Vaccines to Treat Substance Use Disorders: Current Status and Future Directions
Abstract
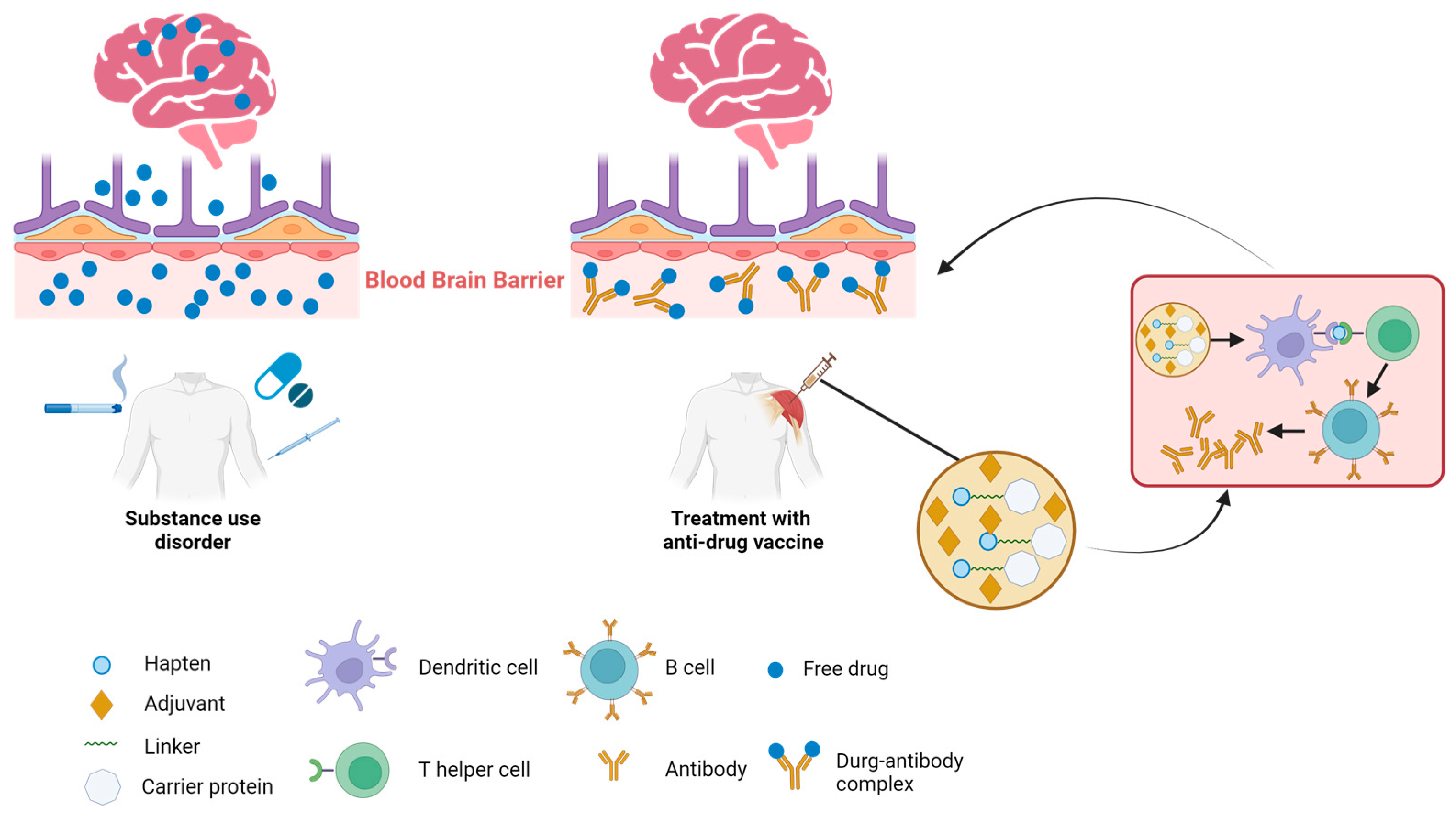
:1. Introduction
2. The Mechanisms of Immunotherapies against SUDs
3. Conjugate Vaccines
4. The Key of Anti-Drug Vaccine Design
5. Recent Progress in Anti-Drug Vaccine Research
5.1. Opioid Vaccines
5.2. Nicotine Vaccines
5.3. Cocaine Vaccines
5.4. Methamphetamine Vaccine
6. Conclusions and Outlook
- Non-interaction with receptors in the CNS (do not act on the brain): The immunotherapeutic strategy has the potential to inhibit the drug’s activity without directly interacting with receptors (MORs, etc.). This approach does not elicit pharmacological effects that could potentially result in dependence or withdrawal. Moreover, these vaccines can be employed in conjunction with other medications, such as antidepressants and anti-craving agents;
- Reduced side effects: Anti-drug antibodies do not directly engage with drug receptors in the brain or periphery. Consequently, the side effect profiles of these vaccines are significantly reduced compared to conventional pharmacological interventions, rendering them more tolerable for patients;
- Long-lasting effects: The administered vaccine generates circulating antibodies that can remain in the body for up to a year, provided that strategically spaced booster injections are administered. This sustained effect offers extended protection against addiction and overdose risks, obviating the need for frequent dosing or patient compliance;
- Reversible nature: the gradual attenuation of anti-drug antibodies over time confers on patients the capability to manage and potentially discontinue their vaccination regimen as necessitated.
