoformulated cancer diagnostics and therapeutics are summarized in Table 2.
Recent scientific breakthroughs including the development of biomarker initiatives have seen a paradigm shift towards personalized medicine, and with this the emergence of theranostics [19]. This evolving field of theranostics essentially refers to the combined use of therapeutic and diagnostics, with the sole purpose of optimizing efficacy and safety, and by identifying patients that are most likely to benefit from a tailored form of therapeutics. In this regard, multifunctional nanoparticle agents are now being used to integrate both therapeutics and diagnostics, with theranostics, and several are now in various stages of preclinical and clinical development [104-105]. While this nascent field has much potential in improving patient-care, there are a number of challenges that need to be overcome before it can be translated into routine clinic use [106,107]. Key among these include the development of a single platform for use in theranostics, and, elegant and sensitive methodologies to decipher the fate of nanoformulated therapeutics tailored specifically for patients, that can provide information on distribution and drug release as well as treatment efficacy after administration [108,109].
8. Conclusions
Thus, future challenges in nano-based DDS include (a) enhancing specificity for target cells; (b) regulating of bioavailability once these delivery systems reach the target; (c) enhancing their ability to deliver therapeutic molecules to specific sites within the target cells, and (d) lowering toxicity. Finally, nano-materials conjugated with target specific ligand and chemo-therapeutic drugs have the potential to selectively kill cancer cells leaving healthy non-diseased cells intact, and with PEGylation, these complexes can be rendered more hydrophilic and aqueous-dispersible, allowing for a prolonged desired effect and without toxicity.
| Platform | Characteristics | References |
|---|---|---|
| Liposomes | Drug encapsulation, hydrophilic interior, individual lipids can be changed to accommodate particular functionality (surface charge, etc). | [12,22,23] |
| Dendrimers | Large number of peripheral functional groups allows for the multiple drug, label, ligand functionalization. | [24-26] |
| Polymers | Most widely used drug delivery vehicles, some are self- assembling, can be coated with solubilizing agents, non-immunogenic and highly versatile. | [27-29] |
| Metallic particles | Generally used as diagnostic agents, drug delivery, thermal-ablation via laser excitation, multifunctional. | [10,19,30] |
| Carbon nanotubes | High functionality, limited solubility, functionalized CNT acts as an inert bioconjugate in vivo, drug delivery “missiles”. | [31-34] |
| Lipoproteins | Biocompatible protein-lipid based molecules which can carry hydrophobic drugs to tumor targets with minimal toxicity. | [35,36] |
| Preclinical | Features | References |
|---|---|---|
| Kipps et al. | Stimulation of the immune system using a chemically engineered adenovirus nanoparticle in the treatment of leukemia | [91] |
| Davis et al. | Delivery of siRNA using cyclodextrin-based nanoparticles in cancer treatment | [91] |
| Davis et al. | Administration of camptothecin bound to cyclodextrin-based polymer for treatment of solid tumors | [91] |
| Mirkin et al. | Detection of drug levels in body using nanosensors, applicable also to cancer biomarkers | [91] |
| Heath et al. | Measurement of miRNA levels in melanoma patients pre- and post-treatment using the Integrated Blood Barcode (IBBC) chip | [91] |
| Langer and Farokhzad | Multifunctional drug delivery using a polymer matrix, therapeutic payload(s), surface moieties, and targeting ligands | [91] |
| Clinical | Features | References |
| Shimada et al. | INGN-201 (Vivante GMP Solutions) a liposomal nanoformulation for lung cancer treatment administered intravenously is in Phase I. | [92,93] |
| Schwartz et al. | AuraShell (Nanospectra Biosciences) a gold-coated silica NP based drug formulation for treatment of solid tumors, now under Phase I evaluation. | [94] |
Acknowledgements
Preparation of this review was supported by the Intramural Research Program of the National Institute of Dental and Craniofacial Research and the National Institute of Biomedical Imaging and Bioengineering, NIH, and jointly by PHS grant ES013557 from NIEHS/NIH, by the National Science Foundation (DMR-0604815), and by a Walton Research Fellowship awarded by Science Foundation Ireland to JFR.
