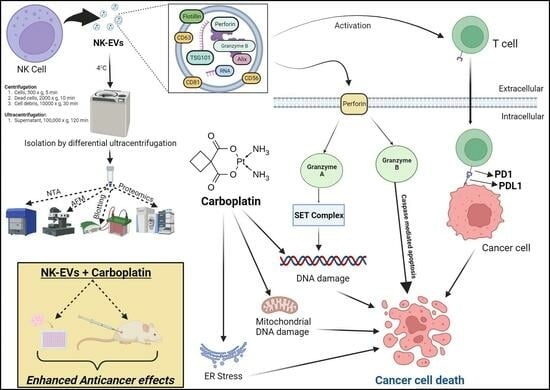
Combined Role of Interleukin-15 Stimulated Natural Killer Cell-Derived Extracellular Vesicles and Carboplatin in Osimertinib-Resistant H1975 Lung Cancer Cells with EGFR Mutations
Abstract
:1. Introduction
2. Materials
3. Methods
3.1. NK92 Cell Culture in 500 mL PBS-Vertical Wheel (VW) Bioreactor
3.2. NK-EV Isolation and Purification
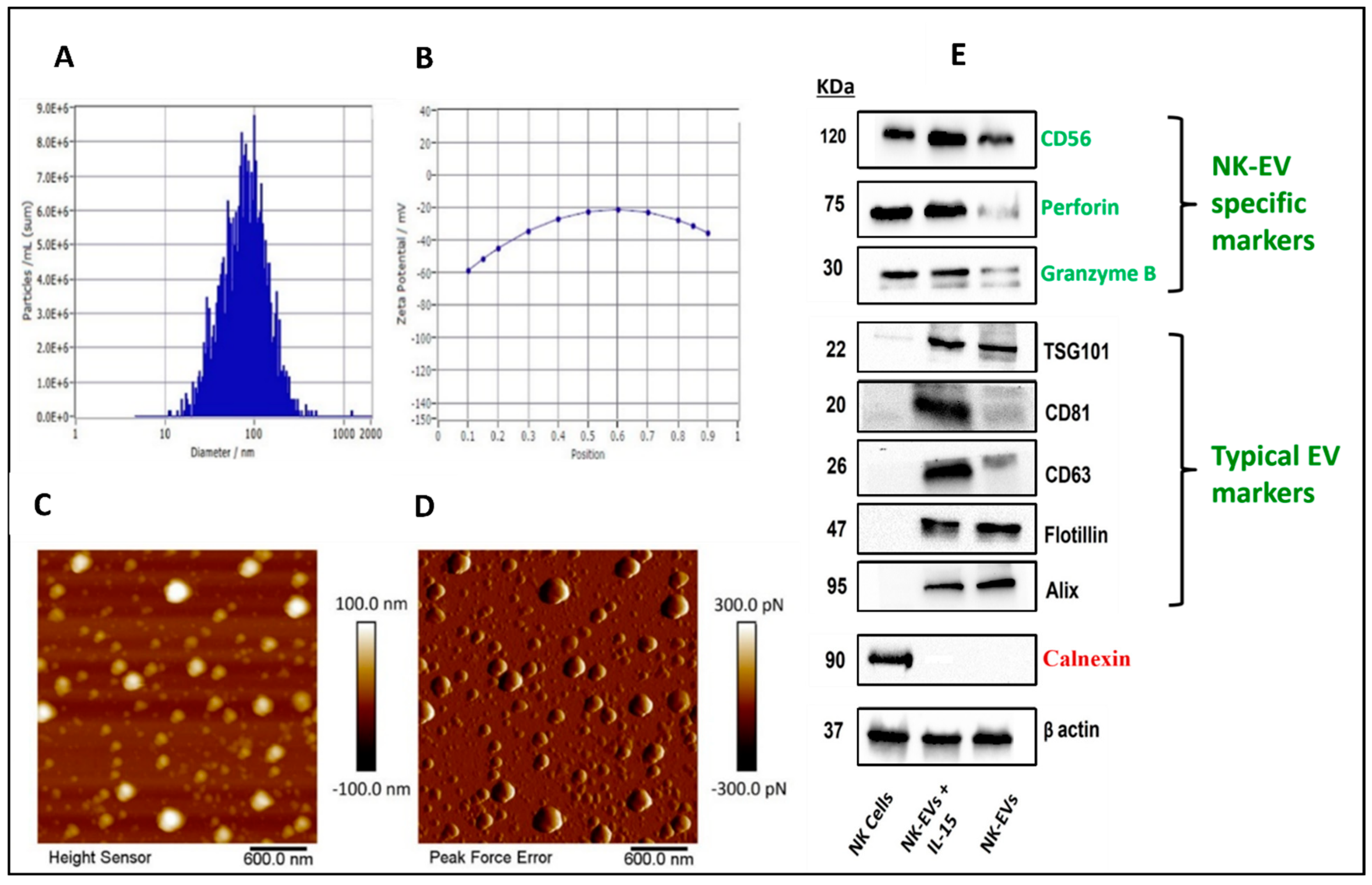
3.3. Characterization of NK-EVs
3.3.1. Particle Size, Zeta Potential, and Concentration
3.3.2. Atomic Force Microscopy (AFM)
3.3.3. Western Blotting of NK-EV Markers
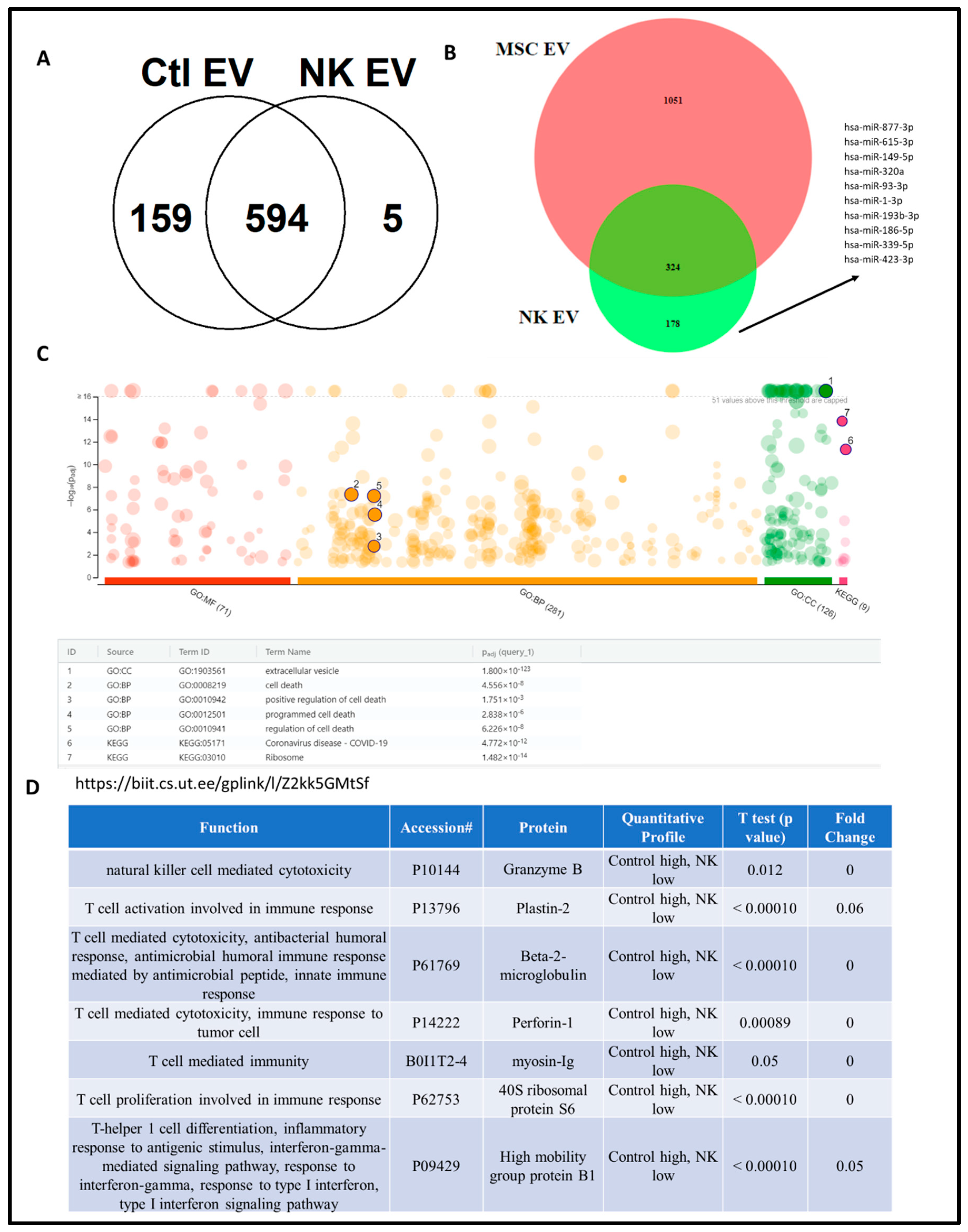
3.3.4. Proteomic Analysis of NK-EVs Followed by Gene Ontology Profiling
3.4. Cell Viability Studies
3.4.1. Two-Dimensional Cytotoxicity Assay
3.4.2. Magnetic Nanoshuttle 3D Cytotoxicity Assay
3.4.3. Dual Acridine Orange/Ethidium Bromide (AO/EB) Fluorescent Staining
3.4.4. Scratch Migration Assay
3.5. Cell Cycle Analysis by Flow Cytometry
3.6. In Vivo Tumor Studies
3.7. Immunoblotting
3.8. Statistical Analysis
4. Results
4.1. NK Cell Growth and Expansion in PBS-Vertical Wheel Bioreactor
4.2. Isolation and Characterization of NK-EVs
4.3. Proteomics Analysis of NK-EV Protein Cargo
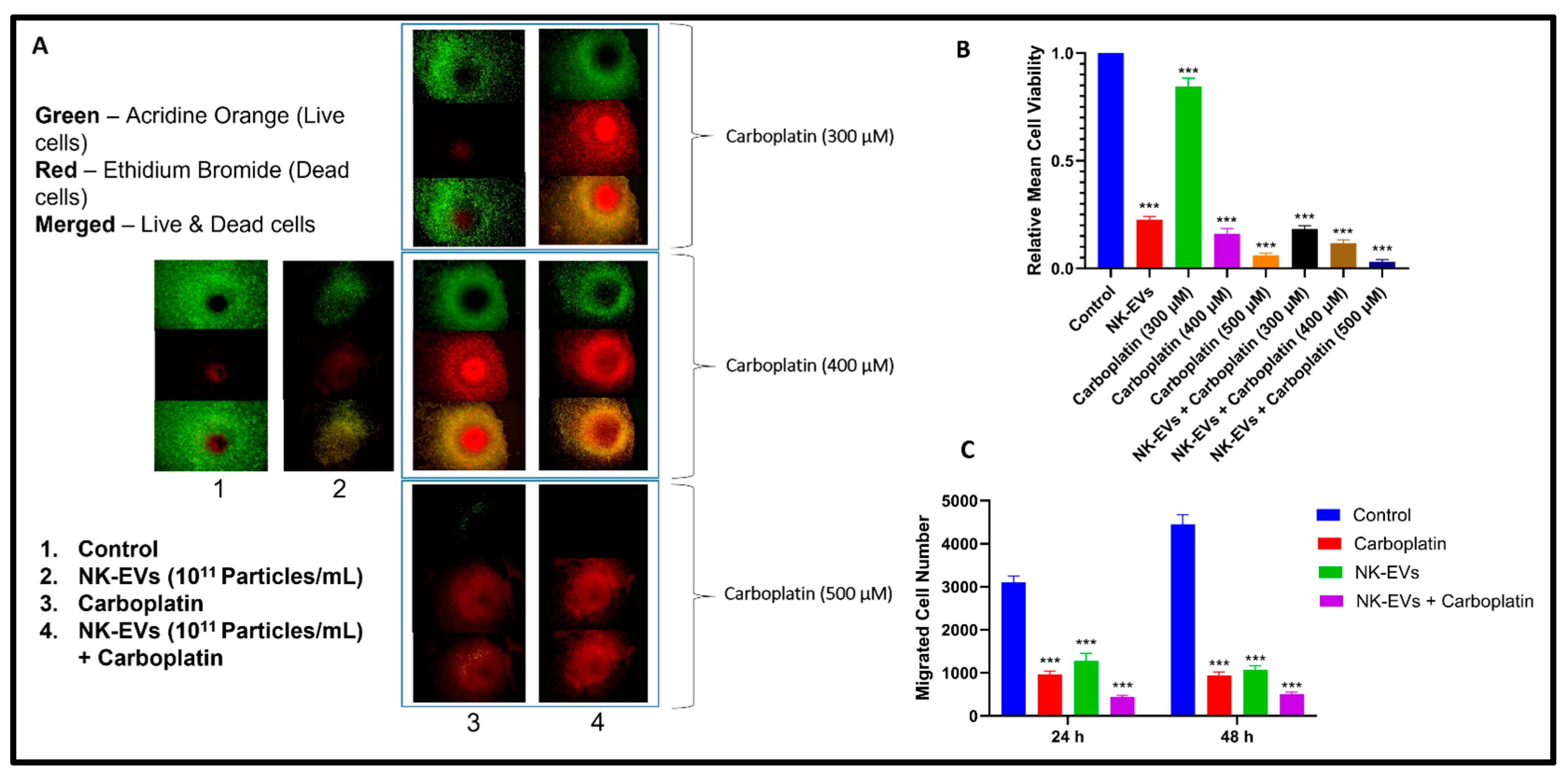
4.4. Effect of NK-EVs on Cell Viability and Migration
4.5. Cell Cycle Analysis
4.6. Effect of NK-EVs, CBP, and NK-EVs + CBP in OSM-Resistant H1975 Lung Cancer Tumor Model
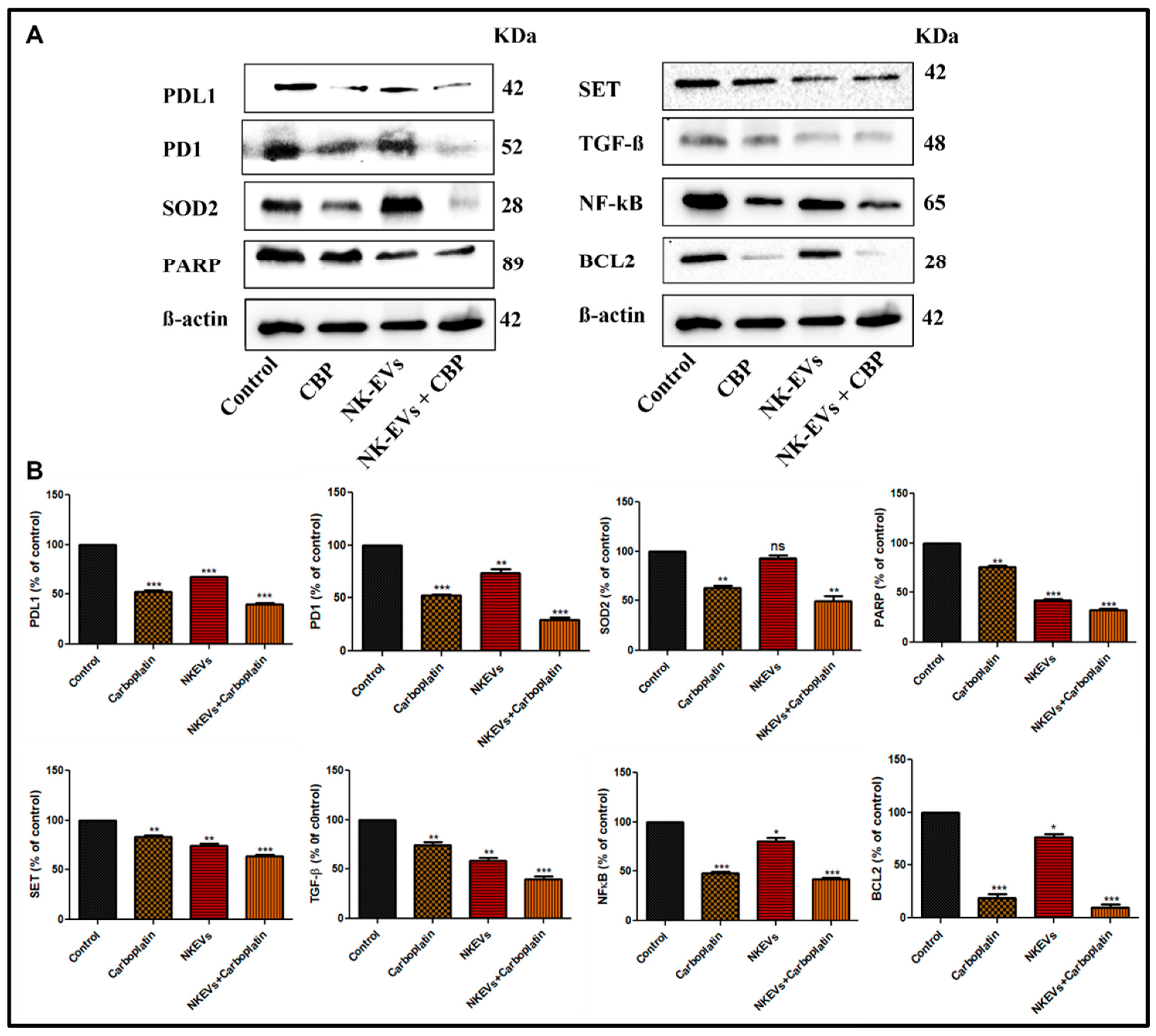
4.7. Effect of NK-EVs, CBP, and NK-EVs + CBP on Apoptotic and Inflammation Markers in OSM-Resistant H1975 Lung Cancer Tumor Model
5. Discussion
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Correction Statement
References
- Kooti, W.; Servatyari, K.; Behzadifar, M.; Asadi-Samani, M.; Sadeghi, F.; Nouri, B.; Marzouni, H.Z. Effective Medicinal Plant in Cancer Treatment, Part 2: Review Study. J. Evid.-Based Complement. Altern. Med. 2017, 22, 982–995. [Google Scholar] [CrossRef] [PubMed]
- Hirsch, F.R.; Scagliotti, G.V.; Mulshine, J.L.; Kwon, R.; Curran, W.J., Jr.; Wu, Y.-L.; Paz-Ares, L. Lung cancer: Current therapies and new targeted treatments. Lancet 2017, 389, 299–311. [Google Scholar] [CrossRef] [PubMed]
- Nurgali, K.; Jagoe, R.T.; Abalo, R. Editorial: Adverse Effects of Cancer Chemotherapy: Anything New to Improve Tolerance and Reduce Sequelae? Front. Pharmacol. 2018, 9, 245. [Google Scholar] [CrossRef]
- Schirrmacher, V. From chemotherapy to biological therapy: A review of novel concepts to reduce the side effects of systemic cancer treatment (Review). Int. J. Oncol. 2019, 54, 407–419. [Google Scholar] [CrossRef]
- Whiteside, T.L.; Demaria, S.; Rodriguez-Ruiz, M.E.; Zarour, H.M.; Melero, I. Emerging Opportunities and Challenges in Cancer Immunotherapy. Clin. Cancer Res. 2016, 22, 1845–1855. [Google Scholar] [CrossRef] [PubMed]
- Farkona, S.; Diamandis, E.P.; Blasutig, I.M. Cancer immunotherapy: The beginning of the end of cancer? BMC Med. 2016, 14, 73. [Google Scholar] [CrossRef]
- Vahedi, F.; Nham, T.; Poznanski, S.M.; Chew, M.V.; Shenouda, M.M.; Lee, D.; Ashkar, A.A. Ex Vivo Expanded Human NK Cells Survive and Proliferate in Humanized Mice with Autologous Human Immune Cells. Sci. Rep. 2017, 7, 12083. [Google Scholar] [CrossRef]
- Carrega, P.; Ferlazzo, G. Natural killer cell distribution and trafficking in human tissues. Front. Immunol. 2012, 3, 347. [Google Scholar] [CrossRef]
- Paul, S.; Lal, G. The Molecular Mechanism of Natural Killer Cells Function and Its Importance in Cancer Immunotherapy. Front. Immunol. 2017, 8, 1124. [Google Scholar] [CrossRef]
- Sonar, S.; Lal, G. Role of tumor necrosis factor superfamily in neuroinflammation and autoimmunity. Front. Immunol. 2015, 6, 364. [Google Scholar] [CrossRef]
- Choi, J.-W.; Lim, S.; Kang, J.H.; Hwang, S.H.; Hwang, K.-C.; Kim, S.W.; Lee, S. Proteome analysis of human natural killer cell derived extracellular vesicles for identification of anticancer effectors. Molecules 2020, 25, 5216. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Ruiz, Y.; Valitutti, S.; Dupre, L. Stepwise Maturation of Lytic Granules during Differentiation and Activation of Human CD8+ T Lymphocytes. PLoS ONE 2011, 6, e27057. [Google Scholar] [CrossRef]
- Robbins, P.D.; Morelli, A.E. Regulation of immune responses by extracellular vesicles. Nat. Rev. Immunol. 2014, 14, 195–208. [Google Scholar] [CrossRef]