- Focus on antibody function: Place greater emphasis on assessing the function of antibodies, particularly their affinity or avidity. The antigen’s structure, including the hapten, plays a crucial role in determining antibody function [42];
- Explore novel adjuvants: consider using new adjuvants that have shown promise in boosting both antibody quantity and affinity [104];
- Alternative administration routes: explore alternative administration methods beyond intramuscular injection, such as intranasal immunization, which could play a vital role in preventing cocaine’s direct entry into the brain through the olfactory bulb [106];
- Combination: Recognizing the multifaceted complexity of SUDs is essential, as they result from the interplay of various environmental and individual factors. This understanding implies that a multidisciplinary approach should be adopted in their treatment, involving the combination of vaccines with other addiction interventions (such as medications, monitoring, behavioral therapy, and peer support), or the use of multiple vaccines.
- The pursuit of effective anti-drug vaccines, although challenging, holds immense potential benefits, including the reduction of drug-related harm and enhancement of the quality of life for affected individuals. In the future, these vaccines may serve as a testament to the power of medical innovation in addressing one of the most pressing public health concerns of our time.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Friedman, J.; Shover, C. Charting the fourth wave: Geographic, temporal, race/ethnicity and demographic trends in polysubstance fentanyl overdose deaths in the United States, 2010–2021. Addiction 2023, 118, 2477–2485. [Google Scholar] [CrossRef] [PubMed]
- Ciccarone, D. The rise of illicit fentanyls, stimulants and the fourth wave of the opioid overdose crisis. Curr. Opin. Psychiatry 2021, 34, 344–350. [Google Scholar] [CrossRef] [PubMed]
- Cano, M.; Huang, Y. Overdose deaths involving psychostimulants with abuse potential, excluding cocaine: State-level differences and the role of opioids. Drug Alcohol Depend. 2021, 218, 108384. [Google Scholar] [CrossRef] [PubMed]
- Guenzel, N.; McChargue, D. Addiction Relapse Prevention; StatPearls Publishing: St. Petersburg, FL, USA, 2023. [Google Scholar]
- Lee, Y.; Gold, M.; Fuehrlein, B. Looking beyond the opioid receptor: A desperate need for new treatments for opioid use disorder. J. Neurol. Sci. 2022, 432, 120094. [Google Scholar] [CrossRef]
- Zeigler, D.; Roque, R.; Clegg, C. Optimization of a multivalent peptide vaccine for nicotine addiction. Vaccine 2019, 37, 1584–1590. [Google Scholar] [CrossRef] [PubMed]
- Stephenson, R.; Toth, I. Anti-cocaine vaccine development: Where are we now and where are we going? J. Med. Chem. 2023, 66, 7086–7100. [Google Scholar] [CrossRef] [PubMed]
- Zimmermann, P.; Curtis, N. Factors That influence the immune response to vaccination. Clin. Microbiol. Rev. 2019, 32, e00084-18. [Google Scholar] [CrossRef] [PubMed]
- Luba, R.; Martinez, S.; Jones, J.; Pravetoni, M.; Comer, S. Immunotherapeutic strategies for treating opioid use disorder and overdose. Expert Opin. Investig. Drugs 2023, 32, 77–87. [Google Scholar] [CrossRef]
- Truong, T.; Kosten, T. Current status of vaccines for substance use disorders: A brief review of human studies. J. Neurol. Sci. 2022, 434, 120098. [Google Scholar] [CrossRef]
- Clinical Trials of Multivalent Opioid Vaccine Components. Available online: https://classic.clinicaltrials.gov/ct2/show/NCT04458545 (accessed on 4 August 2023).