References
- Caruthers, S.D.; Wickline, S.A.; Lanza, G.M. Nanotechnological applications in medicine. Curr. Opin. Biotechnol. 2007, 18, 26–30. [Google Scholar]
- Grobmyer, S.R.; Iwakuma, N.; Sharma, P.; Moudgil, B.M. What is cancer nanotechnology? Methods Mol. Biol. 2010, 624, 1–9. [Google Scholar]
- Emerich, D.F.; Thanos, C.G. Nanotechnology and medicine. Expert Opin. Biol. Ther. 2003, 3, 655–663. [Google Scholar]
- Rao, C.N.R.; Muller, A.; Cheetham, A.K. The Chemistry of Nanomaterials: Synthesis, Properties and Applications; John Wiley & Sons: Oxford, England, 2004; p. 761. [Google Scholar]
- Farokhzad, O.C.; Langer, R. Nanomedicine: Developing smarter therapeutic and diagnostic modalities. Adv. Drug Delivery Rev. 2006, 58, 1456–1459. [Google Scholar]
- Roco, M.C. Nanotechnology: Convergence with modern biology and medicine. Curr. Opin. Biotechnol. 2003, 14, 337–346. [Google Scholar]
- Ferrari, M. Cancer nanotechnology: Opportunities and challenges. Nat. Rev. Cancer 2005, 5, 161–171. [Google Scholar]
- Moghimi, S.M.; Hunter, A.C.; Murray, J.C. Nanomedicine: Current status and future prospects. FASEB J. 2005, 19, 311–330. [Google Scholar]
- Jemal, A.; Siegel, R.; Ward, E.; Hao, Y.; Xu, J.; Thun, M.J. Cancer statistics, 2009. CA Cancer J. Clin. 2009, 59, 225–249. [Google Scholar]
- Petros, R.A.; DeSimone, J.M. Strategies in the design of nanoparticles for therapeutic applications. Nat. Rev. Drug Discov. 2010, 9, 615–627. [Google Scholar]
- Gaitanis, A.; Staal, S. Liposomal doxorubicin and nab-paclitaxel: Nanoparticle cancer chemotherapy in current clinical use. Methods Mol. Biol. 2010, 624, 385–392. [Google Scholar]
- Fanciullino, R.; Ciccolini, J. Liposome-encapsulated anticancer drugs: Still waiting for the magic bullet? Curr. Med. Chem. 2009, 16, 4361–4371. [Google Scholar]
- Ali, I. Nano Anti-Cancer Drugs: Pros and Cons and Future Perspectives. Curr. Cancer Drug Targets 2010. In Press. [Google Scholar]
- Utreja, P.; Jain, S.; Tiwary, A.K. Novel drug delivery systems for sustained and targeted delivery of anti- cancer drugs: Current status and future prospects. Curr. Drug Deliv. 2010, 7, 152–161. [Google Scholar]
- Bryant, H.E.; Schultz, N.; Thomas, H.D.; Parker, K.M.; Flower, D.; Lopez, E.; Kyle, S.; Meuth, M.; Curtin, N.J.; Helleday, T. Specific killing of BRCA2-deficient tumours with inhibitors of poly(ADP-ribose) polymerase. Nature 2005, 434, 913–917. [Google Scholar]
- Farmer, H.; McCabe, N.; Lord, C.J.; Tutt, A.N.; Johnson, D.A.; Richardson, T.B.; Santarosa, M.; Dillon, K.J.; Hickson, I.; Knights, C.; Martin, N.M.; Jackson, S.P.; Smith, G.C.; Ashworth, A. Targeting the DNA repair defect in BRCA mutant cells as a therapeutic strategy. Nature 2005, 434, 917–921. [Google Scholar]
- Helleday, T.; Bryant, H.E.; Schultz, N. Poly(ADP-ribose) polymerase (PARP-1) in homologous recombination and as a target for cancer therapy. Cell Cycle 2005, 4, 1176–1178. [Google Scholar]
- Torchilin, V.P. Passive and active drug targeting: Drug delivery to tumors as an example. Handb. Exp. Pharmacol. 2010, 197, 3–53. [Google Scholar]
- Xie, J.; Lee, S.; Chen, X. Nanoparticle-based theranostic agents. Adv. Drug Delivery Rev. 2010, 62, 1064–1079. [Google Scholar]
- Wang, H.; Chen, X. Applications for site-directed molecular imaging agents coupled with drug delivery potential. Expert Opin. Drug Deliv. 2009, 6, 745–768. [Google Scholar]
