Effect of Stabilizers on Encapsulation Efficiency and Release Behavior of Exenatide-Loaded PLGA Microsphere Prepared by the W/O/W Solvent Evaporation Method
Abstract
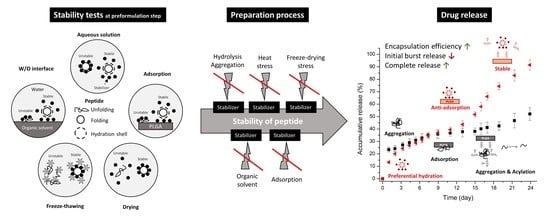
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Quantification of Exenatide
2.3. Stability Tests
2.3.1. Aqueous Solution Stability of Exenatide
2.3.2. W/O Interface-Induced Stability
2.3.3. Freeze-Thawing Stability
2.3.4. Freeze-Drying Stability
2.4. Adsorption Tests of Exenatide to PLGA
2.4.1. Effect of pH in the Aqueous Phase
2.4.2. The Effect of Hydrophilic Additives in the Aqueous Phase
2.4.3. The Effect of Amphipathic Additives Blended into PLGA
2.5. Preparation of Exenatide-Loaded Microspheres
2.6. Characterization of Lyophilized Microsphere Morphology
2.7. Particle Size Analysis
2.8. Drug Loading Capacity (LC) and Encapsulation Efficiency (EE)
2.9. In Vitro Release of Exenatide
2.10. Water Vapor Sorption Analysis
2.11. Differential Scanning Calorimetry (DSC)
2.12. Fourier-Transform Infrared (FT-IR) Spectroscopy
2.13. Circular Dichroism (CD)
3. Results and Discussion
3.1. Effects of Additives on Various Stability of Exenatide
3.1.1. Aqueous Solution Stability
3.1.2. W/O Interface-Induced Instability
3.1.3. Freeze-Thawing Stability
3.1.4. Freeze-Drying Stability
3.2. Effects of Additives on Adsorption of Exenatide to PLGA
3.2.1. Effect of pH
3.2.2. Effects of Hydrophilic Additives
3.2.3. Effect of Amphipathic Additives Blended in PLGA
3.3. Effects of Additives on Pharmaceutical Characteristics of Exenatide-Loaded PLGA Microsphere
3.3.1. Particle Size and Morphology
3.3.2. Encapsulation Efficiency (EE) and In Vitro Release of Exenatide-Loaded Microspheres
3.3.3. Secondary Structure Stability of Exenatide in PLGA Microsphere
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Ye, M.; Kim, S.; Park, K. Issues in long-term protein delivery using biodegradable microparticles. J. Control. Release 2010, 146, 241–260. [Google Scholar] [CrossRef] [PubMed]
- Emami, F.; Vatanara, A.; Park, E.J.; Na, D.H. Drying technologies for the stability and bioavailability of biopharmaceuticals. Pharmaceutics 2018, 10, 131. [Google Scholar] [CrossRef] [PubMed]
- Wang, W. Instability, stabilization, and formulation of liquid protein pharmaceuticals. Int. J. Pharm. 1999, 185, 129–188. [Google Scholar] [CrossRef]
- O’Donnell, P.B.; McGinity, J.W. Preparation of microspheres by the solvent evaporation technique. Adv. Drug Deliv. Rev. 1997, 28, 25–42. [Google Scholar] [CrossRef]
- Van de Weert, M.; Hennink, W.E.; Jiskoot, W. Protein instability in poly (lactic-co-glycolic acid) microparticles. Pharm. Res. 2000, 17, 1159–1167. [Google Scholar] [CrossRef] [PubMed]
- Giteau, A.; Venier-Julienne, M.-C.; Aubert-Pouëssel, A.; Benoit, J.-P. How to achieve sustained and complete protein release from PLGA-based microparticles? Int. J. Pharm. 2008, 350, 14–26. [Google Scholar] [CrossRef] [PubMed]
- Wang, W. Protein aggregation and its inhibition in biopharmaceutics. Int. J. Pharm. 2005, 289, 1–30. [Google Scholar] [CrossRef]
- Arakawa, T.; Prestrelski, S.J.; Kenney, W.C.; Carpenter, J.F. Factors affecting short-term and long-term stabilities of proteins. Adv. Drug Deliv. Rev. 2001, 46, 307–326. [Google Scholar] [CrossRef]