- Pravetoni, M.; Le Naour, M.; Tucker, A.; Harmon, T.; Hawley, T.; Portoghese, P.; Pentel, P. Reduced antinociception of opioids in rats and mice by vaccination with immunogens containing oxycodone and hydrocodone haptens. J. Med. Chem. 2013, 56, 915–923. [Google Scholar] [CrossRef]
- Kimishima, A.; Wenthur, C.; Zhou, B.; Janda, K. An advance in prescription opioid vaccines: Overdose mortality reduction and extraordinary alteration of drug half-life. ACS Chem. Biol. 2017, 12, 36–40. [Google Scholar] [CrossRef] [PubMed]
- Townsend, E.; Blake, S.; Faunce, K.; Hwang, C.; Natori, Y.; Zhou, B.; Bremer, P.; Janda, K.; Banks, M. Conjugate vaccine produces long-lasting attenuation of fentanyl vs. food choice and blocks expression of opioid withdrawal-induced increases in fentanyl choice in rats. Neuropsychopharmacol. Off. Publ. Am. Coll. Neuropsychopharmacol. 2019, 44, 1681–1689. [Google Scholar] [CrossRef] [PubMed]
- Haile, C.; Baker, M.; Sanchez, S.; Lopez Arteaga, C.; Duddupudi, A.; Cuny, G.; Norton, E.; Kosten, T.; Kosten, T. An immunconjugate vaccine alters distribution and reduces the antinociceptive, behavioral and physiological effects of fentanyl in male and female rats. Pharmaceutics 2022, 14, 2290. [Google Scholar] [CrossRef] [PubMed]
- Méndez, S.; Matus-Ortega, M.; Miramontes, R.; Salazar-Juárez, A. Effect of the morphine/heroin vaccine on opioid and non-opioid drug-induced antinociception in mice. Eur. J. Pharmacol. 2021, 891, 173718. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; Zhou, Q.; Zheng, H.; Li, S. Preparation and characterization of anti-morphine vaccine antibody. Xi Bao Yu Fen Zi Mian Yi Xue Za Zhi = Chin. J. Cell. Mol. Immunol. 2006, 22, 368–370. [Google Scholar]
- Raleigh, M.; Pravetoni, M.; Harris, A.; Birnbaum, A.; Pentel, P. Selective effects of a morphine conjugate vaccine on heroin and metabolite distribution and heroin-induced behaviors in rats. J. Pharmacol. Exp. Ther. 2013, 344, 397–406. [Google Scholar] [CrossRef]
- Kosten, T.; Shen, X.; O’Malley, P.; Kinsey, B.; Lykissa, E.; Orson, F.; Kosten, T. A morphine conjugate vaccine attenuates the behavioral effects of morphine in rats. Prog. Neuropsychopharmacol. Biol. Psychiatry 2013, 45, 223–229. [Google Scholar] [CrossRef]
- Esterlis, I.; Hannestad, J.; Perkins, E.; Bois, F.; D’Souza, D.; Tyndale, R.; Seibyl, J.; Hatsukami, D.; Cosgrove, K.; O’Malley, S. Effect of a nicotine vaccine on nicotine binding to β2*-nicotinic acetylcholine receptors in vivo in human tobacco smokers. Am. J. Psychiatry 2013, 170, 399–407. [Google Scholar] [CrossRef]
- Tonstad, S.; Heggen, E.; Giljam, H.; Lagerbäck, P.; Tønnesen, P.; Wikingsson, L.; Lindblom, N.; de Villiers, S.; Svensson, T.; Fagerström, K. Niccine®, a nicotine vaccine, for relapse prevention: A phase II, randomized, placebo-controlled, multicenter clinical trial. Nicotine Tob. Res. Off. J. Soc. Res. Nicotine Tob. 2013, 15, 1492–1501. [Google Scholar] [CrossRef]
- Cornuz, J.; Zwahlen, S.; Jungi, W.; Osterwalder, J.; Klingler, K.; van Melle, G.; Bangala, Y.; Guessous, I.; Müller, P.; Willers, J.; et al. A vaccine against nicotine for smoking cessation: A randomized controlled trial. PLoS ONE 2008, 3, e2547. [Google Scholar] [CrossRef]
- Hu, Y.; Smith, D.; Frazier, E.; Hoerle, R.; Ehrich, M.; Zhang, C. The next-generation nicotine vaccine: A novel and potent hybrid nanoparticle-based nicotine vaccine. Biomaterials 2016, 106, 228–239. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Li, Z.; Zhu, X.; Cao, Y.; Chen, X. Improving immunogenicity and safety of flagellin as vaccine carrier by high-density display on virus-like particle surface. Biomaterials 2020, 249, 120030. [Google Scholar] [CrossRef] [PubMed]
- Martell, B.; Orson, F.; Poling, J.; Mitchell, E.; Rossen, R.; Gardner, T.; Kosten, T. Cocaine vaccine for the treatment of cocaine dependence in methadone-maintained patients: A randomized, double-blind, placebo-controlled efficacy trial. Arch. Gen. Psychiatry 2009, 66, 1116–1123. [Google Scholar] [CrossRef] [PubMed]
- Multisite Controlled Trial of Cocaine Vaccine (TA-CD). Available online: https://classic.clinicaltrials.gov/ct2/show/NCT00969878?term=TA-CD&draw=2&rank=1 (accessed on 1 September 2023).
- Safety Study of a Disrupted Adenovirus (Ad) Serotype Cocaine Vaccine for Cocaine-Dependent Individuals. Available online: https://classic.clinicaltrials.gov/ct2/show/NCT02455479 (accessed on 31 January 2023).