- Alexis, F.; Pridgen, E.M.; Langer, R.; Farokhzad, O.C. Nanoparticle technologies for cancer therapy. Handb. Exp. Pharmacol. 2010, 197, 55–86. [Google Scholar]
- Kaasgaard, T.; Andresen, T.L. Liposomal cancer therapy: Exploiting tumor characteristics. Expert Opin. Drug Deliv. 2010, 7, 225–243. [Google Scholar]
- Plosker, G.L. Pegylated liposomal Doxorubicin: A review of its use in the treatment of relapsed or refractory multiple myeloma. Drugs 2008, 68, 2535–2551. [Google Scholar]
- Astruc, D.; Boisselier, E.; Ornelas, C. Dendrimers designed for functions: From physical, photophysical, and supramolecular properties to applications in sensing, catalysis, molecular electronics, photonics, and nanomedicine. Chem. Rev. 2010, 110, 1857–1959. [Google Scholar]
- Calderon, M.; Quadir, M.A.; Sharma, S.K.; Haag, R. Dendritic polyglycerols for biomedical applications. Adv. Mater. 2010, 22, 190–218. [Google Scholar]
- Gajbhiye, V.; Palanirajan, V.K.; Tekade, R.K.; Jain, N.K. Dendrimers as therapeutic agents: A systematic review. J. Pharm. Pharmacol. 2009, 61, 989–1003. [Google Scholar]
- De Souza, R.; Zahedi, P.; Allen, C.J.; Piquette-Miller, M. Polymeric drug delivery systems for localized cancer chemotherapy. Drug Deliv. 2010, 17, 365–375. [Google Scholar]
- Joralemon, M.J.; McRae, S.; Emrick, T. PEGylated polymers for medicine: From conjugation to self-assembled systems. Chem. Commun. (Camb) 2010, 46, 1377–1393. [Google Scholar]
- Luten, J.; van Nostrum, C.F.; De Smedt, S.C.; Hennink, W.E. Biodegradable polymers as nonviral carriers for plasmid DNA delivery. J. Control. Release 2008, 126, 97–110. [Google Scholar]
- Ahmad, M.Z.; Akhter, S.; Jain, G.K.; Rahman, M.; Pathan, S.A.; Ahmad, F.J.; Khar, R.K. Metallic nanoparticles: Technology overview & drug delivery applications in oncology. Expert Opin. Drug Deliv. 2010, 7, 927–942. [Google Scholar]
- Prato, M.; Kostarelos, K.; Bianco, A. Functionalized carbon nanotubes in drug design and discovery. Acc. Chem. Res. 2008, 41, 60–68. [Google Scholar]
- Kostarelos, K.; Bianco, A.; Prato, M. Promises, facts and challenges for carbon nanotubes in imaging and therapeutics. Nat. Nanotechnol. 2009, 4, 627–633. [Google Scholar]
- Moser, G.; Vacariu-Granser, G.V.; Schneider, C.; Abatzi, T.A.; Pokieser, P.; Stacher-Janotta, G.; Gaupmann, G.; Weber, U.; Wenzel, T.; Roden, M.; et al. High incidence of esophageal motor disorders in consecutive patients with globus sensation. Gastroenterology 1991, 101, 1512–1521. [Google Scholar]
- Akbar, S.; Taimoor, A.A. Functionalization of carbon nanotubes: Manufacturing techniques and properties of customized nanocomponents for molecular-level technology. Recent Patents Nanotechnol. 2009, 3, 154–161. [Google Scholar]
- Tulenko, T.N.; Sumner, A.E. The physiology of lipoproteins. J. Nucl. Cardiol. 2002, 9, 638–649. [Google Scholar]
- Lacko, A.G.; Nair, M.; Prokai, L.; McConathy, W.J. Prospects and challenges of the development of lipoprotein-based formulations for anti-cancer drugs. Expert Opin. Drug Deliv. 2007, 4, 665–675. [Google Scholar]
- Carrero-Sanchez, J.C.; Elias, A.L.; Mancilla, R.; Arrellin, G.; Terrones, H.; Laclette, J.P.; Terrones, M. Biocompatibility and toxicological studies of carbon nanotubes doped with nitrogen. Nano Lett. 2006, 6, 1609–1616. [Google Scholar]
