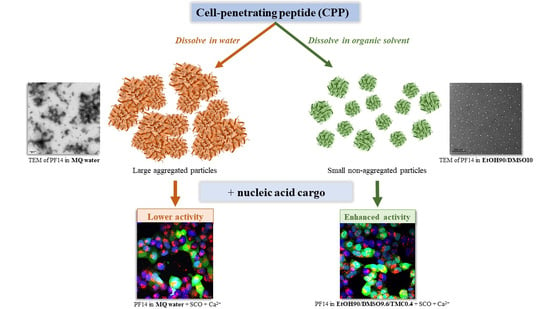
Choosing an Optimal Solvent Is Crucial for Obtaining Cell-Penetrating Peptide Nanoparticles with Desired Properties and High Activity in Nucleic Acid Delivery
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. PF14-SCO Nanoparticle Formation
2.3. Dynamic Light Scattering Analysis
2.4. Cell Culture
2.5. Luminescence Measurement
2.6. Cytotoxicity Assay
2.7. Confocal Microscopy
2.8. Flow Cytometry
2.9. Transmission Electron Microscopy (TEM)
2.10. Atomic Force Microscopy (AFM)
2.11. Scanning Electron Microscopy (SEM)
2.12. Circular Dichroism Spectroscory (CD)
2.13. Energy-Dispersive X-ray Spectrometry (EDX)
2.14. Statistical Analysis
3. Results and Discussion
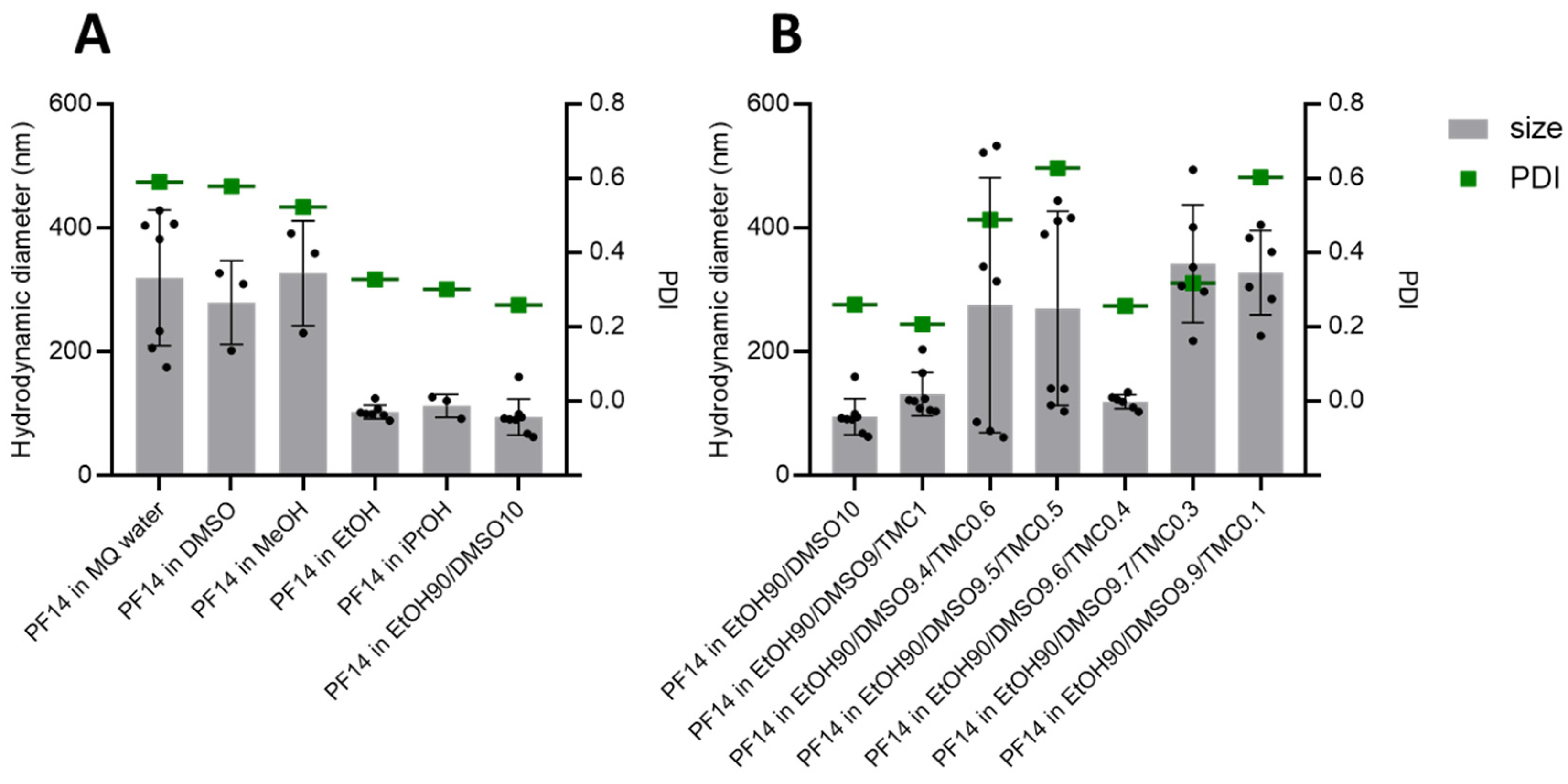
3.1. PF14 Dissolved in Polar Organic Solvents Assembles into Nanoparticles in Water