- Sabato, B.; Augusto, P.; Lima Gonçalves Pereira, R.; Coutinho Batista Esteves, F.; Caligiorne, S.; Rodrigues Dias Assis, B.; Apolo Correia Marcelino, S.; Pires do Espírito Santo, L.; Dias Dos Reis, K.; Da Silva Neto, L.; et al. Safety and immunogenicity of the anti-cocaine vaccine UFMG-VAC-V4N2 in a non-human primate model. Vaccine 2023, 41, 2127–2136. [Google Scholar] [CrossRef]
- Miller, M.; Moreno, A.; Aarde, S.; Creehan, K.; Vandewater, S.; Vaillancourt, B.; Wright, M.; Janda, K.; Taffe, M. A methamphetamine vaccine attenuates methamphetamine-induced disruptions in thermoregulation and activity in rats. Biol. Psychiatry 2013, 73, 721–728. [Google Scholar] [CrossRef]
- Rüedi-Bettschen, D.; Wood, S.; Gunnell, M.; West, C.; Pidaparthi, R.; Carroll, F.; Blough, B.; Owens, S. Vaccination protects rats from methamphetamine-induced impairment of behavioral responding for food. Vaccine 2013, 31, 4596–4602. [Google Scholar] [CrossRef]
- Haile, C.; Kosten, T.; Shen, X.; O’Malley, P.; Winoske, K.; Kinsey, B.; Wu, Y.; Huang, Z.; Lykissa, E.; Naidu, N.; et al. Altered methamphetamine place conditioning in mice vaccinated with a succinyl-methamphetamine-tetanus-toxoid vaccine. Am. J. Addict. 2015, 24, 748–755. [Google Scholar] [CrossRef]
- Xiaoshan, T.; Junjie, Y.; Wenqing, W.; Yunong, Z.; Jiaping, L.; Shanshan, L.; Kutty Selva, N.; Kui, C. Immunotherapy for treating methamphetamine, heroin and cocaine use disorders. Drug Discov. Today 2020, 25, 610–619. [Google Scholar] [CrossRef]
- Hossain, M.; Davidson, M.; Kypreos, E.; Feehan, J.; Muir, J.; Nurgali, K.; Apostolopoulos, V. Immunotherapies for the treatment of drug addiction. Vaccines 2022, 10, 1778. [Google Scholar] [CrossRef]
- Wolfe, D.; Saucier, R. Biotechnologies and the future of opioid addiction treatments. Int. J. Drug Policy 2021, 88, 103041. [Google Scholar] [CrossRef]
- Lee, J.; Janda, K. Development of effective therapeutics for polysubstance use disorders. Curr. Opin. Chem. Biol. 2022, 66, 102105. [Google Scholar] [CrossRef] [PubMed]
- Wenthur, C.; Zhou, B.; Janda, K. Vaccine-driven pharmacodynamic dissection and mitigation of fenethylline psychoactivity. Nature 2017, 548, 476–479. [Google Scholar] [CrossRef] [PubMed]
- Davidson, M.; Mayer, M.; Habib, A.; Rashidi, N.; Filippone, R.; Fraser, S.; Prakash, M.; Sinnayah, P.; Tangalakis, K.; Mathai, M.; et al. Methamphetamine induces systemic inflammation and anxiety: The role of the gut-immune-brain axis. Int. J. Mol. Sci. 2022, 23, 11224. [Google Scholar] [CrossRef] [PubMed]
- Davidson, M.; Rashidi, N.; Nurgali, K.; Apostolopoulos, V. The role of tryptophan metabolites in neuropsychiatric disorders. Int. J. Mol. Sci. 2022, 23, 9968. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Z.; Ma, M.; Jia, Y.; Cui, Y.; Zhao, R.; Li, S.; Wenthur, C.; Li, L.; Li, G. Expedited evaluation of conformational stability-heterogeneity associations for crude polyclonal antibodies in response to conjugate vaccines. Anal. Chem. 2023, 95, 10895–10902. [Google Scholar] [CrossRef]
- Lee, J.; Janda, K. Immunopharmacotherapeutic advancements in addressing methamphetamine abuse. RSC Chem. Biol. 2021, 2, 77–93. [Google Scholar] [CrossRef]
- Hossain, M.; Hassanzadeganroudsari, M.; Nurgali, K.; Apostolopoulos, V. Vaccine development against methamphetamine drug addiction. Expert Rev. Vaccines 2020, 19, 1105–1114. [Google Scholar] [CrossRef]
- Hossain, M.; Davidson, M.; Feehan, J.; Deraos, G.; Nurgali, K.; Matsoukas, J.; Apostolopoulos, V. Development of methamphetamine conjugated vaccine through hapten design: In vitro and in vivo characterization. Vaccines 2023, 11, 340. [Google Scholar] [CrossRef]
- Kamal Hossain, M.; Davidson, M.; Feehan, J.; Deraos, G.; Nurgali, K.; Matsoukas, J.; Apostolopoulos, V. Development and characterization of a novel conjugated methamphetamine vaccine. Vaccine 2022, 40, 5882–5891. [Google Scholar] [CrossRef]
- Liao, F.; Wang, H.; Dao, Y.; Yuan, K.; Lu, J.; Shi, J.; Han, Y.; Dong, S.; Lu, L. Synthesis and biological evaluation of a lipopeptide-based methamphetamine vaccine. Chin. Chem. Lett. 2021, 32, 1575–1579. [Google Scholar] [CrossRef]