- Hoshino, A.; Costa-Silva, B.; Shen, T.-L.; Rodrigues, G.; Hashimoto, A.; Mark, M.T.; Molina, H.; Kohsaka, S.; Di Giannatale, A.; Ceder, S.; et al. Tumour exosome integrins determine organotropic metastasis. Nature 2015, 527, 329–335. [Google Scholar] [CrossRef] [PubMed]
- Tominaga, N.; Kosaka, N.; Ono, M.; Katsuda, T.; Yoshioka, Y.; Tamura, K.; Lötvall, J.; Nakagama, H.; Ochiya, T. Brain metastatic cancer cells release microRNA-181c-containing extracellular vesicles capable of destructing blood–brain barrier. Nat. Commun. 2015, 6, 6716. [Google Scholar] [CrossRef]
- Daneshpour, H.; Youk, H. Modeling cell–cell communication for immune systems across space and time. Curr. Opin. Syst. Biol. 2019, 18, 44–52. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Jiang, H.; Wang, K.; Liu, C.; Man, X.; Fu, Q. Hypoxia enhances the production and antitumor effect of exosomes derived from natural killer cells. Ann. Transl. Med. 2021, 9, 473. [Google Scholar] [CrossRef]
- Cochran, A.M.; Kornbluth, J. Extracellular Vesicles From the Human Natural Killer Cell Line NK3.3 Have Broad and Potent Anti-Tumor Activity. Front. Cell Dev. Biol. 2021, 9, 698639. [Google Scholar] [CrossRef]
- Zhu, L.; Kalimuthu, S.; Gangadaran, P.; Oh, J.M.; Lee, H.W.; Baek, S.H.; Jeong, S.Y.; Lee, S.-W.; Lee, J.; Ahn, B.-C. Exosomes Derived From Natural Killer Cells Exert Therapeutic Effect in Melanoma. Theranostics 2017, 7, 2732–2745. [Google Scholar] [CrossRef]
- Kim, H.Y.; Min, H.-K.; Song, H.-W.; Yoo, A.; Lee, S.; Kim, K.-P.; Park, J.-O.; Choi, Y.H.; Choi, E. Delivery of human natural killer cell-derived exosomes for liver cancer therapy: An in vivo study in subcutaneous and orthotopic animal models. Drug Deliv. 2022, 29, 2897–2911. [Google Scholar] [CrossRef]
- Szefler, B.; Czeleń, P. Will the Interactions of Some Platinum (II)-Based Drugs with B-Vitamins Reduce Their Therapeutic Effect in Cancer Patients? Comparison of Chemotherapeutic Agents such as Cisplatin, Carboplatin and Oxaliplatin—A Review. Int. J. Mol. Sci. 2023, 24, 1548. [Google Scholar] [CrossRef] [PubMed]
- de Sousa, G.F.; Wlodarczyk, S.R.; Monteiro, G. Carboplatin: Molecular mechanisms of action associated with chemoresistance. Braz J. Pharm. Sci. 2014, 50, 693–701. [Google Scholar] [CrossRef]
- Ghaferi, M.; Raza, A.; Koohi, M.; Zahra, W.; Akbarzadeh, A.; Shahmabadi, H.E.; Alavi, S.E. Impact of PEGylated Liposomal Doxorubicin and Carboplatin Combination on Glioblastoma. Pharmaceutics 2022, 14, 2183. [Google Scholar] [CrossRef] [PubMed]
- Ebrahimifar, M.; Rashidi, S.; Taghvaei, F.; Rastin, S.J.; Milani, A.T.; Khameneh, Z.R.; Hamini, F.; Rasouli, M.A.; Asghari, K.; Shishavan, A.M.R. Carboplatin and epigallocatechin-3-gallate synergistically induce cytotoxic effects in esophageal cancer cells. Res. Pharm. Sci. 2021, 16, 240–249. [Google Scholar] [CrossRef] [PubMed]
- Pignata, S.; Lorusso, D.; Joly, F.; Gallo, C.; Colombo, N.; Sessa, C.; Bamias, A.; Salutari, V.; Selle, F.; Frezzini, S.; et al. Carboplatin-based doublet plus bevacizumab beyond progression versus carboplatin-based doublet alone in patients with platinum-sensitive ovarian cancer: A randomised, phase 3 trial. Lancet Oncol. 2021, 22, 267–276. [Google Scholar] [CrossRef]
- Porter, L.H.; Zhu, J.J.; Lister, N.L.; Harrison, S.G.; Keerthikumar, S.; Goode, D.L.; Urban, R.Q.; Byrne, D.J.; Azad, A.; Vela, I.; et al. Low-dose carboplatin modifies the tumor microenvironment to augment CAR T cell efficacy in human prostate cancer models. Nat. Commun. 2023, 14, 5346. [Google Scholar] [CrossRef]
- Danışman-Kalındemirtaş, F.; Kariper, A.; Erdemir, G.; Sert, E.; Erdem-Kuruca, S. Evaluation of anticancer effects of carboplatin–gelatin nanoparticles in different sizes synthesized with newly self-assembly method by exposure to IR light. Sci. Rep. 2022, 12, 10686. [Google Scholar] [CrossRef]
- Poy, D.; Ebrahimi Shahemabadi, H.; Akbarzadeh, A.; Moradi-Sardareh, H.; Ebrahimifar, M. Carboplatin liposomal nanoparticles: Preparation, characterization, and cytotoxicity effects on lung cancer in vitro environment. Int. J. Polym. Mater. Polym. Biomater. 2018, 67, 367–370. [Google Scholar] [CrossRef]
- Parrish-Novak, J.; Dillon, S.R.; Nelson, A.; Hammond, A.; Sprecher, C.; Gross, J.A.; Johnston, J.; Madden, K.; Xu, W.; West, J.; et al. Interleukin 21 and its receptor are involved in NK cell expansion and regulation of lymphocyte function. Nature 2000, 408, 57–63. [Google Scholar] [CrossRef]