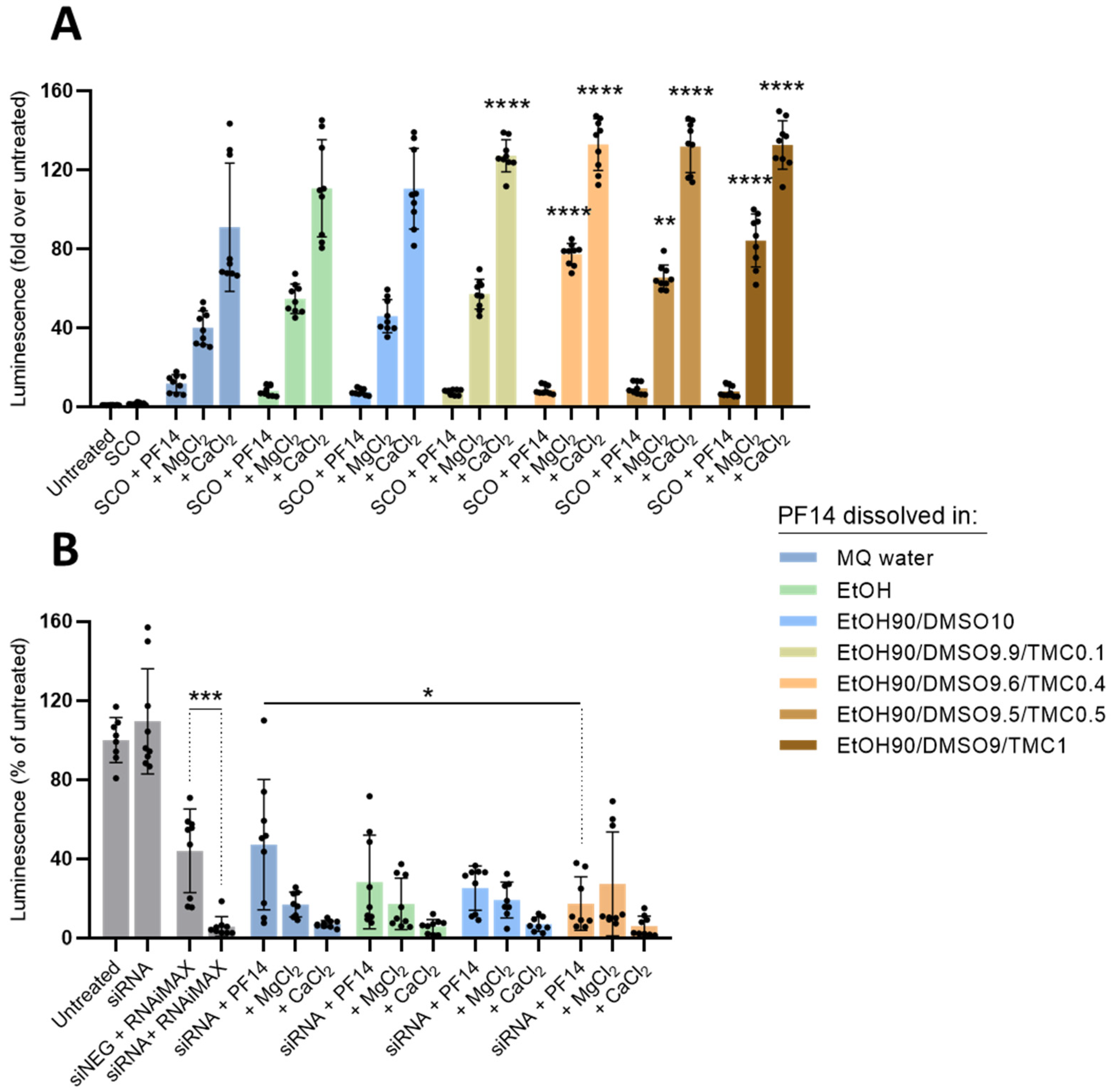
3.2. Dissolution of PF14 in the Mixture of Polar Organic Solvents Does Not Compromise the Ability of Peptide to Transduce Oligonucleotides into Cells
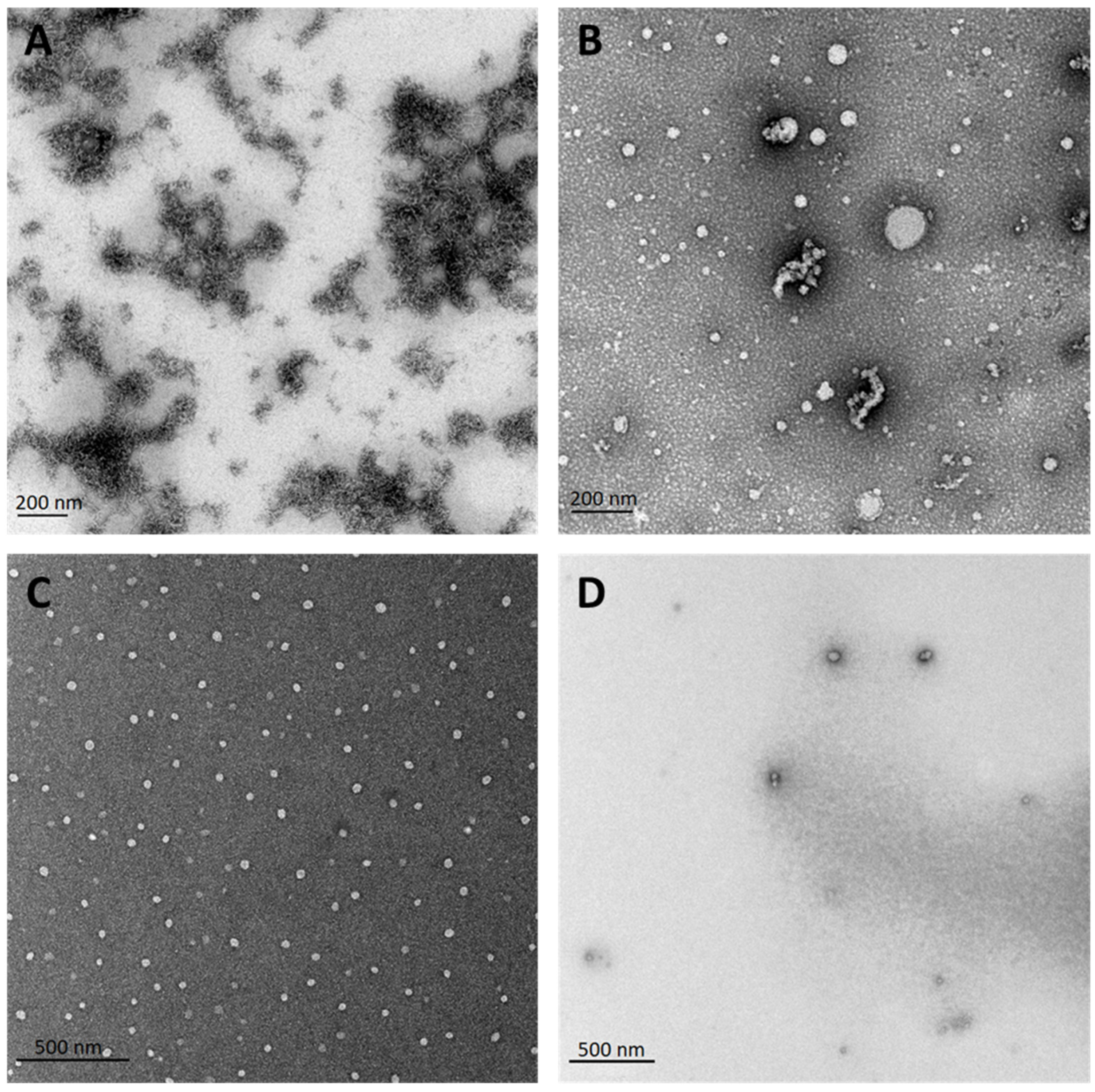
3.3. Electron Microscopy Confirms the Beneficial Effect of Dissolving PF14 in Organic Solvents, Compared to Dissolving in Water
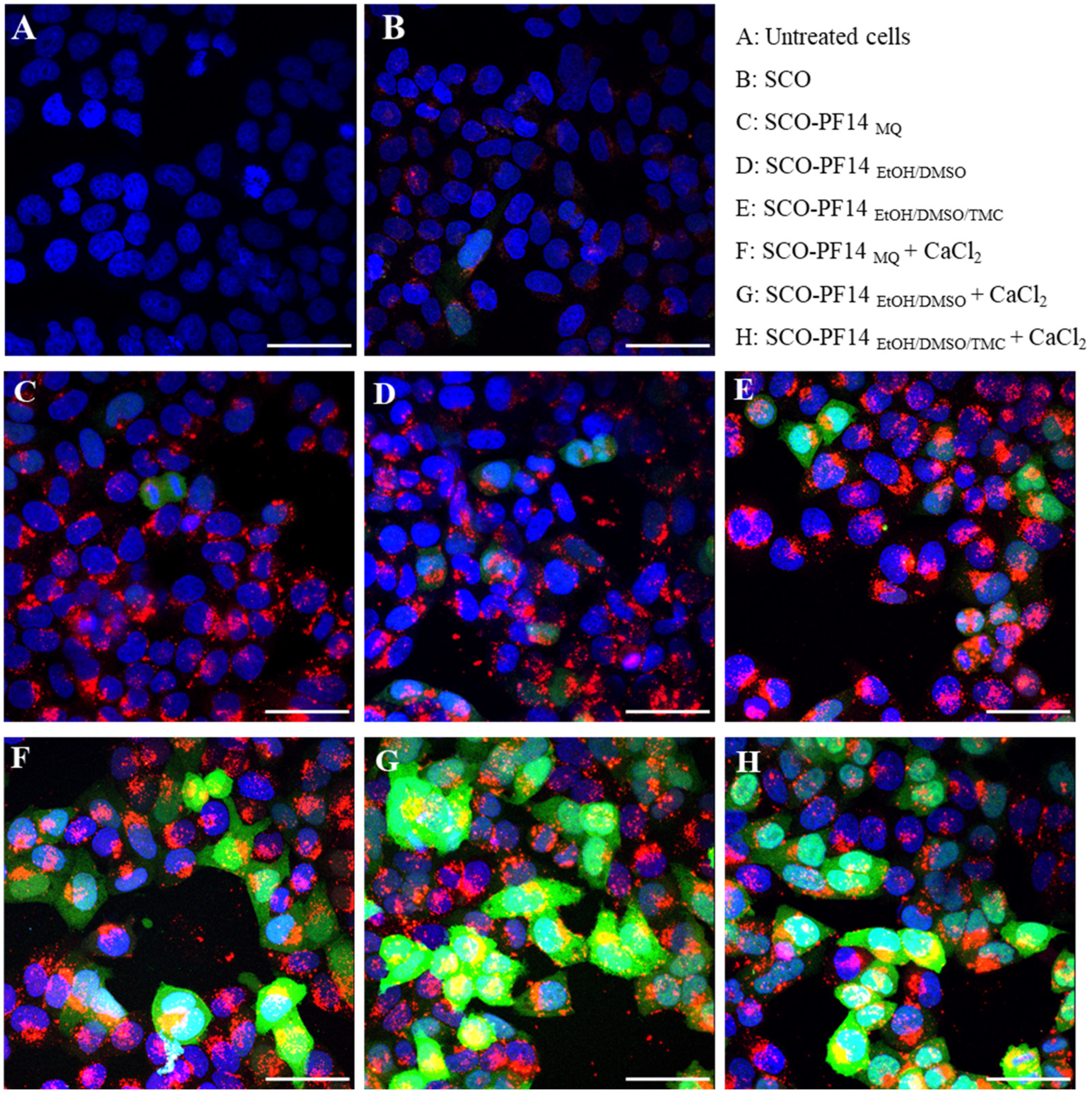
3.4. Confocal Microscopy Analysis Corroborates Efficient Oligonucleotide Delivery and Splicing Correction with PF14 Dissolved in the Mixture of Organic Solvents
3.5. Other Organic Solvents Can Also Be Beneficial for Dissolving PF14, and Hydrophobic CPPs
3.6. Nanoparticles of PF14 and Its Complexes with SCO Are Spherical
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Damase, T.R.; Sukhovershin, R.; Boada, C.; Taraballi, F.; Pettigrew, R.I.; Cooke, J.P. The Limitless Future of RNA Therapeutics. Front. Bioeng. Biotechnol. 2021, 9, 628137. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, G.A.R.; de Melo Alves Paiva, R. Gene Therapy: Advances, Challenges and Perspectives. Einstein 2017, 15, 369–375. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dunbar, C.E.; High, K.A.; Joung, J.K.; Kohn, D.B.; Ozawa, K.; Sadelain, M. Gene Therapy Comes of Age. Science 2018, 359, eaan4672. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- U.S. Food and Drug Administration. What Is Gene Therapy? Available online: https://www.fda.gov/vaccines-blood-biologics/cellular-gene-therapy-products/what-gene-therapy (accessed on 1 January 2023).