- Bhirde, A.A.; Patel, S.; Sousa, A.A.; Patel, V.; Molinolo, A.A.; Ji, Y.; Leapman, R.D.; Gutkind, J.S.; Rusling, J.F. Distribution and clearance of PEG-single-walled carbon nanotube cancer drug delivery vehicles in mice. Nanomedicine (Lond) 2010, 5, 1535–1546. [Google Scholar]
- Edri, E.; Regev, O. “Shaken, not stable”: Dispersion mechanism and dynamics of protein-dispersed nanotubes studied via spectroscopy. Langmuir 2009, 25, 10459–10465. [Google Scholar]
- Karousis, N.; Tagmatarchis, N.; Tasis, D. Current progress on the chemical modification of carbon nanotubes. Chem. Rev. 2010, 110, 5366–5397. [Google Scholar]
- Scheinberg, D.A.; Villa, C.H.; Escorcia, F.E.; McDevitt, M.R. Conscripts of the infinite armada: Systemic cancer therapy using nanomaterials. Nat. Rev. Clin. Oncol. 2010, 7, 266–276. [Google Scholar]
- Kim, J.; Piao, Y.; Hyeon, T. Multifunctional nanostructured materials for multimodal imaging, and simultaneous imaging and therapy. Chem. Soc. Rev. 2009, 38, 372–390. [Google Scholar]
- Subbiah, R.; Veerapandian, M.; Yun, K.S. Nanoparticles: Functionalization and Multifunctional Applications in Biomedical Sciences. Curr. Med. Chem. 2010, 17, 4559–4577. [Google Scholar]
- Hart, S.L. Multifunctional nanocomplexes for gene transfer and gene therapy. Cell Biol. Toxicol. 2010, 26, 69–81. [Google Scholar]
- Dharmapuri, S.; Peruzzi, D.; Aurisicchio, L. Engineered adenovirus serotypes for overcoming anti-vector immunity. Expert Opin. Biol. Ther. 2009, 9, 1279–1287. [Google Scholar]
- Goyal, A.K.; Khatri, K.; Vyas, S.P. Patents on non-viral mediated gene delivery. Recent Patents DNA Gene Seq. 2008, 2, 44–60. [Google Scholar]
- Khurana, B.; Goyal, A.K.; Budhiraja, A.; Arora, D.; Vyas, S.P. siRNA delivery using nanocarriers - an efficient tool for gene silencing. Curr. Gene Ther. 2010, 10, 139–155. [Google Scholar]
- Thomas, C.E.; Ehrhardt, A.; Kay, M.A. Progress and problems with the use of viral vectors for gene therapy. Nat. Rev. Genet. 2003, 4, 346–358. [Google Scholar]
- Heidel, J.D.; Yu, Z.; Liu, J.Y.; Rele, S.M.; Liang, Y.; Zeidan, R.K.; Kornbrust, D.J.; Davis, M.E. Administration in non-human primates of escalating intravenous doses of targeted nanoparticles containing ribonucleotide reductase subunit M2 siRNA. Proc. Natl. Acad. Sci. USA 2007, 104, 5715–5721. [Google Scholar]
- Takeshita, F.; Minakuchi, Y.; Nagahara, S.; Honma, K.; Sasaki, H.; Hirai, K.; Teratani, T.; Namatame, N.; Yamamoto, Y.; Hanai, K.; Kato, T.; Sano, A.; Ochiya, T. Efficient delivery of small interfering RNA to bone-metastatic tumors by using atelocollagen in vivo. Proc. Natl. Acad. Sci. USA 2005, 102, 12177–12182. [Google Scholar]
- Ferrari, M. Frontiers in cancer nanomedicine: Directing mass transport through biological barriers. Trends Biotechnol. 2010, 28, 181–188. [Google Scholar]
- Tanaka, T.; Mangala, L.S.; Vivas-Mejia, P.E.; Nieves-Alicea, R.; Mann, A.P.; Mora, E.; Han, H.D.; Shahzad, M.M.; Liu, X.; Bhavane, R.; Gu, J.; Fakhoury, J.R.; Chiappini, C.; Lu, C.; Matsuo, K.; Godin, B.; Stone, R.L.; Nick, A.M.; Lopez-Berestein, G.; Sood, A.K.; Ferrari, M. Sustained small interfering RNA delivery by mesoporous silicon particles. Cancer Res. 2010, 70, 3687–3696. [Google Scholar]