- Meazza, R.; Azzarone, B.; Orengo, A.M.; Ferrini, S. Role of Common-Gamma Chain Cytokines in NK Cell Development and Function: Perspectives for Immunotherapy. BioMed Res. Int. 2011, 2011, e861920. [Google Scholar] [CrossRef]
- Enomoto, Y.; Li, P.; Jenkins, L.M.; Anastasakis, D.; Lyons, G.C.; Hafner, M.; Leonard, W.J. Cytokine-enhanced cytolytic activity of exosomes from NK Cells. Cancer Gene Ther. 2021, 29, 734–749. [Google Scholar] [CrossRef] [PubMed]
- Patel, N.; Kommineni, N.; Surapaneni, S.K.; Kalvala, A.; Yaun, X.; Gebeyehu, A.; Arthur, P.; Duke, L.C.; York, S.B.; Bagde, A.; et al. Cannabidiol loaded extracellular vesicles sensitize triple-negative breast cancer to doxorubicin in both in-vitro and in vivo models. Int. J. Pharm. 2021, 607, 120943. [Google Scholar] [CrossRef]
- Jeske, R.; Liu, C.; Duke, L.; Castro, M.L.C.; Muok, L.; Arthur, P.; Singh, M.; Jung, S.; Sun, L.; Li, Y. Upscaling human mesenchymal stromal cell production in a novel vertical-wheel bioreactor enhances extracellular vesicle secretion and cargo profile. Bioact. Mater. 2023, 25, 732–747. [Google Scholar] [CrossRef]
- Hurwitz, S.N.; Nkosi, D.; Conlon, M.M.; York, S.B.; Liu, X.; Tremblay, D.C.; Meckes, D.G., Jr. CD63 Regulates Epstein-Barr Virus LMP1 Exosomal Packaging, Enhancement of Vesicle Production, and Noncanonical NF-κB Signaling. J. Virol. 2017, 91, e02251-16. [Google Scholar] [CrossRef]
- Hurwitz, S.N.; Cheerathodi, M.R.; Nkosi, D.; York, S.B.; Meckes, D.G. Tetraspanin CD63 Bridges Autophagic and Endosomal Processes To Regulate Exosomal Secretion and Intracellular Signaling of Epstein-Barr Virus LMP1. J. Virol. 2018, 92, e01969-17. [Google Scholar] [CrossRef] [PubMed]
- Mehdiani, A.; Maier, A.; Pinto, A.; Barth, M.; Akhyari, P.; Lichtenberg, A. An Innovative Method for Exosome Quantification and Size Measurement. JoVE J. Vis. Exp. 2015, 95, e50974. [Google Scholar]
- Kalvala, A.K.; Bagde, A.; Arthur, P.; Kulkarni, T.; Bhattacharya, S.; Surapaneni, S.; Patel, N.K.; Nimma, R.; Gebeyehu, A.; Kommineni, N.; et al. Cannabidiol-Loaded Extracellular Vesicles from Human Umbilical Cord Mesenchymal Stem Cells Alleviate Paclitaxel-Induced Peripheral Neuropathy. Pharmaceutics 2023, 15, 554. [Google Scholar] [CrossRef] [PubMed]
- Gulati, M.; Grover, M.; Singh, M.; Singh, S. Study of azathioprine encapsulation into liposomes. J. Microencapsul. 1998, 15, 485–494. [Google Scholar] [CrossRef]
- Hedrick, E.; Lee, S.-O.; Doddapaneni, R.; Singh, M.; Safe, S. NR4A1 Antagonists Inhibit β1-Integrin-Dependent Breast Cancer Cell Migration. Mol. Cell. Biol. 2016, 36, 1383–1394. [Google Scholar] [CrossRef]
- Gangadaran, P.; Rajendran, R.L.; Lee, H.W.; Kalimuthu, S.; Hong, C.M.; Jeong, S.Y.; Lee, S.-W.; Lee, J.; Ahn, B.-C. Extracellular vesicles from mesenchymal stem cells activates VEGF receptors and accelerates recovery of hindlimb ischemia. J. Control. Release 2017, 264, 112–126. [Google Scholar] [CrossRef]
- Hurwitz, S.N.; Meckes, D.G. An Adaptable Polyethylene Glycol-Based Workflow for Proteomic Analysis of Extracellular Vesicles. In Extracellular Vesicles: Methods and Protocols; Kuo, W.P., Jia, S., Eds.; Methods in Molecular Biology; Springer: New York, NY, USA, 2017; pp. 303–317. [Google Scholar] [CrossRef]
- Hurwitz, S.N.; Sun, L.; Cole, K.Y.; Ford, C.R.; Olcese, J.M.; Meckes, D.G. An optimized method for enrichment of whole brain-derived extracellular vesicles reveals insight into neurodegenerative processes in a mouse model of Alzheimer’s disease. J. Neurosci. Methods 2018, 307, 210–220. [Google Scholar] [CrossRef]
- Arthur, P.; Patel, N.; Surapaneni, S.K.; Mondal, A.; Gebeyehu, A.; Bagde, A.; Kutlehria, S.; Nottingham, E.; Singh, M. Targeting lung cancer stem cells using combination of Tel and Docetaxel liposomes in 3D cultures and tumor xenografts. Toxicol. Appl. Pharmacol. 2020, 401, 115112. [Google Scholar] [CrossRef]
- Baillargeon, P.; Shumate, J.; Hou, S.; Fernandez-Vega, V.; Marques, N.; Souza, G.; Seldin, J.; Spicer, T.P.; Scampavia, L. Automating a Magnetic 3D Spheroid Model Technology for High-Throughput Screening. SLAS Technol. 2019, 24, 420–428. [Google Scholar] [CrossRef] [PubMed]
- Abdullah, M.; Bela, B.; Syam, A.F.; Simadibrata, M.; Meilany, S.; Annisa, F.; Amirulloh, D.; Makmun, D.; Rani, A.A. Establishment of primary 3D cell culture based on magnetic bioprinting for colorectal cancer cells from patients in Cipto Mangunkusumo National Hospital Indonesia. AIP Conf. Proc. 2019, 2155, 020025. [Google Scholar]