- Thomas, C.E.; Ehrhardt, A.; Kay, M.A. Progress and Problems with the Use of Viral Vectors for Gene Therapy. Nat. Rev. Genet. 2003, 4, 346–358. [Google Scholar] [CrossRef]
- Nayak, S.; Herzog, R.W. Progress and Prospects: Immune Responses to Viral Vectors. Gene Ther. 2010, 17, 295–304. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kofron, M.D.; Laurencin, C.T. Bone Tissue Engineering by Gene Delivery. Adv. Drug Deliv. Rev. 2006, 58, 555–576. [Google Scholar] [CrossRef] [PubMed]
- Davé, U.P.; Jenkins, N.A.; Copeland, N.G. Gene Therapy Insertional Mutagenesis Insights. Science 2004, 303, 333. [Google Scholar] [CrossRef] [Green Version]
- Al-Dosari, M.S.; Gao, X. Nonviral Gene Delivery: Principle, Limitations, and Recent Progress. AAPS J. 2009, 11, 671. [Google Scholar] [CrossRef] [Green Version]
- Piotrowski-Daspit, A.S.; Kauffman, A.C.; Bracaglia, L.G.; Saltzman, W.M. Polymeric Vehicles for Nucleic Acid Delivery. Adv. Drug Deliv. Rev. 2020, 156, 119–132. [Google Scholar] [CrossRef]
- Ramamoorth, M.; Narvekar, A. Non Viral Vectors in Gene Therapy—An Overview. J. Clin. Diagn. Res. 2015, 9, GE01–GE06. [Google Scholar] [CrossRef]
- Filion, M.C.; Phillips, N.C. Toxicity and Immunomodulatory Activity of Liposomal Vectors Formulated with Cationic Lipids toward Immune Effector Cells. Biochim. Biophys. Acta (BBA)-Biomembr. 1997, 1329, 345–356. [Google Scholar] [CrossRef] [Green Version]
- Kurrikoff, K.; Veiman, K.-L.; Künnapuu, K.; Peets, E.M.; Lehto, T.; Pärnaste, L.; Arukuusk, P.; Langel, Ü. Effective In Vivo Gene Delivery with Reduced Toxicity, Achieved by Charge and Fatty Acid-Modified Cell Penetrating Peptide. Sci. Rep. 2017, 7, 17056. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xie, J.; Bi, Y.; Zhang, H.; Dong, S.; Teng, L.; Lee, R.J.; Yang, Z. Cell-Penetrating Peptides in Diagnosis and Treatment of Human Diseases: From Preclinical Research to Clinical Application. Front. Pharmacol. 2020, 11, 697. [Google Scholar] [CrossRef]
- Saar, K.; Lindgren, M.; Hansen, M.; Eiríksdóttir, E.; Jiang, Y.; Rosenthal-Aizman, K.; Sassian, M.; Langel, Ü. Cell-Penetrating Peptides: A Comparative Membrane Toxicity Study. Anal. Biochem. 2005, 345, 55–65. [Google Scholar] [CrossRef] [PubMed]
- El Andaloussi, S.; Lehto, T.; Mäger, I.; Rosenthal-Aizman, K.; Oprea, I.I.; Simonson, O.E.; Sork, H.; Ezzat, K.; Copolovici, D.M.; Kurrikoff, K. Design of a Peptide-Based Vector, PepFect6, for Efficient Delivery of SiRNA in Cell Culture and Systemically In Vivo. Nucleic Acids Res. 2011, 39, 3972–3987. [Google Scholar] [CrossRef] [Green Version]
- Pooga, M.; Langel, Ü. Classes of Cell-Penetrating Peptides. In Cell-Penetrating Peptides; Springer: New York, NY, USA, 2015; pp. 3–28. [Google Scholar] [CrossRef]
- Kristensen, M.; Birch, D.; Mørck Nielsen, H. Applications and Challenges for Use of Cell-Penetrating Peptides as Delivery Vectors for Peptide and Protein Cargos. Int. J. Mol. Sci. 2016, 17, 185. [Google Scholar] [CrossRef] [Green Version]
- Kalafatovic, D.; Giralt, E. Cell-Penetrating Peptides: Design Strategies beyond Primary Structure and Amphipathicity. Molecules 2017, 22, 1929. [Google Scholar] [CrossRef] [Green Version]
- Biswas, A.; Chakraborty, K.; Dutta, C.; Mukherjee, S.; Gayen, P.; Jan, S.; Mallick, A.M.; Bhattacharyya, D.; Sinha Roy, R. Engineered Histidine-Enriched Facial Lipopeptides for Enhanced Intracellular Delivery of Functional SiRNA to Triple Negative Breast Cancer Cells. ACS Appl. Mater. Interfaces 2019, 11, 4719–4736. [Google Scholar] [CrossRef]
- Porosk, L.; Arukuusk, P.; Põhako, K.; Kurrikoff, K.; Kiisholts, K.; Padari, K.; Pooga, M.; Langel, Ü. Enhancement of SiRNA Transfection by the Optimization of Fatty Acid Length and Histidine Content in the CPP. Biomater. Sci. 2019, 7, 4363–4374. [Google Scholar] [CrossRef] [Green Version]
- Beloor, J.; Zeller, S.; Choi, C.S.; Lee, S.-K.; Kumar, P. Cationic Cell-Penetrating Peptides as Vehicles for SiRNA Delivery. Ther. Deliv. 2015, 6, 491–507. [Google Scholar] [CrossRef]