- Chakravarty, P.; Qian, W.; El-Sayed, M.A.; Prausnitz, M.R. Delivery of molecules into cells using carbon nanoparticles activated by femtosecond laser pulses. Nat. Nanotechnol. 2010, 5, 607–611. [Google Scholar]
- Gao, X.; Zhang, P. Transgenic RNA interference in mice. Physiology (Bethesda) 2007, 22, 161–166. [Google Scholar]
- Takahashi, Y.; Nishikawa, M.; Takakura, Y. Nonviral vector-mediated RNA interference: Its gene silencing characteristics and important factors to achieve RNAi-based gene therapy. Adv. Drug Delivery Rev. 2009, 61, 760–766. [Google Scholar]
- Tao, W.; Davide, J.P.; Cai, M.; Zhang, G.J.; South, V.J.; Matter, A.; Ng, B.; Zhang, Y.; Sepp-Lorenzino, L. Noninvasive imaging of lipid nanoparticle-mediated systemic delivery of small-interfering RNA to the liver. Mol. Ther. 2010, 18, 1657–1666. [Google Scholar]
- Davis, M.E.; Zuckerman, J.E.; Choi, C.H.; Seligson, D.; Tolcher, A.; Alabi, C.A.; Yen, Y.; Heidel, J.D.; Ribas, A. Evidence of RNAi in humans from systemically administered siRNA via targeted nanoparticles. Nature 2010, 464, 1067–1070. [Google Scholar]
- Davis, M.E. The first targeted delivery of siRNA in humans via a self-assembling, cyclodextrin polymer-based nanoparticle: From concept to clinic. Mol. Pharm 2009, 6, 659–668. [Google Scholar]
- Singh, R.; Pantarotto, D.; McCarthy, D.; Chaloin, O.; Hoebeke, J.; Partidos, C.D.; Briand, J.P.; Prato, M.; Bianco, A.; Kostarelos, K. Binding and condensation of plasmid DNA onto functionalized carbon nanotubes: Toward the construction of nanotube-based gene delivery vectors. J. Am. Chem. Soc. 2005, 127, 4388–4396. [Google Scholar]
- Hanahan, D.; Weinberg, R.A. The hallmarks of cancer. Cell 2000, 100, 57–70. [Google Scholar]
- Bhirde, A.A.; Patel, V.; Gavard, J.; Zhang, G.; Sousa, A.A.; Masedunskas, A.; Leapman, R.D.; Weigert, R.; Gutkind, J.S.; Rusling, J.F. Targeted killing of cancer cells in vivo and in vitro with EGF-directed carbon nanotube-based drug delivery. ACS Nano 2009, 3, 307–316. [Google Scholar]
- Bhirde, A.A.; Sousa, A.A.; Patel, V.; Azari, A.A.; Gutkind, J.S.; Leapman, R.D.; Rusling, J.F. Imaging the distribution of individual platinum-based anticancer drug molecules attached to single-wall carbon nanotubes. Nanomedicine (Lond) 2009, 4, 763–772. [Google Scholar]
- Tarahovsky, Y.S. “Smart” liposomal nanocontainers in biology and medicine”. Biochemistry (Mosc) 2010, 75, 811–824. [Google Scholar]
- Quan, C.Y.; Chen, J.X.; Wang, H.Y.; Li, C.; Chang, C.; Zhang, X.Z.; Zhuo, R.X. Core-shell nanosized assemblies mediated by the alpha-beta cyclodextrin dimer with a tumor-triggered targeting property. ACS Nano 2010, 4, 4211–4219. [Google Scholar]
- Medina, O.P.; Zhu, Y.; Kairemo, K. Targeted liposomal drug delivery in cancer. Curr. Pharm. Des. 2004, 10, 2981–2989. [Google Scholar]
- Koivunen, E.; Arap, W.; Valtanen, H.; Rainisalo, A.; Medina, O.P.; Heikkila, P.; Kantor, C.; Gahmberg, C.G.; Salo, T.; Konttinen, Y.T.; Sorsa, T.; Ruoslahti, E.; Pasqualini, R. Tumor targeting with a selective gelatinase inhibitor. Nat. Biotechnol. 1999, 17, 768–774. [Google Scholar]
- O'Neal, D.P.; Hirsch, L.R.; Halas, N.J.; Payne, J.D.; West, J.L. Photo-thermal tumor ablation in mice using near infrared-absorbing nanoparticles. Cancer Lett. 2004, 209, 171–176. [Google Scholar]