- Kalvala, A.K.; Yerra, V.G.; Sherkhane, B.; Gundu, C.; Arruri, V.; Kumar, R.; Kumar, A. Chronic hyperglycemia impairs mitochondrial unfolded protein response and precipitates proteotoxicity in experimental diabetic neuropathy: Focus on LonP1 mediated mitochondrial regulation. Pharmacol. Rep. 2020, 72, 1627–1644. [Google Scholar] [CrossRef]
- Kalvala, A.K.; Nimma, R.; Bagde, A.; Surapaneni, S.K.; Patel, N.; Arthur, P.; Sun, L.; Singh, R.; Kommineni, N.; Nathani, A.; et al. The role of Cannabidiol and tetrahydrocannabivarin to overcome doxorubicin resistance in MDA-MB-231 xenografts in athymic nude mice. Biochimie 2023, 208, 19–30. [Google Scholar] [CrossRef]
- Khan, I.; Preeti, K.; Kumar, R.; Khatri, D.K.; Singh, S.B. Piceatannol promotes neuroprotection by inducing mitophagy and mitobiogenesis in the experimental diabetic peripheral neuropathy and hyperglycemia-induced neurotoxicity. Int. Immunopharmacol. 2023, 116, 109793. [Google Scholar] [CrossRef]
- Non Communicable Diseases. Available online: https://www.who.int/news-room/fact-sheets/detail/noncommunicable-diseases (accessed on 3 December 2023).
- Brianna; Lee, S.H. Chemotherapy: How to reduce its adverse effects while maintaining the potency? Med. Oncol. 2023, 40, 88. [Google Scholar] [CrossRef]
- Cheng, C.-F.; Lu, C.-W.; Wu, W.-J.; Su, L.-Y.; Nguyen, T.K.N.; Shen, S.-C.; Lien, C.-Y.; Chuang, W.-C.; Lee, M.-C.; Wu, C.-H. Therapeutic Effects of Plant Extracts of Anoectochilus roxburghii on Side Effects of Chemotherapy in BALB/c Breast Cancer Mice. Plants 2023, 12, 2494. [Google Scholar] [CrossRef]
- Frenkel, M. Refusing Treatment. Oncologist 2013, 18, 634–636. [Google Scholar] [CrossRef]
- Patel, D.B.; Santoro, M.; Born, L.J.; Fisher, J.P.; Jay, S.M. Towards rationally designed biomanufacturing of therapeutic extracellular vesicles: Impact of the bioproduction microenvironment. Biotechnol. Adv. 2018, 36, 2051–2059. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, H.; Gu, J.; Zhang, J.; Shi, H.; Qian, H.; Wang, D.; Xu, W.; Pan, J.; Santos, H.A. Engineered Extracellular Vesicles for Cancer Therapy. Adv. Mater. 2021, 33, 2005709. [Google Scholar] [CrossRef]
- Ozturk, S.S.; Jorjani, P.; Taticek, R.; Lowe, B.; Shackleford, S.; Ladehoff-Guiles, D.; Thrift, J.; Blackie, J.; Naveh, D. Kinetics of Glucose Metabolism and Utilization of Lactate in Mammalian Cell Cultures. In Animal Cell Technology: From Vaccines to Genetic Medicine; Carrondo, M.J.T., Griffiths, B., Moreira, J.L.P., Eds.; Springer: Dordrecht, The Netherlands, 1997; pp. 355–360. [Google Scholar]
- Rabinowitz, J.D.; Enerbäck, S. Lactate: The ugly duckling of energy metabolism. Nat. Metab. 2020, 2, 566–571. [Google Scholar] [CrossRef] [PubMed]
- Kalimuthu, S.; Gangadaran, P.; Li, X.J.; Oh, J.M.; Lee, H.W.; Jeong, S.Y.; Lee, S.-W.; Lee, J.; Ahn, B.-C. In Vivo therapeutic potential of mesenchymal stem cell-derived extracellular vesicles with optical imaging reporter in tumor mice model. Sci. Rep. 2016, 6, 30418. [Google Scholar] [CrossRef]
- Kim, J.; Lee, H.; Park, K.; Shin, S. Rapid and Efficient Isolation of Exosomes by Clustering and Scattering. J. Clin. Med. 2020, 9, 650. [Google Scholar] [CrossRef]
- Luo, H.; Zhou, Y.; Zhang, J.; Zhang, Y.; Long, S.; Lin, X.; Yang, A.; Duan, J.; Yang, N.; Yang, Z.; et al. NK cell-derived exosomes enhance the anti-tumor effects against ovarian cancer by delivering cisplatin and reactivating NK cell functions. Front. Immunol. 2023, 13, 1087689. [Google Scholar] [CrossRef]
- Pietrangelo, T.; Di Filippo, E.S.; Locatelli, M.; Piacenza, F.; Farina, M.; Pavoni, E.; Di Donato, A.; Innosa, D.; Provinciali, M.; Fulle, S. Extracellular Guanosine 5′-Triphosphate Induces Human Muscle Satellite Cells to Release Exosomes Stuffed With Guanosine. Front. Pharmacol. 2018, 9, 152. [Google Scholar] [CrossRef]
- Chopra, N.; Arya, B.D.; Jain, N.; Yadav, P.; Wajid, S.; Singh, S.P.; Choudhury, S. Biophysical Characterization and Drug Delivery Potential of Exosomes from Human Wharton’s Jelly-Derived Mesenchymal Stem Cells. ACS Omega 2019, 4, 13143–13152. [Google Scholar] [CrossRef]
- Woo, J.; Sharma, S.; Gimzewski, J. The Role of Isolation Methods on a Nanoscale Surface Structure and its Effect on the Size of Exosomes. J. Circ. Biomark. 2016, 5, 11. [Google Scholar] [CrossRef]