- Boisguérin, P.; Deshayes, S.; Gait, M.J.; O’Donovan, L.; Godfrey, C.; Betts, C.A.; Wood, M.J.; Lebleu, B. Delivery of Therapeutic Oligonucleotides with Cell Penetrating Peptides. Adv. Drug Deliv. Rev. 2015, 87, 52–67. [Google Scholar] [CrossRef] [PubMed]
- Lehto, T.; Ezzat, K.; Wood, M.J.A.; EL Andaloussi, S. Peptides for Nucleic Acid Delivery. Adv. Drug Deliv. Rev. 2016, 106, 172–182. [Google Scholar] [CrossRef] [PubMed]
- Oba, M.; Ito, Y.; Umeno, T.; Kato, T.; Tanaka, M. Plasmid DNA Delivery Using Cell-Penetrating Peptide Foldamers Composed of Arg-Arg-Aib Repeating Sequences. ACS Biomater. Sci. Eng. 2019, 5, 5660–5668. [Google Scholar] [CrossRef] [PubMed]
- Kato, T.; Yamashita, H.; Misawa, T.; Nishida, K.; Kurihara, M.; Tanaka, M.; Demizu, Y.; Oba, M. Plasmid DNA Delivery by Arginine-Rich Cell-Penetrating Peptides Containing Unnatural Amino Acids. Bioorg. Med. Chem. 2016, 24, 2681–2687. [Google Scholar] [CrossRef]
- Yokoo, H.; Oba, M.; Uchida, S. Cell-Penetrating Peptides: Emerging Tools for MRNA Delivery. Pharmaceutics 2021, 14, 78. [Google Scholar] [CrossRef] [PubMed]
- Dinca, A.; Chien, W.-M.; Chin, M.T. Intracellular Delivery of Proteins with Cell-Penetrating Peptides for Therapeutic Uses in Human Disease. Int. J. Mol. Sci. 2016, 17, 263. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Freimann, K.; Arukuusk, P.; Kurrikoff, K.; Pärnaste, L.; Raid, R.; Piirsoo, A.; Pooga, M.; Langel, Ü. Formulation of Stable and Homogeneous Cell-Penetrating Peptide NF55 Nanoparticles for Efficient Gene Delivery in Vivo. Mol. Ther.-Nucleic Acids 2018, 10, 28–35. [Google Scholar] [CrossRef] [Green Version]
- Sharma, A.; Madhunapantula, S.V.; Robertson, G.P. Toxicological Considerations When Creating Nanoparticle Based Drugs and Drug Delivery Systems? Expert Opin. Drug Metab. Toxicol. 2012, 8, 47–69. [Google Scholar] [CrossRef]
- Ravichandran, R. Nanoparticles in Drug Delivery: Potential Green Nanobiomedicine Applications. Int. J. Green Nanotechnol. Biomed. 2009, 1, B108–B130. [Google Scholar] [CrossRef]
- Whitesides, G.M.; Mathias, J.P.; Seto, C.T. Molecular Self-Assembly and Nanochemistry: A Chemical Strategy for the Synthesis of Nanostructures. Science 1991, 254, 1312–1319. [Google Scholar] [CrossRef]
- Edwards-Gayle, C.J.C.; Hamley, I.W. Self-Assembly of Bioactive Peptides, Peptide Conjugates, and Peptide Mimetic Materials. Org. Biomol. Chem. 2017, 15, 5867–5876. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, Y.; Tao, K.; Ji, W.; Kumar, V.B.; Rencus-Lazar, S.; Gazit, E. Histidine as a Key Modulator of Molecular Self-Assembly: Peptide-Based Supramolecular Materials Inspired by Biological Systems. Mater. Today 2022, 60, 106–127. [Google Scholar] [CrossRef]
- Chen, Y.; Guerin, S.; Yuan, H.; O’Donnell, J.; Xue, B.; Cazade, P.-A.; Haq, E.U.; Shimon, L.J.W.; Rencus-Lazar, S.; Tofail, S.A.M.; et al. Guest Molecule-Mediated Energy Harvesting in a Conformationally Sensitive Peptide–Metal Organic Framework. J. Am. Chem. Soc. 2022, 144, 3468–3476. [Google Scholar] [CrossRef]
- van der Vegt, N.F.; Nayar, D. The Hydrophobic Effect and the Role of Cosolvents. J. Phys. Chem. B 2017, 121, 9986–9998. [Google Scholar] [CrossRef]
- Nandakumar, A.; Ito, Y.; Ueda, M. Solvent Effects on the Self-Assembly of an Amphiphilic Polypeptide Incorporating α-Helical Hydrophobic Blocks. J. Am. Chem. Soc. 2020, 142, 20994–21003. [Google Scholar] [CrossRef]
- Ezzat, K.; El Andaloussi, S.; Zaghloul, E.M.; Lehto, T.; Lindberg, S.; Moreno, P.M.; Viola, J.R.; Magdy, T.; Abdo, R.; Guterstam, P. PepFect 14, a Novel Cell-Penetrating Peptide for Oligonucleotide Delivery in Solution and as Solid Formulation. Nucleic Acids Res. 2011, 39, 5284–5298. [Google Scholar] [CrossRef]
