Carbomer Hydrogels with Microencapsulated α-Tocopherol: Focus on the Biocompatibility of the Microcapsules, Topical Application Attributes, and In Vitro Release Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Preparation of Microcapsules with α-Tocopherol
2.2.2. Characterization of α-Tocopherol-Loaded Microcapsules
2.2.3. Preparation of Hydrogels with Microencapsulated α-Tocopherol
2.2.4. Evaluation of the Compatibility of Microencapsulated α-Tocopherol with Carbomer Hydrogel
2.2.5. Evaluation of Topical Application Attributes of the Hydrogels with Microencapsulated α-Tocopherol
2.2.6. In Vitro Release Testing of the Hydrogels with Microencapsulated α-Tocopherol
3. Results
3.1. Influence of the Composition of the Microcapsule Wall on the Characteristics of α-Tocopherol-Loaded Microcapsules
3.2. Compatibility of Microencapsulated α-Tocopherol with Carbomer Hydrogel
3.3. Application Attributes of Hydrogels with Microencapsulated α-Tocopherol
3.4. In Vitro Release of α-Tocopherol
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wallert, M.; Börmel, L.; Lorkowski, S. Inflammatory Diseases and Vitamin E—What Do We Know and Where Do We Go? Mol. Nutr. Food Res. 2021, 65, 2000097:1–2000097:16. [Google Scholar] [CrossRef] [PubMed]
- Di Pasquale, M.; Nguyen, M.H.L.; Marquardt, D. The antioxidant vitamin E as a membrane raft modulator: Tocopherols do not abolish lipid domains. Biochim. Biophys. Acta Biomembr. 2020, 1862, 183189:1–183189:8. [Google Scholar] [CrossRef] [PubMed]
- Waghmode, M.; Kamble, M.; Shruti, A. Pharmaceutical profile of alpha-tocopherol—A brief review. Int. J. Pharm. Chem. Biol. Sci. 2012, 1, 1023–1039. [Google Scholar]
- CosIng—Cosmetics Ingredients. Ingredient: TOCOPHEROL. Available online: https://ec.europa.eu/growth/tools-databases/cosing/details/80273 (accessed on 5 March 2024).
- Sheskey, P.; Hancock, B.; Moss, G.; Goldfarb, D. (Eds.) Handbook of Pharmaceutical Excipients, 9th ed.; Pharmaceutical Press: London, UK, 2020; pp. 93–96. ISBN 978-08-5711-375-7. [Google Scholar]
- Ungurianu, A.; Zanfirescu, A.; Nitulescu, G.; Margina, D. Vitamin E beyond Its Antioxidant Label. Antioxidants 2021, 10, 634. [Google Scholar] [CrossRef] [PubMed]
- Hobson, R. Vitamin E and wound healing: An evidence-based review. Int. Wound J. 2016, 13, 331–335. [Google Scholar] [CrossRef] [PubMed]
- Ashamalla, L.; Maurice, M.; Sidhom, K. Topical vitamin E in granuloma annulare. Int. J. Dermatol. 1998, 27, 348. [Google Scholar] [CrossRef]
- Chiu, A.; Kimball, A.B. Topical vitamins, minerals and botanical ingredients as modulators of environmental and chronological skin damage. Br. J. Dermatol. 2003, 149, 681–691. [Google Scholar] [CrossRef]
- Baumann, L.S.; Spencer, J. The effects of topical vitamin E on the cosmetic appearance of scars. Dermatol. Surg. 1999, 25, 311–315. [Google Scholar] [CrossRef]
- Keen, M.A.; Hassan, I. Vitamin E in dermatology. Indian Dermatol. Online J. 2016, 7, 311–315. [Google Scholar] [CrossRef]
- Thiele, J.J.; Ekanayake-Mudiyanselage, S. Vitamin E in human skin: Organ-specific physiology and considerations for its use in dermatology. Mol. Asp. Med. 2007, 28, 646–667. [Google Scholar] [CrossRef]
- Bikiaris, N.D.; Koumentakou, I.; Hatzistamatiou, K.; Lykidou, S.; Barmpaxis, P.; Nikolaidis, N. Preparation and Investigation of the SPF and Antioxidant Properties of O/W and W/O Emulsions Containing Vitamins A, C and E for Cosmetic Applications. Cosmetics 2023, 10, 76. [Google Scholar] [CrossRef]
- Cosmile Europe. Ingredient. TOCOPHEROL. Available online: https://cosmileeurope.eu/inci/detail/16234/tocopherol/ (accessed on 5 March 2024).
- Zussman, J.; Ahdout, J.; Kim, J. Vitamins and photoaging: Do scientific data support their use? J. Am. Acad. Dermatol. 2010, 63, 507–525. [Google Scholar] [CrossRef]
- Fiume, M.M.; Bergfeld, W.F.; Belsito, D.V.; Hill, R.A.; Klaassen, C.D.; Liebler, D.C.; Marks, J.G., Jr.; Shank, R.C.; Slaga, T.J.; Snyder, P.W.; et al. Safety assessment of tocopherols and tocotrienols as used in cosmetics. Int. J. Toxicol. 2018, 37, 61S–94S. [Google Scholar] [CrossRef] [PubMed]
- Personal Care Products Council. INCI. Available online: https://www.personalcarecouncil.org/resources/inci/ (accessed on 5 March 2024).
- Burton, G.W.; Ingold, K.U. Vitamine E: Application of the principles of physical organic chemistry to the exploration of its structure and function. Acc. Chem. Res. 1986, 19, 194–201. [Google Scholar] [CrossRef]
- Azzi, A.; Atkinson, J.; Ozer, N.K.; Manor, D.; Wallert, M.; Galli, F. Vitamin E discussion forum position paper on the revision of the nomenclature of vitamin E. Free Radic. Biol. Med. 2023, 207, 178–180. [Google Scholar] [CrossRef]
- Alpha-Tocopherol. 3 Chemical and Physical Properties. 3.2 Experimental Properties. 3.2.1 Physical Description. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Alpha%2DTocopherol#section=Physical-Description (accessed on 5 March 2024).