- Li, P.; Kaslan, M.; Lee, S.H.; Yao, J.; Gao, Z. Progress in Exosome Isolation Techniques. Theranostics 2017, 7, 789–804. [Google Scholar] [CrossRef]
- Qiu, Y.; Su, M.; Liu, L.; Tang, Y.; Pan, Y.; Sun, J. Clinical Application of Cytokines in Cancer Immunotherapy. Drug Des. Dev. Ther. 2021, 15, 2269–2287. [Google Scholar] [CrossRef]
- Chulpanova, D.S.; Gilazieva, Z.E.; Kletukhina, S.K.; Aimaletdinov, A.M.; Garanina, E.E.; James, V.; Rizvanov, A.A.; Solovyeva, V.V. Cytochalasin B-Induced Membrane Vesicles from Human Mesenchymal Stem Cells Overexpressing IL2 Are Able to Stimulate CD8+ T-Killers to Kill Human Triple Negative Breast Cancer Cells. Biology 2021, 10, 141. [Google Scholar] [CrossRef]
- Kim, I.-Y.; Kim, H.Y.; Song, H.-W.; Park, J.-O.; Choi, Y.H.; Choi, E. Functional enhancement of exosomes derived from NK cells by IL-15 and IL-21 synergy against hepatocellular carcinoma cells: The cytotoxicity and apoptosis in vitro study. Heliyon 2023, 9, e16962. [Google Scholar] [CrossRef]
- Zhu, L.; Kalimuthu, S.; Oh, J.M.; Gangadaran, P.; Baek, S.H.; Jeong, S.Y.; Lee, S.-W.; Lee, J.; Ahn, B.-C. Enhancement of antitumor potency of extracellular vesicles derived from natural killer cells by IL-15 priming. Biomaterials 2019, 190–191, 38–50. [Google Scholar] [CrossRef]
- Patel, K.; Doddapaneni, R.; Chowdhury, N.; Boakye, C.H.; Behl, G.; Singh, M. Tumor stromal disrupting agent enhances the anticancer efficacy of docetaxel loaded PEGylated liposomes in lung cancer. Nanomedicine 2016, 11, 1377–1392. [Google Scholar] [CrossRef]
- Doddapaneni, R.; Patel, K.; Owaid, I.H.; Singh, M. Tumor neovasculature-targeted cationic PEGylated liposomes of gambogic acid for the treatment of triple-negative breast cancer. Drug Deliv. 2015, 23, 1232–1241. [Google Scholar] [CrossRef]
- Godugu, C.; Doddapaneni, R.; Singh, M. Honokiol nanomicellar formulation produced increased oral bioavailability and anticancer effects in triple negative breast cancer (TNBC). Colloids Surf. B Biointerfaces 2017, 153, 208–219. [Google Scholar] [CrossRef]
- Ferdous, A.J.; Stembridge, N.Y.; Singh, M. Role of monensin PLGA polymer nanoparticles and liposomes as potentiator of ricin A immunotoxins in vitro. J. Control. Release 1998, 50, 71–78. [Google Scholar] [CrossRef]
- Singh, M.; Ghose, T.; Faulkner, G.; Kralovec, J.; Mezei, M. Targeting of methotrexate-containing liposomes with a monoclonal antibody against human renal cancer. Cancer Res. 1989, 49, 3976–3984. [Google Scholar]
- Haynes, A.; Shaik, M.S.; Chatterjee, A.; Singh, M. Evaluation of an Aerosolized Selective COX-2 Inhibitor as a Potentiator of Doxorubicin in a Non-Small-Cell Lung Cancer Cell Line. Pharm. Res. 2003, 20, 1485–1495. [Google Scholar] [CrossRef]
- Zhu, L.; Oh, J.M.; Gangadaran, P.; Kalimuthu, S.; Baek, S.H.; Jeong, S.Y.; Lee, S.-W.; Lee, J.; Ahn, B.-C. Targeting and Therapy of Glioblastoma in a Mouse Model Using Exosomes Derived From Natural Killer Cells. Front. Immunol. 2018, 9, 824. [Google Scholar] [CrossRef]
- Munagala, R.; Aqil, F.; Jeyabalan, J.; Gupta, R.C. Bovine milk-derived exosomes for drug delivery. Cancer Lett. 2016, 371, 48–61. [Google Scholar] [CrossRef]
- Lai, R.C.; Yeo, R.W.Y.; Tan, K.H.; Lim, S.K. Exosomes for drug delivery—A novel application for the mesenchymal stem cell. Biotechnol. Adv. 2013, 31, 543–551. [Google Scholar] [CrossRef]
- Kaur, S.; Nathani, A.; Singh, M. Exosomal delivery of cannabinoids against cancer. Cancer Lett. 2023, 566, 216243. [Google Scholar] [CrossRef]
- Oh, E.J.; Lee, H.W.; Kalimuthu, S.; Kim, T.J.; Kim, H.M.; Baek, S.H.; Zhu, L.; Oh, J.M.; Son, S.H.; Chung, H.Y.; et al. In vivo migration of mesenchymal stem cells to burn injury sites and their therapeutic effects in a living mouse model. J. Control. Release 2018, 279, 79–88. [Google Scholar] [CrossRef]
- Lee, S.J.; Kang, W.Y.; Yoon, Y.; Jin, J.Y.; Song, H.J.; Her, J.H.; Kang, S.M.; Hwang, Y.K.; Kang, K.J.; Joo, K.M.; et al. Natural killer (NK) cells inhibit systemic metastasis of glioblastoma cells and have therapeutic effects against glioblastomas in the brain. BMC Cancer 2015, 15, 1–13. [Google Scholar] [CrossRef]
- Parolini, I.; Federici, C.; Raggi, C.; Lugini, L.; Palleschi, S.; De Milito, A.; Coscia, C.; Iessi, E.; Logozzi, M.; Molinari, A.; et al. Microenvironmental pH Is a Key Factor for Exosome Traffic in Tumor Cells. J. Biol. Chem. 2009, 284, 34211–34222. [Google Scholar] [CrossRef]