- Hamid, F.; Marker, C.; Raleigh, M.; Khaimraj, A.; Winston, S.; Pentel, P.; Pravetoni, M. Pre-clinical safety and toxicology profile of a candidate vaccine to treat oxycodone use disorder. Vaccine 2022, 40, 3244–3252. [Google Scholar] [CrossRef] [PubMed]
- Rawson, R.; Erath, T.; Clark, H. The fourth wave of the overdose crisis: Examining the prominent role of psychomotor stimulants with and without fentanyl. Prev. Med. 2023, 176, 107625. [Google Scholar] [CrossRef] [PubMed]
- Hu, T.; McCormack, D.; Juurlink, D.; Campbell, T.; Bayoumi, A.; Leece, P.; Kent, J.; Gomes, T. Initiation of opioid agonist therapy after hospital visits for opioid poisonings in Ontario. CMAJ Can. Med. Assoc. J. = J. De L’association Medicale Can. 2023, 195, E1709–E1717. [Google Scholar] [CrossRef]
- Degenhardt, L.; Clark, B.; Macpherson, G.; Leppan, O.; Nielsen, S.; Zahra, E.; Larance, B.; Kimber, J.; Martino-Burke, D.; Hickman, M.; et al. Buprenorphine versus methadone for the treatment of opioid dependence: A systematic review and meta-analysis of randomised and observational studies. Lancet Psychiatry 2023, 10, 386–402. [Google Scholar] [CrossRef]
- Volkow, N.; Blanco, C. Medications for opioid use disorders: Clinical and pharmacological considerations. J. Clin. Investig. 2020, 130, 10–13. [Google Scholar] [CrossRef]
- Fairley, M.; Humphreys, K.; Joyce, V.; Bounthavong, M.; Trafton, J.; Combs, A.; Oliva, E.; Goldhaber-Fiebert, J.; Asch, S.; Brandeau, M.; et al. Cost-effectiveness of treatments for opioid use disorder. JAMA Psychiatry 2021, 78, 767–777. [Google Scholar] [CrossRef]
- Kiluk, B.; Kleykamp, B.; Comer, S.; Griffiths, R.; Huhn, A.; Johnson, M.; Kampman, K.; Pravetoni, M.; Preston, K.; Vandrey, R.; et al. Clinical trial design challenges and opportunities for emerging treatments for opioid use disorder: A review. JAMA Psychiatry 2023, 80, 84–92. [Google Scholar] [CrossRef]
- Raleigh, M.; Peterson, S.; Laudenbach, M.; Baruffaldi, F.; Carroll, F.; Comer, S.; Navarro, H.; Langston, T.; Runyon, S.; Winston, S.; et al. Safety and efficacy of an oxycodone vaccine: Addressing some of the unique considerations posed by opioid abuse. PLoS ONE 2017, 12, e0184876. [Google Scholar] [CrossRef]
- Pravetoni, M.; Vervacke, J.; Distefano, M.; Tucker, A.; Laudenbach, M.; Pentel, P. Effect of currently approved carriers and adjuvants on the pre-clinical efficacy of a conjugate vaccine against oxycodone in mice and rats. PLoS ONE 2014, 9, e96547. [Google Scholar] [CrossRef]
- Ciccarone, D. Fentanyl in the US heroin supply: A rapidly changing risk environment. Int. J. Drug Policy 2017, 46, 107–111. [Google Scholar] [CrossRef]
- Han, Y.; Yan, W.; Zheng, Y.; Khan, M.; Yuan, K.; Lu, L. The rising crisis of illicit fentanyl use, overdose, and potential therapeutic strategies. Transl. Psychiatry 2019, 9, 282. [Google Scholar] [CrossRef] [PubMed]
- Bremer, P.; Kimishima, A.; Schlosburg, J.; Zhou, B.; Collins, K.; Janda, K. Combatting synthetic designer opioids: A conjugate vaccine ablates lethal doses of fentanyl class drugs. Angew. Chem. (Int. Ed. Engl.) 2016, 55, 3772–3775. [Google Scholar] [CrossRef] [PubMed]
- Tenney, R.; Blake, S.; Bremer, P.; Zhou, B.; Hwang, C.; Poklis, J.; Janda, K.; Banks, M. Vaccine blunts fentanyl potency in male rhesus monkeys. Neuropharmacology 2019, 158, 107730. [Google Scholar] [CrossRef] [PubMed]
- Eubanks, L.; Blake, S.; Natori, Y.; Ellis, B.; Bremer, P.; Janda, K. A highly efficacious carfentanil vaccine that blunts opioid-induced antinociception and respiratory depression. ACS Chem. Biol. 2021, 16, 277–282. [Google Scholar] [CrossRef]
- Crouse, B.; Miller, S.; Muelken, P.; Hicks, L.; Vigliaturo, J.; Marker, C.; Guedes, A.; Pentel, P.; Evans, J.; LeSage, M.; et al. A TLR7/8 agonist increases efficacy of anti-fentanyl vaccines in rodent and porcine models. NPJ Vaccines 2023, 8, 107. [Google Scholar] [CrossRef]
- Miller, S.; Crouse, B.; Hicks, L.; Amin, H.; Cole, S.; Bazin, H.; Burkhart, D.; Pravetoni, M.; Evans, J. A lipidated TLR7/8 adjuvant enhances the efficacy of a vaccine against fentanyl in mice. NPJ Vaccines 2023, 8, 97. [Google Scholar] [CrossRef]