- Alpha-Tocopherol. 3 Chemical and Physical Properties. 3.2 Experimental Properties. 3.2.3 Solubility. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Alpha-Tocopherol#section=Solubility (accessed on 5 March 2024).
- Liu, S.; Liu, F.; Xue, Y.; Gao, Y. Evaluation on oxidative stability of walnut beverage emulsions. Food Chem. 2016, 203, 409–416. [Google Scholar] [CrossRef] [PubMed]
- Pinto, F.; de Barros, D.P.C.; Fonseca, P.L. Design of multifunctional nanostructured lipid carriers enriched with α-tocopherol using vegetable oils. Ind. Crops Prod. 2018, 118, 149–159. [Google Scholar] [CrossRef]
- De Vaugelade, S.; Nicol, E.; Vujovic, S.; Bourcier, S.; Pirnay, S.; Bouchonnet, S. UV–vis degradation of α-tocopherol in a model system and in a cosmetic emulsion—Structural elucidation of photoproducts and toxicological consequences. J. Chromatogr. A 2017, 1517, 126–133. [Google Scholar] [CrossRef]
- Manyala, D.L.; Ghosh, M.; Dalai, S. Novel microemulsions formulated from sodium N-Lauroyl sarcosinate as nanocarrier for encapsulation of α-Tocopherol. J. Mol. Liq. 2023, 384, 122323:1–122323:9. [Google Scholar] [CrossRef]
- Pereira, G.G.; Detoni, C.B.; Balducci, A.G.; Rondelli, V.; Colombo, P.; Guterres, S.S.; Sonvico, F. Hyaluronate Nanoparticles Included in Polymer Films for the Prolonged Release of Vitamin E for the Management of Skin Wounds. Eur. J. Pharm. Sci. 2016, 83, 203–211. [Google Scholar] [CrossRef]
- Vaz, S.; Silva, R.; Amaral, M.H.; Martins, E.; Sousa Lobo, J.M.; Silva, A.C. Evaluation of the biocompatibility and skin hydration potential of vitamin E-loaded lipid nanosystems formulations: In vitro and human in vivo studies. Colloids Surf. B Biointerfaces 2019, 179, 242–249. [Google Scholar] [CrossRef]
- Ribeiro, A.M.; Estervinho, B.N.; Rocha, F. The progress and application of vitamin E encapsulation—A review. Food Hydrocoll. 2021, 121, 106998:1–106998:22. [Google Scholar] [CrossRef]
- Casanova, F.; Santos, L. Encapsulation of cosmetic active ingredients for topical application—A Review. J. Microencapsul. 2016, 33, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Lengyel, M.; Kállai-Szabó, N.; Antal, V.; Laki, A.J.; Antal, I. Microparticles, Microspheres, and Microcapsules for Advanced Drug Delivery. Sci. Pharm. 2019, 87, 20. [Google Scholar] [CrossRef]
- Martins, E.; Poncelet, D.; Renard, D. A novel method of oil encapsulation in core-shell alginate microcapsules by dispersion-inverse gelation technique. React. Funct. Polym. 2017, 114, 49–57. [Google Scholar] [CrossRef]
- Bakry, A.M.; Abbas, S.; Ali, B.; Majeed, H.; Abouelwafa, M.Y.; Mousa, A.; Lang, L. Microencapsulation of Oils: A Comprehensive Review of Benefits, Techniques, and Applications. Compr. Rev. Food Sci. Food Saf. 2016, 15, 143–182. [Google Scholar] [CrossRef] [PubMed]
- Frent, O.D.; Vicas, L.G.; Duteanu, N.; Morgovan, C.M.; Jurca, T.; Pallag, A.; Muresan, M.E.; Filip, S.M.; Lucaciu, R.-L.; Marian, E. Sodium Alginate-Natural Microencapsulation Material of Polymeric Microparticles. Int. J. Mol. Sci. 2022, 23, 12108. [Google Scholar] [CrossRef] [PubMed]
- Parente, J.F.; Sousa, V.I.; Marques, J.F.; Forte, M.A.; Tavares, C.J. Biodegradable Polymers for Microencapsulation Systems. Adv. Polym. Technol. 2022, 2022, 4640379:1–4640379:43. [Google Scholar] [CrossRef]
- Elieh-Ali-Komi, D.; Hamblin, M.R. Chitin and Chitosan: Production and Application of Versatile Biomedical Nanomaterials. Int. J. Adv. Res. 2016, 4, 411–427. [Google Scholar] [PubMed]
- Sami El-banna, F.; Mahfouz, M.E.; Leporatti, S.; El-Kemary, M.; Hanafy, A.N.N. Chitosan as a Natural Copolymer with Unique Properties for the Development of Hydrogels. Appl. Sci. 2019, 9, 2193. [Google Scholar] [CrossRef]
- Thongngam, M.; Mc Clements, D.J. Influence of pH, ionic strength, and temperature on self-sssociation and interactions of sodium dodecyl sulfate in the absence and presence of chitosan. Langmuir 2005, 21, 79–86. [Google Scholar] [CrossRef]
- Onésippe, C.; Lagerge, S. Study of the complex formation between sodium dodecyl sulfate and chitosan. Colloids Surf. A Physicochem. Eng. Asp. 2008, 317, 100–108. [Google Scholar] [CrossRef]
- Ren, Y.; Xie, H.; Liu, X.; Yang, F.; Yu, W.; Ma, X. Tuning the formation and stability of microcapsules by environmental conditions and chitosan structure. Int. J. Biol. Macromol. 2016, 91, 1090–1100. [Google Scholar] [CrossRef] [PubMed]
- Carpentier, J.; Conforto, E.; Chaigneau, C.; Vendeville, J.-E.; Maugard, T. Microencapsulation and controlled release of α-tocopherol by complex coacervation between pea protein and tragacanth gum: A comparative study with arabic and tara gums. Innov. Food Sci. Emerg. Technol. 2022, 77, 102951:1–102951:14. [Google Scholar] [CrossRef]
- Estevinho, B.; Rocha, F.; Santos, L.; Alves, A. Microencapsulation with chitosan by spray drying for industry applications—A review. Trends Food Sci. Technol. 2013, 31, 138–155. [Google Scholar] [CrossRef]
- Wang, B.; Akanbi, T.O.; Agyei, D.; Holland, B.J.; Barrow, C.J. Coacervation Technique as an Encapsulation and Delivery Tool for Hydrophobic Biofunctional Compounds. In Role of Materials Science in Food Bioengineering; Grumezescu, A., Holban, A.M., Eds.; Elsevier: Amsterdam, The Netherlands, 2018; pp. 235–261. ISBN 978-0-1281-1448-3. [Google Scholar]
- Hilton, J.; Dearman, R.J.; Harvey, P.; Evans, P.; Basketter, D.A.; Kimber, I. Estimation of relative skin sensitizing potency using the local lymph node assay: A comparison of formaldehyde with glutaraldehyde. Am. J. Contact Dermat. 1998, 9, 29–33. [Google Scholar] [CrossRef]
- COMMISSION REGULATION (EU) 2022/1181 of 8 July 2022 amending the preamble of Annex V to Regulation (EC) No 1223/2009 of the European Parliament and of the Council on cosmetic products. OJEU 2022, 184, L184/3–L184/4.