- Korenevskii, A.V.; Milyutina, Y.P.; Zhdanova, A.A.; Pyatygina, K.M.; Sokolov, D.I.; Sel’kov, S.A. Mass-Spectrometric Analysis of Proteome of Microvesicles Produced by NK-92 Natural Killer Cells. Bull. Exp. Biol. Med. 2018, 165, 564–571. [Google Scholar] [CrossRef]
- Federici, C.; Shahaj, E.; Cecchetti, S.; Camerini, S.; Casella, M.; Iessi, E.; Camisaschi, C.; Paolino, G.; Calvieri, S.; Ferro, S.; et al. Natural-Killer-Derived Extracellular Vesicles: Immune Sensors and Interactors. Front. Immunol. 2020, 11, 262. [Google Scholar] [CrossRef]
- Trapani, J.A.; Smyth, M.J. Functional significance of the perforin/granzyme cell death pathway. Nat. Rev. Immunol. 2002, 2, 735–747. [Google Scholar] [CrossRef]
- Lugini, L.; Cecchetti, S.; Huber, V.; Luciani, F.; Macchia, G.; Spadaro, F.; Paris, L.; Abalsamo, L.; Colone, M.; Molinari, A.; et al. Immune Surveillance Properties of Human NK Cell-Derived Exosomes. J. Immunol. 2012, 189, 2833–2842. [Google Scholar] [CrossRef]
- Fais, S. NK cell-released exosomes. OncoImmunology 2013, 2, e22337. [Google Scholar] [CrossRef]
- Aarsund, M.; Segers, F.M.; Wu, Y.; Inngjerdingen, M. Comparison of characteristics and tumor targeting properties of extracellular vesicles derived from primary NK cells or NK-cell lines stimulated with IL-15 or IL-12/15/18. Cancer Immunol. Immunother. 2022, 71, 2227–2238. [Google Scholar] [CrossRef]
- Guillerey, C.; Huntington, N.D.; Smyth, M.J. Targeting natural killer cells in cancer immunotherapy. Nat. Immunol. 2016, 17, 1025–1036. [Google Scholar] [CrossRef]
- Brodbeck, T.; Nehmann, N.; Bethge, A.; Wedemann, G.; Schumacher, U. Perforin-dependent direct cytotoxicity in natural killer cells induces considerable knockdown of spontaneous lung metastases and computer modelling-proven tumor cell dormancy in a HT29 human colon cancer xenograft mouse model. Mol. Cancer 2014, 13, 244. [Google Scholar] [CrossRef]
- Chavezgalan, L.; Arenas-Del Angel, M.C.; Zenteno, E.; Chavez, R.; Lascurain, R. Cell Death Mechanisms Induced by Cytotoxic Lymphocytes. Cell. Mol. Immunol. 2009, 6, 15–25. [Google Scholar] [CrossRef]
- Slee, E.A.; Harte, M.T.; Kluck, R.M.; Wolf, B.B.; Casiano, C.A.; Newmeyer, D.D.; Wang, H.-G.; Reed, J.C.; Nicholson, D.W.; Alnemri, E.S.; et al. Ordering the Cytochrome c–initiated Caspase Cascade: Hierarchical Activation of Caspases-2, -3, -6, -7, -8, and -10 in a Caspase-9–dependent Manner. J. Cell Biol. 1999, 144, 281–292. [Google Scholar] [CrossRef]
- Ewen, C.L.; Kane, K.P.; Bleackley, R.C. A quarter century of granzymes. Cell Death Differ. 2011, 19, 28–35. [Google Scholar] [CrossRef]
- MacDonald, G.; Shi, L.; Velde, C.V.; Lieberman, J.; Greenberg, A.H. Mitochondria-dependent and -independent Regulation of Granzyme B–induced Apoptosis. J. Exp. Med. 1999, 189, 131–144. [Google Scholar] [CrossRef]
- Wang, C.; Morley, S.C.; Donermeyer, D.; Peng, I.; Lee, W.P.; Devoss, J.; Danilenko, D.M.; Lin, Z.; Zhang, J.; Zhou, J.; et al. Actin-Bundling Protein L-Plastin Regulates T Cell Activation. J. Immunol. 2010, 185, 7487–7497. [Google Scholar] [CrossRef]
- Yang, J.; Zhang, X.; Wang, J.; Qian, J.; Zhang, L.; Wang, M.; Kwak, L.W.; Yi, Q. Anti–β2-microglobulin monoclonal antibodies induce apoptosis in myeloma cells by recruiting MHC class I to and excluding growth and survival cytokine receptors from lipid rafts. Blood 2007, 110, 3028–3035. [Google Scholar] [CrossRef] [PubMed]
- Tsai, C.Y.; Yu, C.L.; Lu, J.Y.; Wu, T.H.; Tsai, Y.Y. Increased Excretions of β2-Microglobulin, IL-6, and IL-8 and Decreased Excretion of Tamm-Horsfall Glycoprotein in Urine of Patients with Active Lupus nephritis. Nephron. 21 June 2000, p. 85. Available online: https://discovery.researcher.life/article/increased-excretions-of-2-microglobulin-il-6-and-il-8-and-decreased-excretion-of-tamm-horsfall-glycoprotein-in-urine-of-patients-with-active-lupus-nephritis/37a7e9f1628a3700b81858f80b50ad73 (accessed on 4 January 2024).
- Yuan, S.; Liu, Z.; Xu, Z.; Liu, J.; Zhang, J. High mobility group box 1 (HMGB1): A pivotal regulator of hematopoietic malignancies. J. Hematol. Oncol. 2020, 13, 91. [Google Scholar] [CrossRef] [PubMed]
- Bunnell, T.M.; Burbach, B.J.; Shimizu, Y.; Ervasti, J.M. β-Actin specifically controls cell growth, migration, and the G-actin pool. Mol. Biol. Cell. 2011, 22, 3917–4204. [Google Scholar] [CrossRef] [PubMed]
- Guo, C.; Liu, S.; Wang, J.; Sun, M.Z.; Greenaway, F.T. ACTB in cancer. Clin. Chim. Acta. 2013, 417, 39–44. [Google Scholar] [CrossRef] [PubMed]