- Juks, C.; Padari, K.; Margus, H.; Kriiska, A.; Etverk, I.; Arukuusk, P.; Koppel, K.; Ezzat, K.; Langel, Ü.; Pooga, M. The Role of Endocytosis in the Uptake and Intracellular Trafficking of PepFect14–Nucleic Acid Nanocomplexes via Class A Scavenger Receptors. Biochim. Biophys. Acta (BBA)-Biomembr. 2015, 1848, 3205–3216. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ezzat, K.; Helmfors, H.; Tudoran, O.; Juks, C.; Lindberg, S.; Padari, K.; El-Andaloussi, S.; Pooga, M.; Langel, Ü. Scavenger Receptor-Mediated Uptake of Cell-Penetrating Peptide Nanocomplexes with Oligonucleotides. FASEB J. 2012, 26, 1172–1180. [Google Scholar] [CrossRef]
- Andaloussi, S.E.L.; Lehto, T.; Lundin, P.; Langel, U. Application of PepFect Peptides for the Delivery of Splice-Correcting Oligonucleotides. Methods Mol. Biol. 2011, 683, 361–373. [Google Scholar] [CrossRef]
- Srimanee, A.; Arvanitidou, M.; Kim, K.; Hällbrink, M.; Langel, Ü. Cell-Penetrating Peptides for SiRNA Delivery to Glioblastomas. Peptides 2018, 104, 62–69. [Google Scholar] [CrossRef]
- Urgard, E.; Brjalin, A.; Langel, Ü.; Pooga, M.; Rebane, A.; Annilo, T. Comparison of Peptide-and Lipid-Based Delivery of MiR-34a-5p Mimic into Ppc-1 Cells. Nucleic Acid Ther. 2017, 27, 295–302. [Google Scholar] [CrossRef] [PubMed]
- Carreras-Badosa, G.; Maslovskaja, J.; Periyasamy, K.; Urgard, E.; Padari, K.; Vaher, H.; Tserel, L.; Gestin, M.; Kisand, K.; Arukuusk, P. NickFect Type of Cell-Penetrating Peptides Present Enhanced Efficiency for MicroRNA-146a Delivery into Dendritic Cells and during Skin Inflammation. Biomaterials 2020, 262, 120316. [Google Scholar] [CrossRef] [PubMed]
- Lorents, A.; Urgard, E.; Runnel, T.; Wawrzyniak, P.; Langel, U.; Pooga, M.; Rebane, A. Functional Delivery of MiR-146a to Human Primary Keratinocytes and Skin Equivalents with Cell-Penetrating Peptides. In Allergy; Wiley-Blackwell: Hoboken, NJ, USA, 2014; Volume 69, p. 618. [Google Scholar]
- Urgard, E.; Lorents, A.; Klaas, M.; Padari, K.; Viil, J.; Runnel, T.; Langel, K.; Kingo, K.; Tkaczyk, E.; Langel, Ü. Pre-Administration of PepFect6-MicroRNA-146a Nanocomplexes Inhibits Inflammatory Responses in Keratinocytes and in a Mouse Model of Irritant Contact Dermatitis. J. Control. Release 2016, 235, 195–204. [Google Scholar] [CrossRef]
- Juks, C.; Lorents, A.; Arukuusk, P.; Langel, Ü.; Pooga, M. Cell-Penetrating Peptides Recruit Type A Scavenger Receptors to the Plasma Membrane for Cellular Delivery of Nucleic Acids. FASEB J. 2017, 31, 975–988. [Google Scholar] [CrossRef] [Green Version]
- Lorents, A.; Kodavali, P.K.; Oskolkov, N.; Langel, Ü.; Hällbrink, M.; Pooga, M. Cell-Penetrating Peptides Split into Two Groups Based on Modulation of Intracellular Calcium Concentration. J. Biol. Chem. 2012, 287, 16880–16889. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rocha, M.C.; Fabri, J.H.T.M.; de Godoy, K.F.; de Castro, P.A.; Hori, J.I.; da Cunha, A.F.; Arentshorst, M.; Ram, A.F.J.; van den Hondel, C.A.M.J.J.; Goldman, G.H.; et al. Aspergillus Fumigatus MADS-Box Transcription Factor RlmA Is Required for Regulation of the Cell Wall Integrity and Virulence. G3 2016, 6, 2983–3002. [Google Scholar] [CrossRef] [Green Version]
- Helmfors, H.; Eriksson, J.; Langel, Ü. Optimized Luciferase Assay for Cell-Penetrating Peptide-Mediated Delivery of Short Oligonucleotides. Anal. Biochem. 2015, 484, 136–142. [Google Scholar] [CrossRef]
- Maloverjan, M.; Padari, K.; Abroi, A.; Rebane, A.; Pooga, M. Divalent Metal Ions Boost Effect of Nucleic Acids Delivered by Cell-Penetrating Peptides. Cells 2022, 11, 756. [Google Scholar] [CrossRef]
- Kang, S.-H.; Cho, M.-J.; Kole, R. Up-Regulation of Luciferase Gene Expression with Antisense Oligonucleotides: Implications and Applications in Functional Assay Development. Biochemistry 1998, 37, 6235–6239. [Google Scholar] [CrossRef]
- Sazani, P.; Kang, S.-H.; Maier, M.A.; Wei, C.; Dillman, J.; Summerton, J.; Manoharan, M.; Kole, R. Nuclear Antisense Effects of Neutral, Anionic and Cationic Oligonucleotide Analogs. Nucleic Acids Res. 2001, 29, 3965–3974. [Google Scholar] [CrossRef] [Green Version]
- Cellosaurus Cell Line HeLa EGFP-654 (CVCL_VS42). Available online: https://www.cellosaurus.org/CVCL_VS42 (accessed on 4 January 2023).