- Krendl, A.; Perry, B. Stigma toward substance dependence: Causes, consequences, and potential interventions. Psychol. Sci. Public Interest A J. Am. Psychol. Soc. 2023, 24, 90–126. [Google Scholar] [CrossRef]
- Bonese, K.; Wainer, B.; Fitch, F.; Rothberg, R.; Schuster, C. Changes in heroin self-administration by a rhesus monkey after morphine immunisation. Nature 1974, 252, 708–710. [Google Scholar] [CrossRef]
- Raleigh, M.; Laudenbach, M.; Baruffaldi, F.; Peterson, S.; Roslawski, M.; Birnbaum, A.; Carroll, F.; Runyon, S.; Winston, S.; Pentel, P.; et al. Opioid dose- and route-dependent efficacy of oxycodone and heroin vaccines in rats. J. Pharmacol. Exp. Ther. 2018, 365, 346–353. [Google Scholar] [CrossRef]
- Raleigh, M.; Pentel, P.; LeSage, M. Pharmacokinetic correlates of the effects of a heroin vaccine on heroin self-administration in rats. PLoS ONE 2014, 9, e115696. [Google Scholar] [CrossRef]
- Stowe, G.; Vendruscolo, L.; Edwards, S.; Schlosburg, J.; Misra, K.; Schulteis, G.; Mayorov, A.; Zakhari, J.; Koob, G.; Janda, K. A vaccine strategy that induces protective immunity against heroin. J. Med. Chem. 2011, 54, 5195–5204. [Google Scholar] [CrossRef] [PubMed]
- Bremer, P.; Schlosburg, J.; Banks, M.; Steele, F.; Zhou, B.; Poklis, J.; Janda, K. Development of a clinically viable heroin vaccine. J. Am. Chem. Soc. 2017, 139, 8601–8611. [Google Scholar] [CrossRef]
- Li, Q.; Luo, Y.; Sun, C.; Xue, Y.; Zhu, W.; Shi, H.; Zhai, H.; Shi, J.; Lu, L. A morphine/heroin vaccine with new hapten design attenuates behavioral effects in rats. J. Neurochem. 2011, 119, 1271–1281. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Sun, C.; Luo, Y.; Xue, Y.; Meng, S.; Xu, L.; Chen, N.; Deng, J.; Zhai, H.; Kosten, T.; et al. A conjugate vaccine attenuates morphine- and heroin-induced behavior in rats. Int. J. Neuropsychopharmacol. 2014, 18, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Belz, T.; Bremer, P.; Zhou, B.; Ellis, B.; Eubanks, L.; Janda, K. Enhancement of a heroin vaccine through hapten deuteration. J. Am. Chem. Soc. 2020, 142, 13294–13298. [Google Scholar] [CrossRef] [PubMed]
- Pravetoni, M.; Raleigh, M.; Le Naour, M.; Tucker, A.; Harmon, T.; Jones, J.; Birnbaum, A.; Portoghese, P.; Pentel, P. Co-administration of morphine and oxycodone vaccines reduces the distribution of 6-monoacetylmorphine and oxycodone to brain in rats. Vaccine 2012, 30, 4617–4624. [Google Scholar] [CrossRef] [PubMed]
- Houston, T.; Chen, J.; Amante, D.; Blok, A.; Nagawa, C.; Wijesundara, J.; Kamberi, A.; Allison, J.; Person, S.; Flahive, J.; et al. Effect of technology-assisted brief abstinence game on long-term smoking cessation in individuals not yet ready to quit: A randomized clinical trial. JAMA Intern. Med. 2022, 182, 303–312. [Google Scholar] [CrossRef]
- Keyler, D.; Hieda, Y.; St Peter, J.; Pentel, P. Altered disposition of repeated nicotine doses in rats immunized against nicotine. Nicotine Tob. Res. Off. J. Soc. Res. Nicotine Tob. 1999, 1, 241–249. [Google Scholar] [CrossRef]
- Hatsukami, D.; Jorenby, D.; Gonzales, D.; Rigotti, N.; Glover, E.; Oncken, C.; Tashkin, D.; Reus, V.; Akhavain, R.; Fahim, R.; et al. Immunogenicity and smoking-cessation outcomes for a novel nicotine immunotherapeutic. Clin. Pharmacol. Ther. 2011, 89, 392–399. [Google Scholar] [CrossRef]
- Hartmann-Boyce, J.; Stead, L.; Cahill, K.; Lancaster, T. Efficacy of interventions to combat tobacco addiction: Cochrane update of 2012 reviews. Addiction 2013, 108, 1711–1721. [Google Scholar] [CrossRef]
- Hoogsteder, P.; Kotz, D.; van Spiegel, P.; Viechtbauer, W.; van Schayck, O. Efficacy of the nicotine vaccine 3′-AmNic-rEPA (NicVAX) co-administered with varenicline and counselling for smoking cessation: A randomized placebo-controlled trial. Addiction 2014, 109, 1252–1259. [Google Scholar] [CrossRef]