- Milinković Budinčić, J.; Petrović, L.; Đekić, L.; Fraj, J.; Bučko, S.; Katona, J.; Spasojević, L. Study of vitamin E microencapsulation and controlled release from chitosan/sodium lauryl ether sulfate microcapsules. Carbohydr. Polym. 2021, 251, 116988:1–116988:8. [Google Scholar] [CrossRef]
- Schreiner, T.B.; Santamaria-Echart, A.; Barreiro, M.F. Saponin-based natural nanoemulsions as alpha-tocopherol delivery systems for dermal applications. J. Mol. Liq. 2023, 391, 123371:1–123371:12. [Google Scholar] [CrossRef]
- Olbińska, E.; Trela-Makowej, A.; Larysz, W.; Orzechowska, A.; Szymańska, R. The effect of α-tocopherol incorporated into different carriers on the oxidative stability of oil in water (O/W) emulsions. Colloids Surf. B Biointerface 2023, 230, 113536:1–113536:7. [Google Scholar] [CrossRef]
- Bahram, M.; Mohseni, N.; Moghtader, M. An Introduction to Hydrogels and Some Recent Applications. In Emerging Concepts in Analysis and Applications of Hydrogels; Majee, S.B., Ed.; IntechOpen: London, UK, 2016. [Google Scholar] [CrossRef]
- Blanco, M.D.; Olmo, R.M.; Teijón, J.M. Hydrogels. In Encyclopedia of Pharmaceutical Technology, 3rd ed.; Swarbrick, J., Ed.; Informa Healthcare: New York, NY, USA, 2007; pp. 2021–2039. ISBN 978-0-8493-9396-9. [Google Scholar]
- Ho, T.C.; Chang, C.C.; Chan, H.P.; Chung, T.W.; Shu, C.W.; Chuang, K.P.; Duh, T.H.; Yang, M.H.; Tyan, Y.C. Hydrogels: Properties and Applications in Biomedicine. Molecules 2022, 27, 2902. [Google Scholar] [CrossRef]
- Bashir, S.; Hina, M.; Iqbal, J.; Rajpar, A.H.; Mujtaba, M.A.; Alghamdi, N.A.; Wageh, S.; Ramesh, K.; Ramesh, S. Fundamental Concepts of Hydrogels: Synthesis, Properties, and Their Applications. Polymers 2020, 12, 2702. [Google Scholar] [CrossRef]
- Parhi, R. Cross-Linked Hydrogel for Pharmaceutical Applications: A Review. Adv. Pharm. Bull. 2017, 7, 515–530. [Google Scholar] [CrossRef]
- Jaworski, Z.; Spychaj, T.; Story, A.; Story, G. Carbomer microgels as model yield-stress fluids. Rev. Chem. Eng. 2022, 38, 881–919. [Google Scholar] [CrossRef]
- Lochhead, R.Y. The use of polymers in cosmetic products. In Cosmetic Science and Technology; Sakamoto, K., Lochhead, R.Y., Maibach, H.I., Yamashita, Y., Eds.; Elsevier: Amsterdam, The Netherlands, 2017; pp. 171–221. ISBN 978-0-12-802005-0. [Google Scholar]
- Carbopol® Ultrez 10 Polymer. Available online: https://www.lubrizol.com/en/Personal-Care/Products/Product-Finder/Products-Data/Carbopol-Ultrez-10-polymer (accessed on 5 March 2024).