- Cellosaurus Cell Line U-87 MG-Luc2 (CVCL_5J15). Available online: https://www.cellosaurus.org/CVCL_5J15 (accessed on 4 January 2023).
- Siebring-van Olst, E.; Vermeulen, C.; de Menezes, R.X.; Howell, M.; Smit, E.F.; van Beusechem, V.W. Affordable Luciferase Reporter Assay for Cell-Based High-Throughput Screening. J. Biomol. Screen. 2013, 18, 453–461. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lehto, T.; Vasconcelos, L.; Margus, H.; Figueroa, R.; Pooga, M.; Hällbrink, M.; Langel, Ü. Saturated Fatty Acid Analogues of Cell-Penetrating Peptide PepFect14: Role of Fatty Acid Modification in Complexation and Delivery of Splice-Correcting Oligonucleotides. Bioconjugate Chem. 2017, 28, 782–792. [Google Scholar] [CrossRef]
- Margus, H.; Arukuusk, P.; Langel, Ü.; Pooga, M. Characteristics of Cell-Penetrating Peptide/Nucleic Acid Nanoparticles. Mol. Pharm. 2016, 13, 172–179. [Google Scholar] [CrossRef]
- Blanco, E.; Shen, H.; Ferrari, M. Principles of Nanoparticle Design for Overcoming Biological Barriers to Drug Delivery. Nat. Biotechnol. 2015, 33, 941–951. [Google Scholar] [CrossRef]
- Center for Drug Evaluation and Research. Q3C Tables and List Rev. 4. Available online: https://www.fda.gov/regulatory-information/search-fda-guidance-documents/q3c-tables-and-list-rev-4 (accessed on 27 November 2022).
- Wang, H.; Zhong, C.-Y.; Wu, J.-F.; Huang, Y.-B.; Liu, C.-B. Enhancement of TAT Cell Membrane Penetration Efficiency by Dimethyl Sulphoxide. J. Control. Release 2010, 143, 64–70. [Google Scholar] [CrossRef]
- Gros, E.; Deshayes, S.; Morris, M.C.; Aldrian-Herrada, G.; Depollier, J.; Heitz, F.; Divita, G. A Non-Covalent Peptide-Based Strategy for Protein and Peptide Nucleic Acid Transduction. Biochim. Biophys. Acta (BBA)-Biomembr. 2006, 1758, 384–393. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, Y.; Lau, S.Y.; Lim, Z.W.; Chang, S.C.; Ghadessy, F.; Partridge, A.; Miserez, A. Phase-Separating Peptides for Direct Cytosolic Delivery and Redox-Activated Release of Macromolecular Therapeutics. Nat. Chem. 2022, 14, 274–283. [Google Scholar] [CrossRef]
- Rong, G.; Wang, C.; Chen, L.; Yan, Y.; Cheng, Y. Fluoroalkylation Promotes Cytosolic Peptide Delivery. Sci. Adv. 2020, 6, eaaz1774. [Google Scholar] [CrossRef] [PubMed]
- Mohajeri, S.; Chen, F.; de Prinse, M.; Phung, T.; Burke-Kleinman, J.; Maurice, D.H.; Amsden, B.G. Liquid Degradable Poly(Trimethylene-Carbonate-Co-5-Hydroxy-Trimethylene Carbonate): An Injectable Drug Delivery Vehicle for Acid-Sensitive Drugs. Mol. Pharm. 2020, 17, 1363–1376. [Google Scholar] [CrossRef] [PubMed]
- Fukushima, K. Poly(Trimethylene Carbonate)-Based Polymers Engineered for Biodegradable Functional Biomaterials. Biomater. Sci. 2016, 4, 9–24. [Google Scholar] [CrossRef]
- Tian, H.; Tang, Z.; Zhuang, X.; Chen, X.; Jing, X. Biodegradable Synthetic Polymers: Preparation, Functionalization and Biomedical Application. Prog. Polym. Sci. 2012, 2, 237–280. [Google Scholar] [CrossRef]
- Bazaz, S.; Lehto, T.; Tops, R.; Gissberg, O.; Gupta, D.; Bestas, B.; Bost, J.; Wiklander, O.P.B.; Sork, H.; Zaghloul, E.M.; et al. Novel Orthogonally Hydrocarbon-Modified Cell-Penetrating Peptide Nanoparticles Mediate Efficient Delivery of Splice-Switching Antisense Oligonucleotides In Vitro and In Vivo. Biomedicines 2021, 9, 1046. [Google Scholar] [CrossRef]
- Crombez, L.; Aldrian-Herrada, G.; Konate, K.; Nguyen, Q.N.; McMaster, G.K.; Brasseur, R.; Heitz, F.; Divita, G. A New Potent Secondary Amphipathic Cell–Penetrating Peptide for SiRNA Delivery Into Mammalian Cells. Mol. Ther. 2009, 17, 95–103. [Google Scholar] [CrossRef] [PubMed]
- Tai, W.; Gao, X. Functional Peptides for SiRNA Delivery. Adv. Drug Deliv. Rev. 2017, 110–111, 157–168. [Google Scholar] [CrossRef] [Green Version]
- Rydström, A.; Deshayes, S.; Konate, K.; Crombez, L.; Padari, K.; Boukhaddaoui, H.; Aldrian, G.; Pooga, M.; Divita, G. Direct Translocation as Major Cellular Uptake for CADY Self-Assembling Peptide-Based Nanoparticles. PLoS ONE 2011, 6, e25924. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barbar, E.; Nyarko, A. NMR Characterization of Self-Association Domains Promoted by Interactions with LC8 Hub Protein. Comput. Struct. Biotechnol. J. 2014, 9, e201402003. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Asadi, J.; Ferguson, S.; Raja, H.; Hacker, C.; Marius, P.; Ward, R.; Pliotas, C.; Naismith, J.; Lucocq, J. Enhanced Imaging of Lipid Rich Nanoparticles Embedded in Methylcellulose Films for Transmission Electron Microscopy Using Mixtures of Heavy Metals. Micron 2017, 99, 40–48. [Google Scholar] [CrossRef] [Green Version]