- Kallupi, M.; Xue, S.; Zhou, B.; Janda, K.; George, O. An enzymatic approach reverses nicotine dependence, decreases compulsive-like intake, and prevents relapse. Sci. Adv. 2018, 4, eaat4751. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Hu, Y.; Harmon, T.; Pentel, P.; Ehrich, M.; Zhang, C. Rationalization of a nanoparticle-based nicotine nanovaccine as an effective next-generation nicotine vaccine: A focus on hapten localization. Biomaterials 2017, 138, 46–56. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Powers, K.; Hu, Y.; Raleigh, M.; Pentel, P.; Zhang, C. Engineering of a hybrid nanoparticle-based nicotine nanovaccine as a next-generation immunotherapeutic strategy against nicotine addiction: A focus on hapten density. Biomaterials 2017, 123, 107–117. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Harris, B.; Hu, Y.; Harmon, T.; Pentel, P.; Ehrich, M.; Zhang, C. Rational incorporation of molecular adjuvants into a hybrid nanoparticle-based nicotine vaccine for immunotherapy against nicotine addiction. Biomaterials 2018, 155, 165–175. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Zhao, Z.; Harmon, T.; Pentel, P.; Ehrich, M.; Zhang, C. Paradox of PEGylation in fabricating hybrid nanoparticle-based nicotine vaccines. Biomaterials 2018, 182, 72–81. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Zhao, Z.; Ehrich, M.; Zhang, C. Formulation of nanovaccines toward an extended immunity against nicotine. ACS Appl. Mater. Interfaces 2021, 13, 27972–27982. [Google Scholar] [CrossRef]
- Alzhrani, R.; Xu, H.; Valdes, S.; Cui, Z. Intranasal delivery of a nicotine vaccine candidate induces antibodies in mouse blood and lung mucosal secretions that specifically neutralize nicotine. Drug Dev. Ind. Pharm. 2020, 46, 1656–1664. [Google Scholar] [CrossRef]
- Scendoni, R.; Bury, E.; Ribeiro, I.; Cameriere, R.; Cingolani, M. Vaccines as a preventive tool for substance use disorder: A systematic review including a meta-analysis on nicotine vaccines’ immunogenicity. Hum. Vaccines Immunother. 2022, 18, 2140552. [Google Scholar] [CrossRef]
- Fraleigh, N.; Lewicky, J.; Martel, A.; Diaz-Mitoma, F.; Le, H. Assessing neutralized nicotine distribution using mice vaccinated with the mucosal conjugate nicotine vaccine. Vaccines 2021, 9, 118. [Google Scholar] [CrossRef]
- Fahim, R.; Kessler, P.; Kalnik, M. Therapeutic vaccines against tobacco addiction. Expert Rev. Vaccines 2013, 12, 333–342. [Google Scholar] [CrossRef]
- Bagasra, O.; Forman, L.; Howeedy, A.; Whittle, P. A potential vaccine for cocaine abuse prophylaxis. Immunopharmacology 1992, 23, 173–179. [Google Scholar] [CrossRef]
- Koetzner, L.; Deng, S.; Sumpter, T.; Weisslitz, M.; Abner, R.; Landry, D.; Woods, J. Titer-dependent antagonism of cocaine following active immunization in rhesus monkeys. J. Pharmacol. Exp. Ther. 2001, 296, 789–796. [Google Scholar]
- St John, A.; Choi, H.; Walker, Q.; Blough, B.; Kuhn, C.; Abraham, S.; Staats, H. Novel mucosal adjuvant, mastoparan-7, improves cocaine vaccine efficacy. NPJ Vaccines 2020, 5, 12. [Google Scholar] [CrossRef] [PubMed]
- Kimishima, A.; Olson, M.; Natori, Y.; Janda, K. In vivo efficient syntheses of cocaine vaccines and their evaluation. ACS Med. Chem. Lett. 2018, 9, 411–416. [Google Scholar] [CrossRef] [PubMed]
- Carrera, M.; Ashley, J.; Zhou, B.; Wirsching, P.; Koob, G.; Janda, K. Cocaine vaccines: Antibody protection against relapse in a rat model. Proc. Natl. Acad. Sci. USA 2000, 97, 6202–6206. [Google Scholar] [CrossRef] [PubMed]
- Cai, X.; Tsuchikama, K.; Janda, K. Modulating cocaine vaccine potency through hapten fluorination. J. Am. Chem. Soc. 2013, 135, 2971–2974. [Google Scholar] [CrossRef]
- Wee, S.; Hicks, M.; De, B.; Rosenberg, J.; Moreno, A.; Kaminsky, S.; Janda, K.; Crystal, R.; Koob, G. Novel cocaine vaccine linked to a disrupted adenovirus gene transfer vector blocks cocaine psychostimulant and reinforcing effects. Neuropsychopharmacol. Off. Publ. Am. Coll. Neuropsychopharmacol. 2012, 37, 1083–1091. [Google Scholar] [CrossRef]
- Hicks, M.; Kaminsky, S.; De, B.; Rosenberg, J.; Evans, S.; Foltin, R.; Andrenyak, D.; Moody, D.; Koob, G.; Janda, K.; et al. Fate of systemically administered cocaine in nonhuman primates treated with the dAd5GNE anticocaine vaccine. Hum. Gene Ther. Clin. Dev. 2014, 25, 40–49. [Google Scholar] [CrossRef]
- Maoz, A.; Hicks, M.; Vallabhjosula, S.; Synan, M.; Kothari, P.; Dyke, J.; Ballon, D.; Kaminsky, S.; De, B.; Rosenberg, J.; et al. Adenovirus capsid-based anti-cocaine vaccine prevents cocaine from binding to the nonhuman primate CNS dopamine transporter. Neuropsychopharmacol. Off. Publ. Am. Coll. Neuropsychopharmacol. 2013, 38, 2170–2178. [Google Scholar] [CrossRef]