- Milinković, J.; Petrović, L.; Fraj, J.; Bučko, S.; Katona, J.; Spasojević, L. Interfacial and emulsifying properties of chitosan/sodium lauryl ether sulfate system. Colloids Surf. A Physicochem. Eng. Asp. 2018, 557, 9–13. [Google Scholar] [CrossRef]
- Yuan, Y.; Chesnutt, B.M.; Haggard, W.O.; Bumgardner, J.D. Deacetylation of chitosan: Material characterization and in vitro evaluation via albumin adsorption and pre-osteoblastic cell cultures. Materials 2011, 4, 1399–1416. [Google Scholar] [CrossRef]
- United States Pharmacopeia. USP 43-NF 38 Chapter <659> Packaging and Storage Requirements. In The United States Pharmacopoeia 43, The National Formulary 38; United States Pharmacopoeial Convention: Rockville, MD, USA, 2020; p. 6880. ISBN 978-1-936424-95-5. [Google Scholar]
- Djekic, L.; Martinović, M.; Dobričić, V.; Čalija, B.; Medarević, Đ.; Primorac, M. Comparison of the Effect of Bioadhesive Polymers on Stability and Drug Release Kinetics of Biocompatible Hydrogels for Topical Application of Ibuprofen. J. Pharm. Sci. 2019, 108, 1326–1333. [Google Scholar] [CrossRef]
- Shimamura, T.; Tairabune, T.; Kogo, T.; Ueda, H.; Numajiri, S.; Kobayashi, D.; Morimoto, Y. Investigation of the Release Test Method for the Topical Application of Pharmaceutical Preparations: Release Test of Cataplasm Including Nonsteroidal Anti-inflammatory Drugs Using Artificial Sweat. Chem. Pharm. Bull. 2004, 52, 167–171. [Google Scholar] [CrossRef]
- United States Pharmacopeia. USP 43-NF 38 chapter <1724> Semisolid Drug Products—Performance Tests. In The United States Pharmacopoeia 43, The National Formulary 38; United States Pharmacopoeial Convention: Rockville, MD, USA, 2020; pp. 8473–8484. ISBN 978-1-936424-95-5. [Google Scholar]
- Zhang, Y.; Huo, M.; Zhou, J.; Zou, A.; Li, W.; Yao, C.; Xie, S. DDSolver: An add-in program for modeling and comparison of drug dissolution profiles. AAPS J. 2010, 12, 263–271. [Google Scholar] [CrossRef]
- Diaz, D.A.; Colgan, S.T.; Langer, C.S.; Bandi, N.T.; Likar, M.D.; Van Alstine, L. Dissolution Similarity Requirements: How Similar or Dissimilar Are the Global Regulatory Expectations? AAPS J. 2016, 18, 15–22. [Google Scholar] [CrossRef]