- El-Sayed, I.H.; Huang, X.; El-Sayed, M.A. Selective laser photo-thermal therapy of epithelial carcinoma using anti-EGFR antibody conjugated gold nanoparticles. Cancer Lett. 2006, 239, 129–135. [Google Scholar]
- Kim, K.; Kim, J.H.; Park, H.; Kim, Y.S.; Park, K.; Nam, H.; Lee, S.; Park, J.H.; Park, R.W.; Kim, I.S.; Choi, K.; Kim, S.Y.; Kwon, I.C. Tumor-homing multifunctional nanoparticles for cancer theragnosis: Simultaneous diagnosis, drug delivery, and therapeutic monitoring. J. Control. Release 2010, 146, 219–227. [Google Scholar]
- Quintana, M.; Prato, M. Supramolecular aggregation of functionalized carbon nanotubes. Chem. Commun. (Camb) 2009, 40, 6005–6007. [Google Scholar]
- Kostarelos, K. The long and short of carbon nanotube toxicity. Nat. Biotechnol. 2008, 26, 774–776. [Google Scholar]
- De Jong, W.H.; Borm, P.J. Drug delivery and nanoparticles: Applications and hazards. Int. J. Nanomedicine 2008, 3, 133–149. [Google Scholar]
- Bhirde, A.; Xie, J.; Swierczewska, M.; Chen, X. Nanoparticles for cell labeling. Nanoscale 2010. [Google Scholar] [CrossRef]
- Poland, C.A.; Duffin, R.; Kinloch, I.; Maynard, A.; Wallace, W.A.; Seaton, A.; Stone, V.; Brown, S.; Macnee, W.; Donaldson, K. Carbon nanotubes introduced into the abdominal cavity of mice show asbestos-like pathogenicity in a pilot study. Nat. Nanotechnol. 2008, 3, 423–428. [Google Scholar]
- Cherukuri, P.; Bachilo, S.M.; Litovsky, S.H.; Weisman, R.B. Near-infrared fluorescence microscopy of single-walled carbon nanotubes in phagocytic cells. J. Am. Chem. Soc. 2004, 126, 15638–15639. [Google Scholar]
- Sayes, C.M.; Liang, F.; Hudson, J.L.; Mendez, J.; Guo, W.; Beach, J.M.; Moore, V.C.; Doyle, C.D.; West, J.L.; Billups, W.E.; Ausman, K.D.; Colvin, V.L. Functionalization density dependence of single-walled carbon nanotubes cytotoxicity in vitro. Toxicol. Lett. 2006, 161, 135–142. [Google Scholar]
- Dumortier, H.; Lacotte, S.; Pastorin, G.; Marega, R.; Wu, W.; Bonifazi, D.; Briand, J.P.; Prato, M.; Muller, S.; Bianco, A. Functionalized carbon nanotubes are non-cytotoxic and preserve the functionality of primary immune cells. Nano Lett. 2006, 6, 1522–1528. [Google Scholar]
- Cui, H.F.; Vashist, S.K.; Al-Rubeaan, K.; Luong, J.H.; Sheu, F.S. Interfacing carbon nanotubes with living mammalian cells and cytotoxicity issues. Chem. Res. Toxicol. 2010, 23, 1131–1147. [Google Scholar]
- Veronese, F.M.; Pasut, G. PEGylation, successful approach to drug delivery. Drug Discov. Today 2005, 10, 1451–1458. [Google Scholar]
- Liu, Z.; Sun, X.; Nakayama-Ratchford, N.; Dai, H. Supramolecular chemistry on water-soluble carbon nanotubes for drug loading and delivery. ACS Nano 2007, 1, 50–56. [Google Scholar]
- Owens, D.E.; Peppas, N.A. Opsonization, biodistribution, and pharmacokinetics of polymeric nanoparticles. Int. J. Pharm. 2006, 307, 93–102. [Google Scholar]
- Ryan, S.M.; Mantovani, G.; Wang, X.; Haddleton, D.M.; Brayden, D.J. Advances in PEGylation of important biotech molecules: Delivery aspects. Expert Opin. Drug Deliv. 2008, 5, 371–383. [Google Scholar]
- Wang, A.Z.; Gu, F.; Zhang, L.; Chan, J.M.; Radovic-Moreno, A.; Shaikh, M.R.; Farokhzad, O.C. Biofunctionalized targeted nanoparticles for therapeutic applications. Expert Opin. Biol. Ther. 2008, 8, 1063–1070. [Google Scholar]