- Havlicek, D.; Rosenberg, J.; De, B.; Hicks, M.; Sondhi, D.; Kaminsky, S.; Crystal, R. Cocaine vaccine dAd5GNE protects against moderate daily and high-dose “binge” cocaine use. PLoS ONE 2020, 15, e0239780. [Google Scholar] [CrossRef] [PubMed]
- da Silva Neto, L.; da Silva Maia, A.; Godin, A.; de Almeida Augusto, P.; Pereira, R.; Caligiorne, S.; Alves, R.; Fernandes, S.; Cardoso, V.; Goulart, G.; et al. nCalix[]arene-based immunogens: A new non-proteic strategy for anti-cocaine vaccine. J. Adv. Res. 2022, 38, 285–298. [Google Scholar] [CrossRef]
- Moreno, A.; Mayorov, A.; Janda, K. Impact of distinct chemical structures for the development of a methamphetamine vaccine. J. Am. Chem. Soc. 2011, 133, 6587–6595. [Google Scholar] [CrossRef]
- Shen, X.; Kosten, T.; Lopez, A.; Kinsey, B.; Kosten, T.; Orson, F. A vaccine against methamphetamine attenuates its behavioral effects in mice. Drug Alcohol Depend. 2013, 129, 41–48. [Google Scholar] [CrossRef] [PubMed]
- Haile, C.; Varner, K.; Huijing, X.; Arora, R.; Orson, F.; Kosten, T.; Kosten, T. Active and passive immunization with an anti-methamphetamine vaccine attenuates the behavioral and cardiovascular effects of methamphetamine. Vaccines 2022, 10, 1508. [Google Scholar] [CrossRef] [PubMed]
- Shen, X.; Orson, F.; Kosten, T. Vaccines against drug abuse. Clin. Pharmacol. Ther. 2012, 91, 60–70. [Google Scholar] [CrossRef]
- Orson, F.; Wang, R.; Brimijoin, S.; Kinsey, B.; Singh, R.; Ramakrishnan, M.; Wang, H.; Kosten, T. The future potential for cocaine vaccines. Expert Opin. Biol. Ther. 2014, 14, 1271–1283. [Google Scholar] [CrossRef]
- Orson, F.; Rossen, R.; Shen, X.; Lopez, A.; Wu, Y.; Kosten, T. Spontaneous development of IgM anti-cocaine antibodies in habitual cocaine users: Effect on IgG antibody responses to a cocaine cholera toxin B conjugate vaccine. Am. J. Addict. 2013, 22, 169–174. [Google Scholar] [CrossRef]
- Xu, A.; Kosten, T. Current status of immunotherapies for addiction. Ann. New York Acad. Sci. 2021, 1489, 3–16. [Google Scholar] [CrossRef]
- Gooyit, M.; Miranda, P.; Wenthur, C.; Ducime, A.; Janda, K. Influencing antibody-mediated attenuation of methamphetamine cns distribution through vaccine linker design. ACS Chem. Neurosci. 2017, 8, 468–472. [Google Scholar] [CrossRef]
- Cornish, K.; de Villiers, S.; Pravetoni, M.; Pentel, P. Immunogenicity of individual vaccine components in a bivalent nicotine vaccine differ according to vaccine formulation and administration conditions. PLoS ONE 2013, 8, e82557. [Google Scholar] [CrossRef] [PubMed]
- Lin, M.; Marin, A.; Ellis, B.; Eubanks, L.; Andrianov, A.; Janda, K. Polyphosphazene: A new adjuvant platform for cocaine vaccine development. Mol. Pharm. 2022, 19, 3358–3366. [Google Scholar] [CrossRef] [PubMed]
| Drug | Clinical Trials | Animal Studies |
|---|---|---|
| Oxycodone/hydrocodone | Oxy(Gly)4-sKLH [11] | 6OXY(Gly)4–KLH [12] OXY-dKLH [12] OXY-TT [13] Hydro-TT [13] |
| Fentanyl | fentanyl-TT [14] FEN-CRM + dmLT [15] | |
| Heroin/morphine | M-TT [16] M-6-S-BSA [17] M-KLH [18] KLH-6-SM [19] | |
| Nicotine | NicVAX [20] Niccine [21] Nic-Qb (NIC002) [22] | NanoNicVac [23] FH VLPs [24] |
| Cocaine | TA-CD [25,26] dAd5GNE [27] | UFMG-VAC-V4N2 [28] |
| Methamphetamine | MH6-KLH [29] SMO9-KLH [30] SMA-TT [31] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lu, T.; Li, X.; Zheng, W.; Kuang, C.; Wu, B.; Liu, X.; Xue, Y.; Shi, J.; Lu, L.; Han, Y. Vaccines to Treat Substance Use Disorders: Current Status and Future Directions. Pharmaceutics 2024, 16, 84. https://doi.org/10.3390/pharmaceutics16010084
Lu T, Li X, Zheng W, Kuang C, Wu B, Liu X, Xue Y, Shi J, Lu L, Han Y. Vaccines to Treat Substance Use Disorders: Current Status and Future Directions. Pharmaceutics. 2024; 16(1):84. https://doi.org/10.3390/pharmaceutics16010084
Chicago/Turabian StyleLu, Tangsheng, Xue Li, Wei Zheng, Chenyan Kuang, Bingyi Wu, Xiaoxing Liu, Yanxue Xue, Jie Shi, Lin Lu, and Ying Han. 2024. "Vaccines to Treat Substance Use Disorders: Current Status and Future Directions" Pharmaceutics 16, no. 1: 84. https://doi.org/10.3390/pharmaceutics16010084