- Sinha, V.; Trehan, A. Biodegradable microspheres for protein delivery. J. Control. Release 2003, 90, 261–280. [Google Scholar] [CrossRef]
- Cai, Y.; Wei, L.; Ma, L.; Huang, X.; Tao, A.; Liu, Z.; Yuan, W. Long-acting preparations of exenatide. Drug Des. Dev. Ther. 2013, 7, 963. [Google Scholar]
- Garber, A.J. Novel GLP-1 receptor agonists for diabetes. Expert Opin. Investig. Drugs 2012, 21, 45–57. [Google Scholar] [CrossRef] [PubMed]
- Andersen, N.H.; Brodsky, Y.; Neidigh, J.W.; Prickett, K.S. Medium-dependence of the secondary structure of exendin-4 and glucagon-like-peptide-1. Bioorg. Med. Chem. 2002, 10, 79–85. [Google Scholar] [CrossRef]
- Yang, H.J.; Park, I.S.; Na, K. Biocompatible microspheres based on acetylated polysaccharide prepared from water-in-oil-in-water (W1/O/W2) double-emulsion method for delivery of type II diabetic drug (exenatide). Colloids Surf. A Physicochem. Eng. Asp. 2009, 340, 115–120. [Google Scholar] [CrossRef]
- Kwak, H.-H.; Shim, W.-S.; Hwang, S.; Son, M.-K.; Kim, Y.-J.; Kim, T.-H.; Yoon, Z.-H.; Youn, H.-J.; Lee, G.-I.; Kang, S.-H. Pharmacokinetics and efficacy of a biweekly dosage formulation of exenatide in Zucker diabetic fatty (ZDF) rats. Pharm. Res. 2009, 26, 2504–2512. [Google Scholar] [CrossRef]
- Kwak, H.-H.; Shim, W.-S.; Son, M.-K.; Kim, Y.-J.; Kim, T.-H.; Youn, H.-J.; Kang, S.-H.; Shim, C.-K. Efficacy of a new sustained-release microsphere formulation of exenatide, DA-3091, in Zucker diabetic fatty (ZDF) rats. Eur. J. Pharm. Sci. 2010, 40, 103–109. [Google Scholar] [CrossRef]
- Liu, B.; Dong, Q.; Wang, M.; Shi, L.; Wu, Y.; Yu, X.; Shi, Y.; Shan, Y.; Jiang, C.; Zhang, X. Preparation, characterization, and pharmacodynamics of exenatide-loaded poly (DL-lactic-co-glycolic acid) microspheres. Chem. Pharm. Bull. 2010, 58, 1474–1479. [Google Scholar] [CrossRef]
- DeYoung, M.B.; MacConell, L.; Sarin, V.; Trautmann, M.; Herbert, P. Encapsulation of exenatide in poly-(D, L-lactide-co-glycolide) microspheres produced an investigational long-acting once-weekly formulation for type 2 diabetes. Diabetes Technol. Ther. 2011, 13, 1145–1154. [Google Scholar] [CrossRef]
- Qi, F.; Wu, J.; Fan, Q.; He, F.; Tian, G.; Yang, T.; Ma, G.; Su, Z. Preparation of uniform-sized exenatide-loaded PLGA microspheres as long-effective release system with high encapsulation efficiency and bio-stability. Colloids Surf. B Biointerfaces 2013, 112, 492–498. [Google Scholar] [CrossRef]
- Liang, R.; Zhang, R.; Li, X.; Wang, A.; Chen, D.; Sun, K.; Liu, W.; Li, Y. Stability of exenatide in poly (d, l-lactide-co-glycolide) solutions: A simplified investigation on the peptide degradation by the polymer. Eur. J. Pharm. Sci. 2013, 50, 502–510. [Google Scholar] [CrossRef]
- Liang, R.; Li, X.; Shi, Y.; Wang, A.; Sun, K.; Liu, W.; Li, Y. Effect of water on exenatide acylation in poly (lactide-co-glycolide) microspheres. Int. J. Pharm. 2013, 454, 344–353. [Google Scholar] [CrossRef]
- Qi, F.; Wu, J.; Hao, D.; Yang, T.; Ren, Y.; Ma, G.; Su, Z. Comparative studies on the influences of primary emulsion preparation on properties of uniform-sized exenatide-loaded PLGA microspheres. Pharm. Res. 2014, 31, 1566–1574. [Google Scholar] [CrossRef] [PubMed]
- Qi, F.; Wu, J.; Yang, T.; Ma, G.; Su, Z. Mechanistic studies for monodisperse exenatide-loaded PLGA microspheres prepared by different methods based on SPG membrane emulsification. Acta Biomater. 2014, 10, 4247–4256. [Google Scholar] [CrossRef] [PubMed]