- Pfisterer, J.; Shannon, C.M.; Baumann, K.; Rau, J.; Harter, P.; Joly, F.; Sehouli, J.; Canzler, U.; Schmalfeldt, B.; Dean, A.P.; et al. Bevacizumab and platinum-based combinations for recurrent ovarian cancer: A randomised, open-label, phase 3 trial. Lancet Oncol. 2020, 21, 699–709. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.J.; Kim, T.G.; Lee, H.J.; Kim, J.H.; Lim, B.H.; Seo, J.W.; Kang, E.M.; Lee, B.U.; Ahn, Y.M.; Roh, Y.H.; et al. A phase II study of combination chemotherapy with docetaxel and carboplatin for elderly patients with advanced non-small cell lung cancer. Lung Cancer. 2010, 68, 248–252. [Google Scholar] [CrossRef]
- Shiono, A.; Imai, H.; Wasamoto, S.; Tsuda, T.; Nagai, Y.; Minemura, H.; Yamada, Y.; Kishikawa, T.; Umeda, Y.; Takechi, H.; et al. Real-world data of atezolizumab plus carboplatin and etoposide in elderly patients with extensive-disease small-cell lung cancer. Cancer Med. 2022, 12, 73–83. [Google Scholar] [CrossRef]
- Nadal, E.; Rodríguez-Abreu, D.; Simó, M.; Massutí, B.; Juan, O.; Huidobro, G.; López, R.; De Castro, J.; Estival, A.; Mosquera, J.; et al. Phase II Trial of Atezolizumab Combined With Carboplatin and Pemetrexed for Patients With Advanced Nonsquamous Non–Small-Cell Lung Cancer With Untreated Brain Metastases (Atezo-Brain, GECP17/05). J. Clin. Oncol. 2023, 41, 4478–4485. [Google Scholar] [CrossRef]
- Shoae-Hassani, A.; Hamidieh, A.A.; Behfar, M.; Mohseni, R.; Mortazavi-Tabatabaei, S.A.; Asgharzadeh, S. NK Cell–derived Exosomes From NK Cells Previously Exposed to Neuroblastoma Cells Augment the Antitumor Activity of Cytokine-activated NK Cells. J. Immunother. 2017, 40, 265–276. [Google Scholar] [CrossRef]
- D’Incecco, A.; Andreozzi, M.; Ludovini, V.; Rossi, E.; Capodanno, A.; Landi, L.; Tibaldi, C.; Minuti, G.; Salvini, J.; Coppi, E.; et al. PD-1 and PD-L1 expression in molecularly selected non-small-cell lung cancer patients. Br. J. Cancer 2015, 112, 95–102. [Google Scholar] [CrossRef]
- Pardoll, D.M. The blockade of immune checkpoints in cancer immunotherapy. Nat. Rev. Cancer 2012, 12, 252–264. [Google Scholar] [CrossRef]
- Zappasodi, R.; Merghoub, T.; Wolchok, J.D. Emerging Concepts for Immune Checkpoint Blockade-Based Combination Therapies. Cancer Cell. 2018, 34, 690. [Google Scholar] [CrossRef] [PubMed]
- Greten, F.R.; Grivennikov, S.I. Inflammation and Cancer: Triggers, Mechanisms, and Consequences. Immunity 2019, 51, 27–41. [Google Scholar] [CrossRef] [PubMed]
- Ridker, P.M.; MacFadyen, J.G.; Thuren, T.; Everett, B.M.; Libby, P.; Glynn, R.J.; Ridker, P.; Lorenzatti, A.; Krum, H.; Varigos, J.; et al. Effect of interleukin-1β inhibition with canakinumab on incident lung cancer in patients with atherosclerosis: Exploratory results from a randomised, double-blind, placebo-controlled trial. Lancet 2017, 390, 1833–1842. [Google Scholar] [CrossRef] [PubMed]
- Ritter, B.; Greten, F.R. Modulating inflammation for cancer therapy. J. Exp. Med. 2019, 216, 1234–1243. [Google Scholar] [CrossRef] [PubMed]
- Rothwell, P.M.; Wilson, M.; Price, J.F.; Belch, J.F.; Meade, T.W.; Mehta, Z. Effect of daily aspirin on risk of cancer metastasis: A study of incident cancers during randomised controlled trials. Lancet 2012, 379, 1591–1601. [Google Scholar] [CrossRef] [PubMed]
- Cullen, S.P.; Brunet, M.; Martin, S.J. Granzymes in cancer and immunity. Cell Death Differ. 2010, 17, 616–623. [Google Scholar] [CrossRef]
- Lieberman, J. Granzyme A activates another way to die. Immunol. Rev. 2010, 235, 93–104. [Google Scholar] [CrossRef]
- Wang, T.; Fu, Y.; Sun, S.; Huang, C.; Yi, Y.; Wang, J.; Deng, Y.; Wu, M. Exosome-based drug delivery systems in cancer therapy. Chin. Chem. Lett. 2023, 34, 107508. [Google Scholar] [CrossRef]
- Shaban, A.M.; Raslan, M.; Sharawi, Z.W.; Abdelhameed, M.S.; Hammouda, O.; El-Masry, H.M.; Elsayed, K.N.M.; El-Magd, M.A. Antibacterial, Antifungal, and Anticancer Effects of Camel Milk Exosomes: An In Vitro Study. Veter- Sci. 2023, 10, 124. [Google Scholar] [CrossRef]
- Adriano, B.; Cotto, N.M.; Chauhan, N.; Jaggi, M.; Chauhan, S.C.; Yallapu, M.M. Milk exosomes: Nature’s abundant nanoplatform for theranostic applications. Bioact. Mater. 2021, 6, 2479–2490. [Google Scholar] [CrossRef]
- Bach, L.G.; Nguyen, T.H.; Ho, M.H.; Nguyen, D.H.; Nguyen, C.K.; Nguyen, C.H.; Nguyen, N.V.; Thi, T.T.H. PEGylated poly(amidoamine) dendrimers-based drug loading vehicles for delivering carboplatin in treatment of various cancerous cells. J. Nanopart. Res. 2019, 21, 43. [Google Scholar] [CrossRef]