- Kaasalainen, M.; Aseyev, V.; von Haartman, E.; Karaman, D.Ş.; Mäkilä, E.; Tenhu, H.; Rosenholm, J.; Salonen, J. Size, Stability, and Porosity of Mesoporous Nanoparticles Characterized with Light Scattering. Nanoscale Res. Lett. 2017, 12, 74. [Google Scholar] [CrossRef] [Green Version]
- Baoum, A.; Xie, S.-X.; Fakhari, A.; Berkland, C. “Soft” Calcium Crosslinks Enable Highly Efficient Gene Transfection Using TAT Peptide. Pharm. Res. 2009, 26, 2619–2629. [Google Scholar] [CrossRef] [Green Version]
- Graham, F.L.; van der Eb, A.J. A New Technique for the Assay of Infectivity of Human Adenovirus 5 DNA. Virology 1973, 52, 456–467. [Google Scholar] [CrossRef] [PubMed]
- Baoum, A.; Ovcharenko, D.; Berkland, C. Calcium Condensed Cell Penetrating Peptide Complexes Offer Highly Efficient, Low Toxicity Gene Silencing. Int. J. Pharm. 2012, 427, 134–142. [Google Scholar] [CrossRef] [PubMed]
- Melikov, K.; Hara, A.; Yamoah, K.; Zaitseva, E.; Zaitsev, E.; Chernomordik, L.V. Efficient Entry of Cell-Penetrating Peptide Nona-Arginine into Adherent Cells Involves a Transient Increase in Intracellular Calcium. Biochem. J. 2015, 471, 221–230. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goldshtein, M.; Forti, E.; Ruvinov, E.; Cohen, S. Mechanisms of Cellular Uptake and Endosomal Escape of Calcium-SiRNA Nanocomplexes. Int. J. Pharm. 2016, 515, 46–56. [Google Scholar] [CrossRef]
- Hori, S.; Yamamoto, T.; Waki, R.; Wada, S.; Wada, F.; Noda, M.; Obika, S. Ca2+ Enrichment in Culture Medium Potentiates Effect of Oligonucleotides. Nucleic Acids Res. 2015, 43, e128. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Delos Santos, R.C.; Bautista, S.; Lucarelli, S.; Bone, L.N.; Dayam, R.M.; Abousawan, J.; Botelho, R.J.; Antonescu, C.N. Selective Regulation of Clathrin-Mediated Epidermal Growth Factor Receptor Signaling and Endocytosis by Phospholipase C and Calcium. Mol. Biol. Cell 2017, 28, 2802–2818. [Google Scholar] [CrossRef] [PubMed]
- Maltseva, D.; Gudbrandsdottir, R.; Kizilsavas, G.; Horinek, D.; Gonella, G. Location and Conformation of the LKα14 Peptide in Water/Ethanol Mixtures. Langmuir 2021, 37, 469–477. [Google Scholar] [CrossRef]
- Kinoshita, M.; Okamoto, Y.; Hirata, F. Peptide Conformations in Alcohol and Water: Analyses by the Reference Interaction Site Model Theory. J. Am. Chem. Soc. 2000, 122, 2773–2779. [Google Scholar] [CrossRef]
- Notman, R.; Noro, M.; O’Malley, B.; Anwar, J. Molecular Basis for Dimethylsulfoxide (DMSO) Action on Lipid Membranes. J. Am. Chem. Soc. 2006, 128, 13982–13983. [Google Scholar] [CrossRef]
- de Menorval, M.-A.; Mir, L.M.; Fernández, M.L.; Reigada, R. Effects of Dimethyl Sulfoxide in Cholesterol-Containing Lipid Membranes: A Comparative Study of Experiments in Silico and with Cells. PLoS ONE 2012, 7, e41733. [Google Scholar] [CrossRef] [Green Version]
- Gironi, B.; Kahveci, Z.; McGill, B.; Lechner, B.-D.; Pagliara, S.; Metz, J.; Morresi, A.; Palombo, F.; Sassi, P.; Petrov, P.G. Effect of DMSO on the Mechanical and Structural Properties of Model and Biological Membranes. Biophys. J. 2020, 119, 274–286. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Biswas, A.; Maloverjan, M.; Padari, K.; Abroi, A.; Rätsep, M.; Wärmländer, S.K.T.S.; Jarvet, J.; Gräslund, A.; Kisand, V.; Lõhmus, R.; et al. Choosing an Optimal Solvent Is Crucial for Obtaining Cell-Penetrating Peptide Nanoparticles with Desired Properties and High Activity in Nucleic Acid Delivery. Pharmaceutics 2023, 15, 396. https://doi.org/10.3390/pharmaceutics15020396
Biswas A, Maloverjan M, Padari K, Abroi A, Rätsep M, Wärmländer SKTS, Jarvet J, Gräslund A, Kisand V, Lõhmus R, et al. Choosing an Optimal Solvent Is Crucial for Obtaining Cell-Penetrating Peptide Nanoparticles with Desired Properties and High Activity in Nucleic Acid Delivery. Pharmaceutics. 2023; 15(2):396. https://doi.org/10.3390/pharmaceutics15020396
Chicago/Turabian StyleBiswas, Abhijit, Maria Maloverjan, Kärt Padari, Aare Abroi, Margus Rätsep, Sebastian K. T. S. Wärmländer, Jüri Jarvet, Astrid Gräslund, Vambola Kisand, Rünno Lõhmus, and et al. 2023. "Choosing an Optimal Solvent Is Crucial for Obtaining Cell-Penetrating Peptide Nanoparticles with Desired Properties and High Activity in Nucleic Acid Delivery" Pharmaceutics 15, no. 2: 396. https://doi.org/10.3390/pharmaceutics15020396