PVP-H2O2 Complex as a New Stressor for the Accelerated Oxidation Study of Pharmaceutical Solids
Abstract
:1. Introduction
2. Materials and Equipment
2.1. Chemicals and Reagents
2.2. Equipment
3. Experimental and Methods
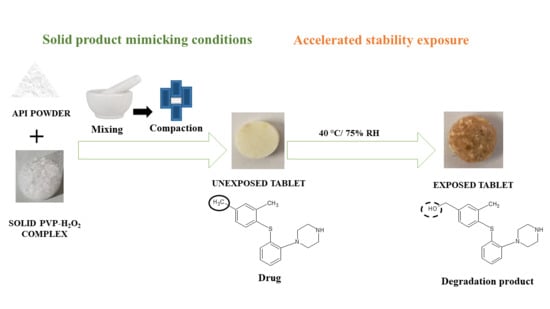
3.1. Oxidative Forced Degradation Study in Solution and Solid State
3.2. Establishment of the Analytical LC Method
3.3. Liquid Chromatography-Mass Spectrometry (LC-MS) Method
3.4. Preparation of Solid State Stress PVP-H2O2 Complex (PHP Complex)
3.5. Measurement of pH of the PHP Complex
3.6. ATR-FTIR Spectral Analysis
3.7. Thermal Analysis
3.8. Preparation of Solid Tablet Compacts and Exposure to Accelerated Storage
3.9. Analysis of the Stored Samples (VOR-PHP Tablet Compacts)
4. Results and Discussion
4.1. LC Analysis
4.2. Speciation of the Oxidative Degradation Product via Forced Degradation Studies
4.3. Physical Characterization and Reproducibility Study of PHP Based Degradation
4.4. Outcome of the Solid State Stress Study Using VOR-PHP Study
4.5. Future Perspective and Practical Relevance of the Stressor
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Hovorka, S.W.; Schöneich, C. Oxidative Degradation of Pharmaceuticals: Theory, Mechanisms and Inhibition. J. Pharm. Sci. 2001, 90, 253–269. [Google Scholar] [CrossRef]
- Wasylaschuk, W.R.; Harmon, P.A.; Wagner, G.; Harman, A.B.; Templeton, A.C.; Xu, H.; Reed, R.A. Evaluation of Hydroperoxides in Common Pharmaceutical Excipients. J. Pharm. Sci. 2007, 96, 106–116. [Google Scholar] [CrossRef] [PubMed]
- Shah, D.D.; Zhang, J.; Hsieh, M.; Sundaram, S.; Maity, H.; Mallela, K.M.G. Effect of Peroxide-Versus Alkoxyl-Induced Chemical Oxidation on the Structure, Stability, Aggregation, and Function of a Therapeutic Monoclonal Antibody. J. Pharm. Sci. 2018, 107, 2789–2803. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Junwal, M.; Modhe, G.; Tiwari, H.; Kurmi, M.; Parashar, N.; Sidduri, P. Forced Degradation Studies to Assess the Stability of Drugs and Products. Trends. Anal. Chem. 2013, 49, 71–88. [Google Scholar] [CrossRef]
- Modhave, D.T.; Handa, T.; Shah, R.P.; Singh, S. Stress Degradation Studies on Lornoxicam Using LC, LC-MS/TOF and LC-MSn. J. Pharm. Biomed. Anal. 2011, 56, 538–545. [Google Scholar] [CrossRef] [PubMed]
- Raijada, D.K.; Bhagwat, P.; Paudel, A.; Shah, R.P.; Singh, S. Characterization of Degradation Products of Amorphous and Polymorphic Forms of Clopidogrel Bisulphate Under Solid State Stress Conditions. J. Pharm. Biomed. Anal. 2010, 52, 332–344. [Google Scholar] [CrossRef] [PubMed]
- Gressl, C.; Brunsteiner, M.; Davis, A.; Landis, M.; Pencheva, K.; Scrivens, G.; Sluggett, G.W.; Wood, G.P.F.; Gruber, W.H.; Khinast, J.G.; et al. Drug-Excipient Interactions in the Solid State: The Role of Different Stress Factors. Mol. Pharm. 2017, 14, 4560–4571. [Google Scholar] [CrossRef] [PubMed]
- Reynolds, D.W.; Galvani, M.; Hicks, S.R.; Joshi, B.J.; Kennedy, G.S.A.; Kleinman, M.H.; Parmar, P.Z. The Use of N-Methylpyrrolidone as a Cosolvent and Oxidant in Pharmaceutical Stress Testing. J. Pharm. Sci. 2012, 101, 761–776. [Google Scholar] [CrossRef] [PubMed]
- Zhu, D.; Zhang, G.G.; George, K.L.; Zhou, D. A Novel Accelerated Oxidative Stability Screening Method for Pharmaceutical Solids. J. Pharm. Sci. 2011, 100, 3529–3538. [Google Scholar] [CrossRef] [PubMed]
- Pourali, A.R.; Ghanei, M. Efficient Epoxidation of α,β-Enones with Polyvinylpyrrolidone Supported Hydrogen Peroxide (PVP-H2O2). Bull. Korean Chem. Soc. 2006, 27, 1674–1676. [Google Scholar] [CrossRef]
- De Diego, M.; Correa, D.; Mennickent, S.; Godoy, R.; Vergara, C. Determination of Vortioxetine and Its Degradation Product in Bulk and Tablets, by LC-DAD and MS/MS Methods. Biomed. Chromatogr. 2018, 32, e4340. [Google Scholar] [CrossRef] [PubMed]
- Nanda, K.K.; Mozziconacci, O.; Small, J.; Allain, L.R.; Helmy, R.; Wuelfing, P. Enrichment of Relevant Oxidative Degradation Products in Pharmaceuticals with Targeted Chemoselective Oxidation. J. Pharm. Sci. 2019, 108, 1466–1475. [Google Scholar] [CrossRef] [PubMed]