- Dejeu, I.L.; Vicaș, L.G.; Vlaia, L.L.; Jurca, T.; Mureșan, M.E.; Pallag, A.; Coneac, G.H.; Olariu, I.V.; Muț, A.M.; Bodea, A.S.; et al. Study for Evaluation of Hydrogels after the Incorporation of Liposomes Embedded with Caffeic Acid. Pharmaceuticals 2022, 15, 175. [Google Scholar] [CrossRef]
- United States Pharmacopeia. USP 43-NF 38 Chapter <1191> Stability Considerations in Dispensing Practice. In The United States Pharmacopoeia 43, The National Formulary 38; United States Pharmacopoeial Convention: Rockville, MD, USA, 2020; p. 8048. ISBN 978-1-936424-95-5. [Google Scholar]
- Allen, L.V. Gels. In The Art, Science, and Technology of Pharmaceutical Compounding, 6th ed.; Allen, L.V., Ed.; APhA PharmacyLibrary: Washington, DC, USA, 2020; ISBN 1-58212-357-8. [Google Scholar] [CrossRef]
- Lein, A.; Oussoren, C. Dermal. In Practical Pharmaceutics; Bouwman-Boer, Y., Fenton-May, V., Le Brun, P., Eds.; Springer: Berlin/Heidelberg, Germany, 2015; pp. 229–263. ISBN 978-3-319-15813-6. [Google Scholar]
- Gutowski, I.A.; Lee, D.; de Bruyn, J.R.; Frisken, B.J. Scaling and mesostructure of Carbopol dispersions. Rheol. Acta 2012, 51, 441–450. [Google Scholar] [CrossRef]
- TDS-237 Neutralizing Carbopol and Pemulen Polymers in Aqueous and Hydroalcoholic Systems. Available online: https://www.academia.edu/42914025/TDS_237_Neutralizing_Carbopol_Pemulen_in_Aqueous_Hydroalcoholic_Systems_PH_1_ (accessed on 5 March 2024).
- Kulkarni, V.S.; Shaw, C. Use of polymers and thickeners in semisolid and liquid formulations. In Essential Chemistry for Formulators of Semisolid and Liquid Dosages; Kulkarni, V.S., Shaw, C., Eds.; Elsevier: Amsterdam, The Netherlands, 2016; pp. 43–69. ISBN 978-0-12-801024-2. [Google Scholar]
- Saba, M.A.; Yosipovitch, G. Skin pH: From Basic Science to Basic Skin Care. Acta Derm. Venereol. 2013, 93, 261–267. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Wen, Y.; Chen, S.; Zhu, L.; Feng, R.; Song, Z. Paclitaxel skin delivery by micelles-embedded Carbopol 940 hydrogel for local therapy of melanoma. Int. J. Pharm. 2020, 587, 119626:1–119626:11. [Google Scholar] [CrossRef] [PubMed]