- Fifis, T.; Gamvrellis, A.; Crimeen-Irwin, B.; Pietersz, G.A.; Li, J.; Mottram, P.L.; McKenzie, I.F.; Plebanski, M. Size-dependent immunogenicity: Therapeutic and protective properties of nanovaccines against tumors. J. Immunol. 2004, 173, 3148–3154. [Google Scholar]
- Linkov, I.; Satterstrom, F.K.; Corey, L.M. Nanotoxicology and nanomedicine: Making hard decisions. Nanomedicine 2008, 4, 167–171. [Google Scholar]
- Canis, L.; Linkov, I.; Seager, T.P. Application of stochastic multiattribute analysis to assessment of single walled carbon nanotube synthesis processes. Environ. Sci. Technol. 2010, 44, 8704–8711. [Google Scholar]
- Nagar, S. Pharmacokinetics of anti-cancer drugs used in breast cancer chemotherapy. Adv. Exp. Med. Biol. 2010, 678, 124–132. [Google Scholar]
- Batist, G. Cardiac safety of liposomal anthracyclines. Cardiovasc. Toxicol. 2007, 7, 72–74. [Google Scholar]
- von Moos, R.; Thuerlimann, B.J.; Aapro, M.; Rayson, D.; Harrold, K.; Sehouli, J.; Scotte, F.; Lorusso, D.; Dummer, R.; Lacouture, M.E.; Lademann, J.; Hauschild, A. Pegylated liposomal doxorubicin-associated hand-foot syndrome: Recommendations of an international panel of experts. Eur. J. Cancer 2008, 44, 781–790. [Google Scholar]
- Iglesias, J. nab-Paclitaxel (Abraxane(R)): An albumin-bound cytotoxic exploiting natural delivery mechanisms into tumors. Breast Cancer Res. 2009, 11 (Suppl. 1), S21. [Google Scholar]
- National Cancer Institute. Nanotechnology in Clinical Trials. Access on http://nano.cancer.gov/learn/now/clinical-trials.asp.
- Shimada, H.; Matsubara, H.; Shiratori, T.; Shimizu, T.; Miyazaki, S.; Okazumi, S.; Nabeya, Y.; Shuto, K.; Hayashi, H.; Tanizawa, T.; Nakatani, Y.; Nakasa, H.; Kitada, M.; Ochiai, T. Phase I/II adenoviral p53 gene therapy for chemoradiation resistant advanced esophageal squamous cell carcinoma. Cancer Sci. 2006, 97, 554–561. [Google Scholar]
- Sharma, A.; Tandon, M.; Bangari, D.S.; Mittal, S.K. Adenoviral vector-based strategies for cancer therapy. Curr. Drug ther. 2009, 4, 117–138. [Google Scholar]
- Schwartz, J.A.; Shetty, A.M.; Price, R.E.; Stafford, R.J.; Wang, J.C.; Uthamanthil, R.K.; Pham, K.; McNichols, R.J.; Coleman, C.L.; Payne, J.D. Feasibility study of particle-assisted laser ablation of brain tumors in orthotopic canine model. Cancer Res. 2009, 69, 1659–1667. [Google Scholar]
- Radu, C.G.; Shu, C.J.; Nair-Gill, E.; Shelly, S.M.; Barrio, J.R.; Satyamurthy, N.; Phelps, M.E.; Witte, O.N. Molecular imaging of lymphoid organs and immune activation by positron emission tomography with a new [18F]-labeled 2′-deoxycytidine analog. Nat. Med. 2008, 14, 783–788. [Google Scholar]
- Ross, R.W.; Zietman, A.L.; Xie, W.; Coen, J.J.; Dahl, D.M.; Shipley, W.U.; Kaufman, D.S.; Islam, T.; Guimaraes, A.R.; Weissleder, R.; Harisinghani, M. Lymphotropic nanoparticle-enhanced magnetic resonance imaging (LNMRI) identifies occult lymph node metastases in prostate cancer patients prior to salvage radiation therapy. Clin. Imag. 2009, 33, 301–305. [Google Scholar]
- Paraskar, A.S.; Soni, S.; Chin, K.T.; Chaudhuri, P.; Muto, K.W.; Berkowitz, J.; Handlogten, M.W.; Alves, N.J.; Bilgicer, B.; Dinulescu, D.M.; Mashelkar, R.A.; Sengupta, S. Harnessing structure-activity relationship to engineer a cisplatin nanoparticle for enhanced antitumor efficacy. Proc. Natl. Acad. Sci. USA 2010, 107, 12435–12440. [Google Scholar]