- Jang, H.J.; Thambi, T.; Sivasubramanian, M.; Byun, J.H.; Ahn, J.Y.; Chae, S.Y.; Jo, D.-G.; Jeong, J.H.; Lee, K.C.; Park, J.H. β-cyclodextrin-bearing glycol chitosan for long-acting formulation of an exenatide derivative. Macromol. Res. 2014, 22, 816–819. [Google Scholar] [CrossRef]
- Liang, R.; Li, X.; Zhang, R.; Shi, Y.; Wang, A.; Chen, D.; Sun, K.; Liu, W.; Li, Y. Acylation of exenatide by glycolic acid and its anti-diabetic activities in db/db mice. Pharm. Res. 2014, 31, 1958–1966. [Google Scholar] [CrossRef]
- Li, X.; Zhao, Z.; Li, L.; Zhou, T.; Lu, W. Pharmacokinetics, in vitro and in vivo correlation, and efficacy of exenatide microspheres in diabetic rats. Drug Deliv. 2015, 22, 86–93. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhong, Y.; Hu, M.; Xiang, N.; Fu, Y.; Gong, T.; Zhang, Z. In vitro and in vivo sustained release of exenatide from vesicular phospholipid gels for type II diabetes. Drug Dev. Ind. Pharm. 2016, 42, 1042–1049. [Google Scholar] [CrossRef]
- Sun, Y.; Wang, M.; Sun, B.; Li, F.; Liu, S.; Zhang, Y.; Zhou, Y.; Chen, Y.; Kong, W. An investigation into the gastrointestinal stability of exenatide in the presence of pure enzymes, everted intestinal rings and intestinal homogenates. Biol. Pharm. Bull. 2016, 39, 42–48. [Google Scholar] [CrossRef]
- Wang, Y.; Sun, T.; Zhang, Y.; Chaurasiya, B.; Huang, L.; Liu, X.; Tu, J.; Xiong, Y.; Sun, C. Exenatide loaded PLGA microspheres for long-acting antidiabetic therapy: Preparation, characterization, pharmacokinetics and pharmacodynamics. RSC Adv. 2016, 6, 37452–37462. [Google Scholar] [CrossRef]
- Lin, X.; Yang, H.; Su, L.; Yang, Z.; Tang, X. Effect of size on the in vitro/in vivo drug release and degradation of exenatide-loaded PLGA microspheres. J. Drug Deliv. Sci. Technol. 2018, 45, 346–356. [Google Scholar] [CrossRef]
- Ruan, S.; Gu, Y.; Liu, B.; Gao, H.; Hu, X.; Hao, H.; Jin, L.; Cai, T. Long-acting release microspheres containing novel GLP-1 analog as an antidiabetic system. Mol. Pharm. 2018, 15, 2857–2869. [Google Scholar] [CrossRef]
- Dong, N.; Zhu, C.; Jiang, J.; Huang, D.; Li, X.; Quan, G.; Liu, Y.; Tan, W.; Pan, X.; Wu, C. Development of composite PLGA microspheres containing exenatide-encapsulated lecithin nanoparticles for sustained drug release. Asian J. Pharm. Sci. 2019. [Google Scholar] [CrossRef]
- Kumat, T.; Samuel, D.; Jayaraman, G.; Srimathi, T.; Yu, C. The role of proline in the prevention of aggregation during protein folding in vitro. IUBMB Life 1998, 46, 509–517. [Google Scholar] [CrossRef] [PubMed]
- Sah, H. Stabilization of proteins against methylene chloride/water interface-induced denaturation and aggregation. J. Control. Release 1999, 58, 143–151. [Google Scholar] [CrossRef]
- Paillard-Giteau, A.; Tran, V.-T.; Thomas, O.; Garric, X.; Coudane, J.; Marchal, S.; Chourpa, I.; Benoît, J.-P.; Montero-Menei, C.; Venier-Julienne, M.-C. Effect of various additives and polymers on lysozyme release from PLGA microspheres prepared by an s/o/w emulsion technique. Eur. J. Pharm. Biopharm. 2010, 75, 128–136. [Google Scholar] [CrossRef]