- Maslii, Y.; Ruban, O.; Kasparaviciene, G.; Kalveniene, Z.; Materiienko, A.; Ivanauskas, L.; Mazurkieviciute, A.; Kopustinskiene, D.M.; Bernatoniene, J. The Influence of pH Values on the Rheological, Textural and Release Properties of Carbomer Polacril® 40P-Based Dental Gel Formulation with Plant-Derived and Synthetic Active Components. Molecules 2020, 25, 5018. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.; Vyas, S.P. Carbopol/Chitosan Based pH Triggered In Situ Gelling System for Ocular Delivery of Timolol Maleate. Sci. Pharm. 2010, 78, 959–976. [Google Scholar] [CrossRef] [PubMed]
- Krieger, I.; Dougherthy, T. A mechanism for non-Newtonian flow in suspensions of rigid spheres. J. Rheol. 1959, 3, 137–152. [Google Scholar] [CrossRef]
- Hui, X.; Anigbogu, A.; Singh, P.; Poblete, N.; Liu, P.; Maibach, H.I. Pharmacokinetic and local tissue disposition of [14C]sodium diclofenac following iontophoresis and systemic administration in rabbits. J. Pharm. Sci. 2001, 90, 1269–1276. [Google Scholar] [CrossRef] [PubMed]
- Gabbanini, S.; Matera, R.; Beltramini, C.; Minghetti, A.; Valgimigli, L. Analysis of in vitro release through reconstructed human epidermis and synthetic membranes of multi-vitamins from cosmetic formulations. J. Pharm. Biomed. Anal. 2010, 52, 461–467. [Google Scholar] [CrossRef]
- Dreijer-van der Glas, S.; Hinrichs, W. Physical Chemistry. In Practical Pharmaceutics; Bouwman-Boer, Y., Fenton-May, V., Le Brun, P., Eds.; Springer: Berlin/Heidelberg, Germany, 2015; pp. 357–382. ISBN 978-3-319-15813-6. [Google Scholar]
- Djekić, L.; Ćirić, A. Modeling of in vitro drug release from polymeric microparticle carriers. ARH Farm 2022, 72, 591–620. [Google Scholar] [CrossRef]

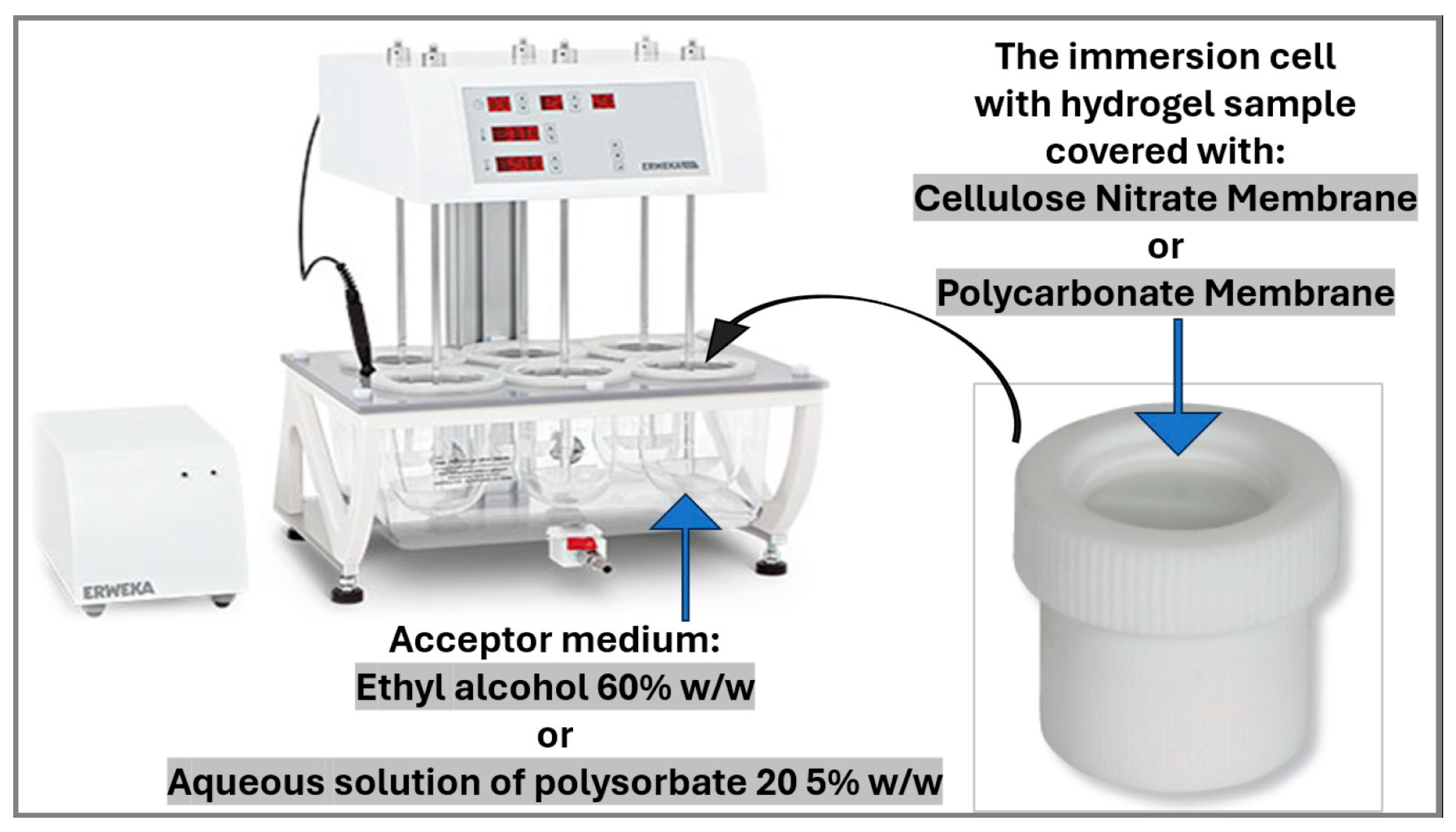
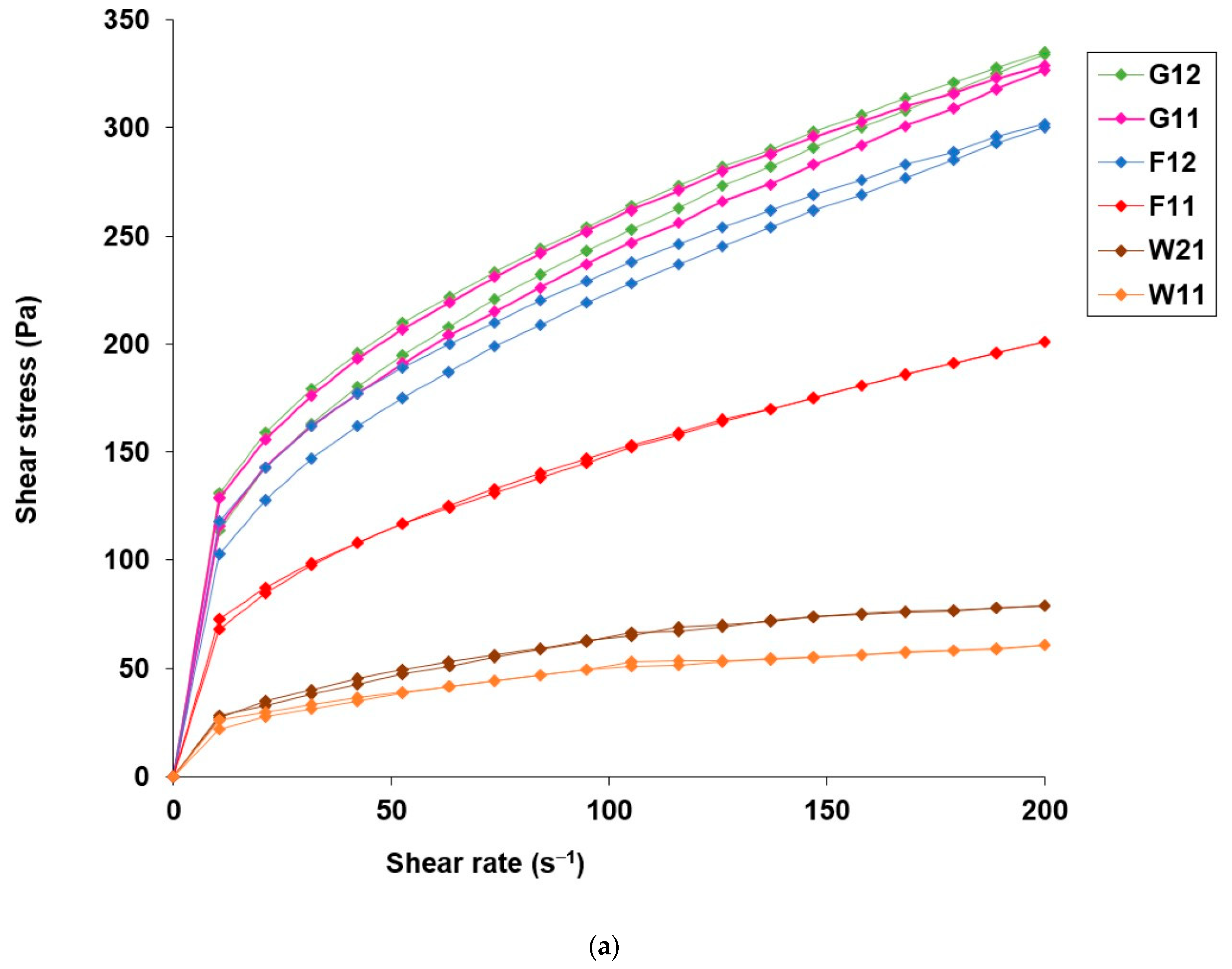
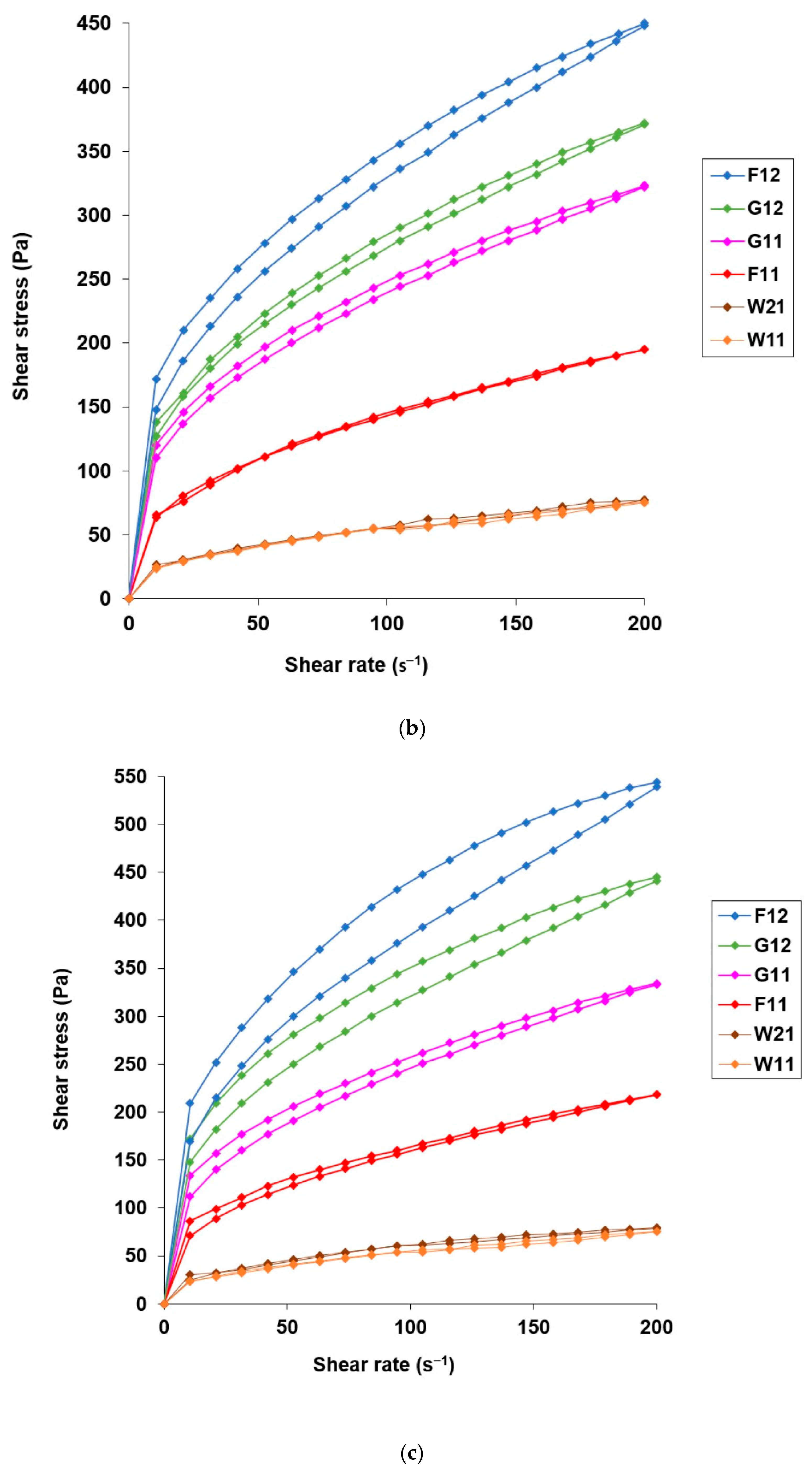

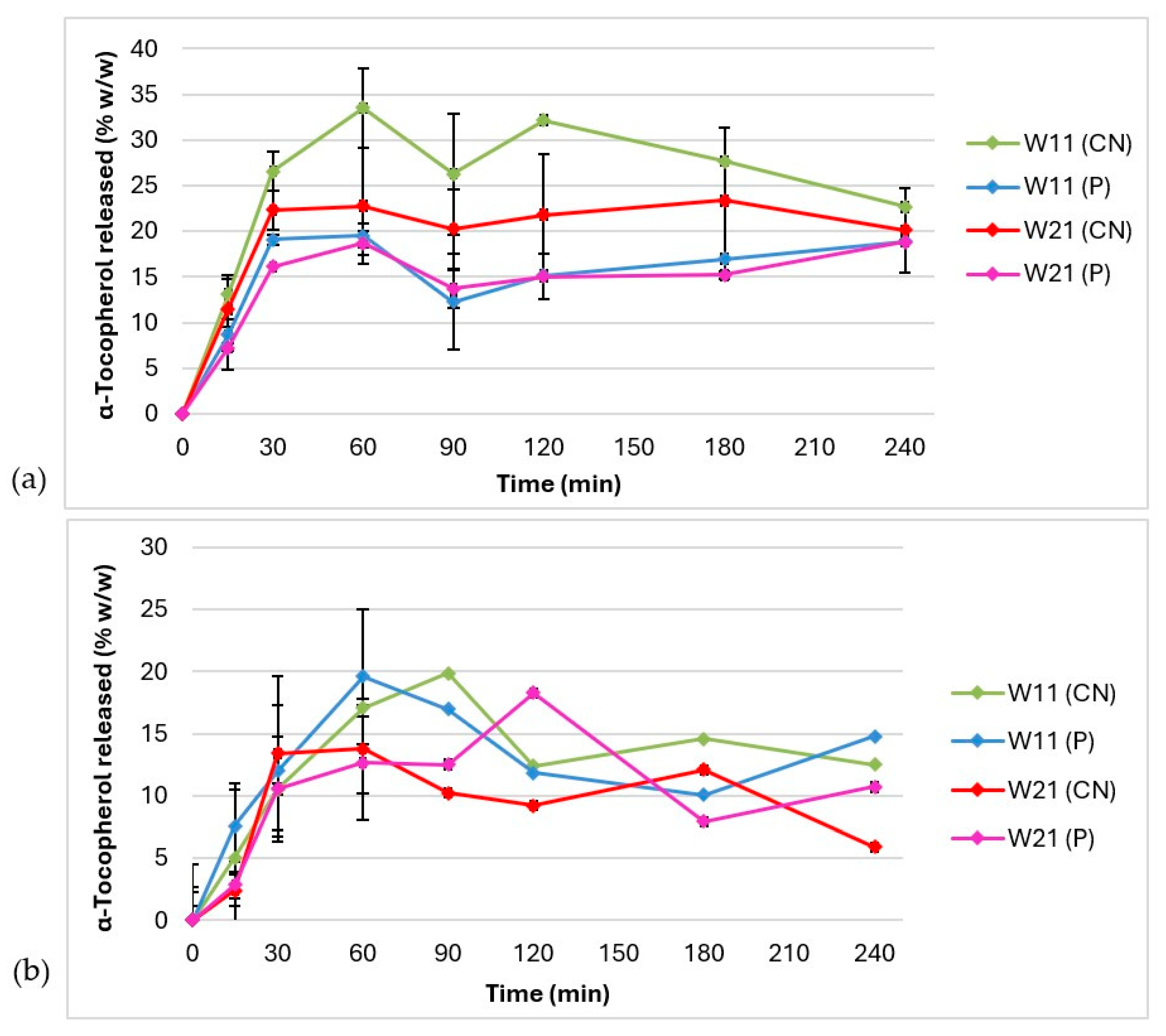
| Composition of the Microcapsule Wall | Moisture Content ± S.D. (% w/w) | Mean Diameter ± S.D. (µm) | EE ± S.D. (% w/w) | Hydrogel Label | Content of α-Tocopherol in Hydrogel (% w/w) |
|---|---|---|---|---|---|