- Liu, Z.; Fan, A.C.; Rakhra, K.; Sherlock, S.; Goodwin, A.; Chen, X.; Yang, Q.; Felsher, D.W.; Dai, H. Supramolecular stacking of doxorubicin on carbon nanotubes for in vivo cancer therapy. Angew Chem. Int. Ed. Engl. 2009, 48, 7668–7672. [Google Scholar]
- Liu, Z.; Tabakman, S.M.; Chen, Z.; Dai, H. Preparation of carbon nanotube bioconjugates for biomedical applications. Nat. Protoc. 2009, 4, 1372–1382. [Google Scholar]
- Prencipe, G.; Tabakman, S.M.; Welsher, K.; Liu, Z.; Goodwin, A.P.; Zhang, L.; Henry, J.; Dai, H. PEG branched polymer for functionalization of nanomaterials with ultralong blood circulation. J. Am. Chem. Soc. 2009, 131, 4783–4787. [Google Scholar]
- Liu, Z.; Chen, K.; Davis, C.; Sherlock, S.; Cao, Q.; Chen, X.; Dai, H. Drug delivery with carbon nanotubes for in vivo cancer treatment. Cancer Res. 2008, 68, 6652–6660. [Google Scholar]
- Schipper, M.L.; Nakayama-Ratchford, N.; Davis, C.R.; Kam, N.W.; Chu, P.; Liu, Z.; Sun, X.; Dai, H.; Gambhir, S.S. A pilot toxicology study of single-walled carbon nanotubes in a small sample of mice. Nat. Nanotechnol. 2008, 3, 216–221. [Google Scholar]
- Liu, Z.; Cai, W.; He, L.; Nakayama, N.; Chen, K.; Sun, X.; Chen, X.; Dai, H. In vivo biodistribution and highly efficient tumour targeting of carbon nanotubes in mice. Nat. Nanotechnol. 2007, 2, 47–52. [Google Scholar]
- Cai, W.; Chen, X. Multimodality molecular imaging of tumor angiogenesis. J. Nucl. Med. 2008, 49 (Suppl. 2), 113S–128S. [Google Scholar]
- Cai, W.; Chen, X. Nanoplatforms for targeted molecular imaging in living subjects. Small 2007, 3, 1840–1854. [Google Scholar]
- Lammers, T.; Kiessling, F.; Hennink, W.E.; Storm, G. Nanotheranostics and image-guided drug delivery: Current concepts and future directions. Mol. Pharm. 2010, 7, 1899–1912. [Google Scholar]
- Xie, J.; Chen, K.; Huang, J.; Lee, S.; Wang, J.; Gao, J.; Li, X.; Chen, X. PET/NIRF/MRI triple functional iron oxide nanoparticles. Biomaterials 2010, 31, 3016–3022. [Google Scholar]
- MacKay, J.A.; Li, Z. Theranostic agents that co-deliver therapeutic and imaging agents? Adv. Drug Deliv. Rev. 2010, 62, 1003–1004. [Google Scholar]
- Xie, J.; Huang, J.; Li, X.; Sun, S.; Chen, X. Iron oxide nanoparticle platform for biomedical applications. Curr. Med. Chem. 2009, 16, 1278–1294. [Google Scholar]
© 2011 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Patel, S.; Bhirde, A.A.; Rusling, J.F.; Chen, X.; Gutkind, J.S.; Patel, V. Nano Delivers Big: Designing Molecular Missiles for Cancer Therapeutics. Pharmaceutics 2011, 3, 34-52. https://doi.org/10.3390/pharmaceutics3010034
Patel S, Bhirde AA, Rusling JF, Chen X, Gutkind JS, Patel V. Nano Delivers Big: Designing Molecular Missiles for Cancer Therapeutics. Pharmaceutics. 2011; 3(1):34-52. https://doi.org/10.3390/pharmaceutics3010034
Chicago/Turabian StylePatel, Sachin, Ashwin A. Bhirde, James F. Rusling, Xiaoyuan Chen, J. Silvio Gutkind, and Vyomesh Patel. 2011. "Nano Delivers Big: Designing Molecular Missiles for Cancer Therapeutics" Pharmaceutics 3, no. 1: 34-52. https://doi.org/10.3390/pharmaceutics3010034
APA StylePatel, S., Bhirde, A. A., Rusling, J. F., Chen, X., Gutkind, J. S., & Patel, V. (2011). Nano Delivers Big: Designing Molecular Missiles for Cancer Therapeutics. Pharmaceutics, 3(1), 34-52. https://doi.org/10.3390/pharmaceutics3010034