- Rezwan, K.; Meier, L.P.; Gauckler, L.J. Lysozyme and bovine serum albumin adsorption on uncoated silica and AlOOH-coated silica particles: The influence of positively and negatively charged oxide surface coatings. Biomaterials 2005, 26, 4351–4357. [Google Scholar] [CrossRef]
- Shubhra, Q.T.; Tóth, J.; Gyenis, J.; Feczkó, T. Surface modification of HSA containing magnetic PLGA nanoparticles by poloxamer to decrease plasma protein adsorption. Colloids Surf. B Biointerfaces 2014, 122, 529–536. [Google Scholar] [CrossRef] [Green Version]
- Kumar Malik, D.; Baboota, S.; Ahuja, A.; Hasan, S.; Ali, J. Recent advances in protein and peptide drug delivery systems. Curr. Drug Deliv. 2007, 4, 141–151. [Google Scholar] [CrossRef]
- Arakawa, T.; Timasheff, S. The stabilization of proteins by osmolytes. Biophys. J. 1985, 47, 411–414. [Google Scholar] [CrossRef]
- Blase, E.; Taylor, K.; Gao, H.Y.; Wintle, M.; Fineman, M. Pharmacokinetics of an oral drug (acetaminophen) administered at various times in relation to subcutaneous injection of exenatide (exendin-4) in healthy subjects. J. Clin. Pharmacol. 2005, 45, 570–577. [Google Scholar] [CrossRef]
- Steiner, S.S. Amylin Formulations. Patent Number WO2008124522A2, 16 October 2008. [Google Scholar]
- Kang, F.; Jiang, G.; Hinderliter, A.; DeLuca, P.P.; Singh, J. Lysozyme stability in primary emulsion for PLGA microsphere preparation: Effect of recovery methods and stabilizing excipients. Pharm. Res. 2002, 19, 629–633. [Google Scholar] [CrossRef]
- Pérez-Rodriguez, C.; Montano, N.; Gonzalez, K.; Griebenow, K. Stabilization of α-chymotrypsin at the CH2Cl2/water interface and upon water-in-oil-in-water encapsulation in PLGA microspheres. J. Control. Release 2003, 89, 71–85. [Google Scholar] [CrossRef]
- Fu, K.; Klibanov, A.; Langer, R. Protein stability in controlled-release systems. Nat. Biotechnol. 2000, 18, 24. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.J.; McAuley, A.; Schilke, K.F.; McGuire, J. Molecular origins of surfactant-mediated stabilization of protein drugs. Adv. Drug Deliv. Rev. 2011, 63, 1160–1171. [Google Scholar] [CrossRef] [PubMed]
- Sah, H. Protein behavior at the water/methylene chloride interface. J. Pharm. Sci. 1999, 88, 1320–1325. [Google Scholar] [CrossRef] [PubMed]
- Van de Weert, M.; Hoechstetter, J.; Hennink, W.E.; Crommelin, D.J. The effect of a water/organic solvent interface on the structural stability of lysozyme. J. Control. Release 2000, 68, 351–359. [Google Scholar] [CrossRef]
- Cleland, J.L.; Jones, A.J. Stable formulations of recombinant human growth hormone and interferon-γ for microencapsulation in biodegradable mircospheres. Pharm. Res. 1996, 13, 1464–1475. [Google Scholar] [CrossRef]
- Carpenter, J.F.; Crowe, J.H. An infrared spectroscopic study of the interactions of carbohydrates with dried proteins. Biochemistry 1989, 28, 3916–3922. [Google Scholar] [CrossRef]
- Ohtake, S.; Kita, Y.; Arakawa, T. Interactions of formulation excipients with proteins in solution and in the dried state. Adv. Drug Deliv. Rev. 2011, 63, 1053–1073. [Google Scholar] [CrossRef]
- Skovgaard, M.; Kodra, J.T.; Gram, D.X.; Knudsen, S.M.; Madsen, D.; Liberles, D.A. Using Evolutionary Information and Ancestral Sequences to Understand the Sequence–Function Relationship in GLP-1 Agonists. J. Mol. Biol. 2006, 363, 977–988. [Google Scholar] [CrossRef]