| LMWC/SLES (mass ratio 2:1) without crosslinker | 1.27 ± 0.081 1 | 5.32 ± 0.252 1 | 73.17 ± 0.64 1 | W21 | 0.37 |
| LMWC/SLES (mass ratio 1:1) without crosslinker | 1.35 ± 0.180 | 4.86 ± 0.29 | 71.12 ± 0.98 | W11 | 0.36 |
| LMWC/SLES (mass ratio 2:1) crosslinked with glutaraldehyde (LMWC/SLES-to-glutaraldehyde mass ratio 1:2) | 1.30 ± 0.173 1 | 5.84 ± 0.256 1 | 99.50 ± 2.27 1 | G12 | 0.50 |
| LMWC/SLES (mass ratio 2:1) crosslinked with glutaraldehyde (LMWC/SLES-to-glutaraldehyde mass ratio 1:1) | 1.66 ± 0.376 1 | 5.04 ± 0.331 1 | 100.00 ± 3.55 1 | G11 | 0.50 |
| LMWC/SLES (mass ratio 2:1) crosslinked with formaldehyde (LMWC/SLES-to-formaldehyde mass ratio 1:2) | 1.76 ± 0.451 1 | 6.21 ± 0.242 1 | 82.30 ± 1.67 1 | F12 | 0.41 |
| LMWC/SLES (mass ratio 2:1) crosslinked with formaldehyde (LMWC/SLES-to-formaldehyde mass ratio 1:1) | 0.97 ± 0.312 1 | 6.25 ± 0.224 1 | 93.50 ± 1.08 1 | F11 | 0.47 |
| pH | |||
|---|---|---|---|
| Sample | 48 h | 1 Month | 2 Months |
| Blank hydrogel | 7.22 | 7.17 | 7.20 |
| W21 | 6.44 | 6.51 | 6.60 |
| W11 | 6.41 | 6.48 | 6.53 |
| F12 | 6.68 | 6.77 | 6.82 |
| F11 | 6.60 | 6.63 | 6.71 |
| G12 | 6.83 | 6.87 | 6.88 |
| G11 | 6.58 | 6.64 | 6.68 |
| 48 h | 1 Month | 2 Months | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Sample | ηmax (Pa∙s) | ηmin (Pa∙s) | H (Pa/s) | ηmax (Pa∙s) | ηmin(Pa∙s) | H (Pa/s) | ηmax (Pa∙s) | ηmin (Pa∙s) | H (Pa/s) |
| Blank hydrogel | 16.4 ± 0.14 | 2.41 ± 0.01 | 2614.99 | 18.7 ± 0.32 | 4.41 ± 0.06 | 2934.09 | 20.5 ± 0.16 | 3.38 ± 0.07 | 3512.08 |
| W21 | 2.54 ± 0.05 | 0.62 ± 0.05 | 7.51 | 2.25 ± 0.34 | 0.51 ± 0.09 | 6.36 | 2.71 ± 0.51 | 0.61 ± 0.02 | 8.33 |
| W11 | 2.18 ± 0.06 | 0.54 ± 0.01 | 6.17 | 2.22 ± 0.07 | 0.54 ± 0.01 | 5.88 | 2.34 ± 0.14 | 0.49 ± 0.03 | 6.24 |
| F12 | 11.2 ± 0.42 | 1.51 ± 0.02 | 1933.9 | 15.4 ± 0.37 | 2.15 ± 0.04 | 2985.64 | 19.8 ± 0.75 | 2.72 ± 0.06 | 8319.54 |
| F11 | 6.9 ± 0.69 | 1.01 ± 0.03 | 8.11 | 6.2 ± 0.08 | 0.98 ± 0.02 | 91.88 | 8.12 ± 0.31 | 1.09 ± 0.02 | 1049.11 |
| G12 | 12.45 ± 0.49 | 1.67 ± 0.01 | 2045.86 | 13.1 ± 0.65 | 1.86 ± 0.07 | 1552.81 | 16.3 ± 0.54 | 2.22 ± 0.07 | 4810.33 |