- Eyjolfsson, R. Diclofenac Sodium: Oxidative Degradation in Solution and Solid State. Drug Dev. Ind. Pham. 2000, 26, 451–453. [Google Scholar] [CrossRef] [PubMed]
- Narang, A.S.; Desai, D.; Badawy, S. Impact of Excipient Interactions on Solid Dosage Form Stability. Pharm. Res. 2012, 29, 2660–2683. [Google Scholar] [CrossRef] [PubMed]
- Yoshioka, S.; Uchiyama, M. Kinetics and Mechanism of the Solid-State Decomposition of Propantheline Bromide. J. Pharm. Sci. 1986, 75, 92–96. [Google Scholar] [CrossRef] [PubMed]






| Oxidative Stressor (s) | Description |
|---|---|
| 2,2-azobisisobutyronitrile (AIBN), 4,4-azobis-4-cyanovaleric acid (ACVA), 2,2′-azobis(2-amidinopropane) dihydrochloride (AAPH) | • Radical induced stressors are not applicable to study the peroxide based degradation mechanisms • Nonselective chemical degradation reactions are expected • Additional safety precautions needed while handling in the laboratory (not environment-friendly) |
| Liquid hydrogen peroxide (H2O2) | • Do not mimic the solid state mechanisms and reactivity, directly • Corrosive to handle |
| Transition metals (Cu2+ & Fe3+) | • Electron transfer mediated (metal catalyzed) redox reaction |
| Fenton Reagent (solution comprises of liquid hydrogen peroxide and iron (II) sulfate) | • Hydroxyl radicle mediated reactions • Solution state degradation |
| Polysorbate 80 and Iron (III) solution | • Radical mediated reactions • Solution state degradation |
| N-methyl pyrrolidone (NMP) as a co-solvent and oxidant | • Solution state degradation • Non-specific degradation, aids side reactions including hydrolysis |
| Parameter | PHP | UHP |
|---|---|---|
| Material attributes for complexation agent | PVP is one of the most commonly used excipients in the pharmaceutical formulations. | Urea is not a common choice in the pharmaceutical formulations. |
| Selectivity and extent of oxidative degradation reaction | Pure PVP as a byproduct (formed upon dissociation of peroxide from the complex during reaction) is chemically neutral and non-reactive. Secondary degradation reactions are thus not expected. | Pure urea as a byproduct (formed upon dissociation of peroxide from the complex during reaction) will lead to the non-oxidative side reactions by impacting micro-environmental pH. Higher extent of degradation and secondary degradates are expected with this type of matrix. |
| Context and application towards screening of reactive impurity mediated excipient incompatibilities | Applicable for the formulation containing vinyl pyrrolidone excipients (by externally spiking and creating oxidative environment), studying lot-to-lot variations for different excipient grades. Effect of plasticization, polymer chain length effect on chemical reactivity is feasible in this type of matrix. The study of the mobility and solubility of peroxide reactive impurity in the polymer matrix is feasible. | Not very relevant in this context. |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Modhave, D.; Barrios, B.; Paudel, A. PVP-H2O2 Complex as a New Stressor for the Accelerated Oxidation Study of Pharmaceutical Solids. Pharmaceutics 2019, 11, 457. https://doi.org/10.3390/pharmaceutics11090457
Modhave D, Barrios B, Paudel A. PVP-H2O2 Complex as a New Stressor for the Accelerated Oxidation Study of Pharmaceutical Solids. Pharmaceutics. 2019; 11(9):457. https://doi.org/10.3390/pharmaceutics11090457
Chicago/Turabian StyleModhave, Dattatray, Brenda Barrios, and Amrit Paudel. 2019. "PVP-H2O2 Complex as a New Stressor for the Accelerated Oxidation Study of Pharmaceutical Solids" Pharmaceutics 11, no. 9: 457. https://doi.org/10.3390/pharmaceutics11090457
APA StyleModhave, D., Barrios, B., & Paudel, A. (2019). PVP-H2O2 Complex as a New Stressor for the Accelerated Oxidation Study of Pharmaceutical Solids. Pharmaceutics, 11(9), 457. https://doi.org/10.3390/pharmaceutics11090457