- Jong, W.H.D.; Borm, P.J. Drug delivery and nanoparticles: Applications and hazards. Int. J. Nanomed. 2008, 3, 133–149. [Google Scholar] [CrossRef]
- Shi, M.; Anantha, M.; Wehbe, M.; Bally, M.B.; Fortin, D.; Roy, L.-O.; Charest, G.; Richer, M.; Paquette, B.; Sanche, L. Liposomal formulations of carboplatin injected by convection-enhanced delivery increases the median survival time of F98 glioma bearing rats. J. Nanobiotechnol. 2018, 16, 133–149. [Google Scholar] [CrossRef]
- Liu, B.; Han, L.; Liu, J.; Han, S.; Chen, Z.; Jiang, L. Co-delivery of paclitaxel and TOS-cisplatin via TAT-targeted solid lipid nanoparticles with synergistic antitumor activity against cervical cancer. Int. J. Nanomed. 2017, 12, 955–968. [Google Scholar] [CrossRef]
- Narayana, R.V.L.; Jana, P.; Tomar, N.; Prabhu, V.; Nair, R.M.; Manukonda, R.; Kaliki, S.; Coupland, S.E.; Alexander, J.; Kalirai, H.; et al. Carboplatin- and Etoposide-Loaded Lactoferrin Protein Nanoparticles for Targeting Cancer Stem Cells in Retinoblastoma In Vitro. Investig. Opthalmol. Vis. Sci. 2021, 62, 13. [Google Scholar] [CrossRef]
- Lee, R.S.; Lin, C.H.; Aljuffali, I.A.; Hu, K.Y.; Fang, J.Y. Passive targeting of thermosensitive diblock copolymer micelles to the lungs: Synthesis and characterization of poly(N-isopropylacrylamide)-block-poly(ε-caprolactone). J. Nanobiotechnol. 2015, 13, 42. [Google Scholar] [CrossRef]
- Haase, D.; Hampel, S.; Kraemer, K.; Kunze, D.; Taylor, A.; Arlt, M.; Thomas, J.; Oswald, S.; Ritschel, M.; Klingeler, R.; et al. Carbon Nanotubes Filled with Carboplatin: Towards Carbon Nanotube-Supported Delivery of Chemotherapeutic Agents. In Carbon Nanotubes for Biomedical Applications; Klingeler, R., Sim, R.B., Eds.; Carbon Nanostructures; Springer: Berlin/Heidelberg, Germany, 2011; pp. 247–528. [Google Scholar]
- Grilley-Olson, J.E.; Keedy, V.L.; Sandler, A.; Moore, D.T.; Socinski, M.A.; Stinchcombe, T.E. A Randomized Phase II Study of Carboplatin With Weekly or Every-3-Week Nanoparticle Albumin-Bound Paclitaxel (Abraxane) in Patients With Extensive-Stage Small Cell Lung Cancer. Oncol. 2015, 20, 105–106. [Google Scholar] [CrossRef]
- Bastos, N.; Ruivo, C.F.; da Silva, S.; Melo, S.A. Exosomes in cancer: Use them or target them? Semin Cell Dev. Biol. 2018, 78, 13–21. [Google Scholar] [CrossRef]
- Escudier, B.; Dorval, T.; Chaput, N.; André, F.; Caby, M.-P.; Novault, S.; Flament, C.; Leboulaire, C.; Borg, C.; Amigorena, S.; et al. Vaccination of metastatic melanoma patients with autologous dendritic cell (DC) derived-exosomes: Results of thefirst phase I clinical trial. J. Transl. Med. 2005, 3, 10. [Google Scholar] [CrossRef]
- Rufino-Ramos, D.; Albuquerque, P.R.; Carmona, V.; Perfeito, R.; Nobre, R.J.; de Almeida, L.P. Extracellular vesicles: Novel promising delivery systems for therapy of brain diseases. J. Control. Release 2017, 262, 247–258. [Google Scholar] [CrossRef]
- Home|Clinical Trials.gov. Available online: https://clinicaltrials.gov/ (accessed on 4 December 2023).



| Treatment | IC50 in 2D (µM) | IC50 in 3D (µM) |
|---|---|---|
| CBP | 195.65 ± 8.12 | 383.64 ± 12.78 |
| NK-EVs | 55% Cell Viability | 68% Cell Viability |
| CBP + NK-EVs | 105.54 ± 4.98 | 226.12 ± 9.16 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nathani, A.; Sun, L.; Khan, I.; Aare, M.; Bagde, A.; Li, Y.; Singh, M. Combined Role of Interleukin-15 Stimulated Natural Killer Cell-Derived Extracellular Vesicles and Carboplatin in Osimertinib-Resistant H1975 Lung Cancer Cells with EGFR Mutations. Pharmaceutics 2024, 16, 83. https://doi.org/10.3390/pharmaceutics16010083
Nathani A, Sun L, Khan I, Aare M, Bagde A, Li Y, Singh M. Combined Role of Interleukin-15 Stimulated Natural Killer Cell-Derived Extracellular Vesicles and Carboplatin in Osimertinib-Resistant H1975 Lung Cancer Cells with EGFR Mutations. Pharmaceutics. 2024; 16(1):83. https://doi.org/10.3390/pharmaceutics16010083
Chicago/Turabian StyleNathani, Aakash, Li Sun, Islauddin Khan, Mounika Aare, Arvind Bagde, Yan Li, and Mandip Singh. 2024. "Combined Role of Interleukin-15 Stimulated Natural Killer Cell-Derived Extracellular Vesicles and Carboplatin in Osimertinib-Resistant H1975 Lung Cancer Cells with EGFR Mutations" Pharmaceutics 16, no. 1: 83. https://doi.org/10.3390/pharmaceutics16010083