| G11 | 12.15 ± 0.35 | 1.65 ± 0.02 | 2481.12 | 11.4 ± 0.29 | 1.61 ± 0.02 | 1533.52 | 12.7 ± 0.41 | 1.67 ± 0.03 | 2227.38 |
| Sample | Φ (mm) ± S.D. | t (min) |
|---|---|---|
| Blank hydrogel | 19.5 ± 0.5 | >5 |
| W21 | 30.7 ± 1.5 | 1–2 |
| W11 | 43.3 ± 2.5 | <1 |
| F12 | 28.7 ± 2.5 | 2–5 |
| F11 | 39.7 ± 1.2 | 2–5 |
| G12 | 36.7 ± 0.6 | 2–5 |
| G11 | 38.3 ± 1.5 | 2–5 |
| Acceptor Medium | Ethyl Alcohol 60% w/w | Polysorbate 20 5% w/w | ||
|---|---|---|---|---|
| Samples | f1 | f2 | f1 | f2 |
| W11 (CN) vs. W11 (P) | 39.25 | 47.36 | 18.12 | 65.24 |
| W21 (CN) vs. W21 (P) | 26.29 | 61.73 | 32.72 | 66.90 |
| W11 (CN) vs. W21 (CN) | 21.92 | 58.76 | 33.39 | 64.87 |
| W11 (P) vs. W21 (P) | 26.29 | 61.73 | 32.54 | 66.03 |
| W11 (CN) vs. W21 (P) | 42.45 | 46.20 | 30.71 | 56.98 |
| W21 (CN) vs. W11 (P) | 22.19 | 64.41 | 52.82 | 57.74 |
| Membrane | Cellulose Nitrate | Polycarbonate | ||
|---|---|---|---|---|
| Samples | f1 | f2 | f1 | f2 |
| W11 (ethyl alcohol 60% w/w) vs. W11 (polysorbate 20 5% w/w) | 49.45 | 46.15 | 24.69 | 60.03 |
| W21 (ethyl alcohol 60% w/w) vs. W21 (polysorbate 20 5% w/w) | 52.82 | 48.99 | 34.08 | 58.11 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Đekić, L.; Milinković Budinčić, J.; Stanić, D.; Fraj, J.; Petrović, L. Carbomer Hydrogels with Microencapsulated α-Tocopherol: Focus on the Biocompatibility of the Microcapsules, Topical Application Attributes, and In Vitro Release Study. Pharmaceutics 2024, 16, 628. https://doi.org/10.3390/pharmaceutics16050628
Đekić L, Milinković Budinčić J, Stanić D, Fraj J, Petrović L. Carbomer Hydrogels with Microencapsulated α-Tocopherol: Focus on the Biocompatibility of the Microcapsules, Topical Application Attributes, and In Vitro Release Study. Pharmaceutics. 2024; 16(5):628. https://doi.org/10.3390/pharmaceutics16050628
Chicago/Turabian StyleĐekić, Ljiljana, Jelena Milinković Budinčić, Dušanka Stanić, Jadranka Fraj, and Lidija Petrović. 2024. "Carbomer Hydrogels with Microencapsulated α-Tocopherol: Focus on the Biocompatibility of the Microcapsules, Topical Application Attributes, and In Vitro Release Study" Pharmaceutics 16, no. 5: 628. https://doi.org/10.3390/pharmaceutics16050628