- Calis, S.; Jeyanthi, R.; Tsai, T.; DeLuca, P.P.; Mehta, R.C. Adsorption of Salmon Calcitonin to PLGA Microspheres. Pharm. Res. 1995, 12, 1072–1076. [Google Scholar] [CrossRef]
- Rabe, M.; Verdes, D.; Seeger, S. Understanding protein adsorption phenomena at solid surfaces. Adv. Colloid Interface Sci. 2011, 162, 87–106. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yoo, J.W.; Mitragotri, S. Polymer particles that switch shape in response to a stimulus. Proc. Natl. Acad. Sci. USA 2010, 107, 11205–11210. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Balmert, S.C.; Zmolek, A.C.; Glowacki, A.J.; Knab, T.D.; Rothstein, S.N.; Wokpetah, J.M.; Fedorchak, M.V.; Little, S.R. Positive charge of “sticky” peptides and proteins impedes release from negatively charged PLGA matrices. J. Mater. Chem. B Mater. Biol. Med. 2015, 3, 4723–4734. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Makadia, H.K.; Siegel, S.J. Poly Lactic-co-Glycolic Acid (PLGA) as Biodegradable Controlled Drug Delivery Carrier. Polymers 2011, 3, 1377–1397. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Schwendeman, S.P. Minimizing acylation of peptides in PLGA microspheres. J. Control. Release 2012, 162, 119–126. [Google Scholar] [CrossRef] [Green Version]
- Sophocleous, A.M.; Zhang, Y.; Schwendeman, S.P. A new class of inhibitors of peptide sorption and acylation in PLGA. J. Control. Release 2009, 137, 179–184. [Google Scholar] [CrossRef] [Green Version]
- Fu, K.; Pack, D.W.; Klibanov, A.M.; Langer, R. Visual Evidence of Acidic Environment Within Degrading Poly(lactic-co-glycolic acid) (PLGA) Microspheres. Pharm. Res. 2000, 17, 100–106. [Google Scholar] [CrossRef]
- Sillero, A.; Ribeiro, J.M. Isoelectric points of proteins: Theoretical determination. Anal. Biochem. 1989, 179, 319–325. [Google Scholar] [CrossRef]
- Houchin, M.L.; Neuenswander, S.A.; Topp, E.M. Effect of excipients on PLGA film degradation and the stability of an incorporated peptide. J. Control. Release 2007, 117, 413–420. [Google Scholar] [CrossRef] [Green Version]
- Quaglia, F.; De Rosa, G.; Granata, E.; Ungaro, F.; Fattal, E.; Immacolata La Rotonda, M. Feeding liquid, non-ionic surfactant and cyclodextrin affect the properties of insulin-loaded poly(lactide-co-glycolide) microspheres prepared by spray-drying. J. Control. Release 2003, 86, 267–278. [Google Scholar] [CrossRef]
- Blanco, M.D.; Alonso, M.J. Development and characterization of protein-loaded poly(lactide-co-glycolide) nanospheres. Eur. J. Pharm. Biopharm. 1997, 43, 287–294. [Google Scholar] [CrossRef]
- Blanco, D.; Alonso, M.J. Protein encapsulation and release from poly(lactide-co-glycolide) microspheres: Effect of the protein and polymer properties and of the co-encapsulation of surfactants. Eur. J. Pharm. Biopharm. 1998, 45, 285–294. [Google Scholar] [CrossRef]
- Rosa, G.D.; Iommelli, R.; La Rotonda, M.I.; Miro, A.; Quaglia, F. Influence of the co-encapsulation of different non-ionic surfactants on the properties of PLGA insulin-loaded microspheres. J. Control. Release 2000, 69, 283–295. [Google Scholar] [CrossRef]
- Brunner, A.; Mäder, K.; Göpferich, A. pH and Osmotic Pressure Inside Biodegradable Microspheres during Erosion. Pharm. Res. 1999, 16, 847–853. [Google Scholar] [CrossRef]
- Bouissou, C.; Rouse, J.J.; Price, R.; van der Walle, C.F. The Influence of Surfactant on PLGA Microsphere Glass Transition and Water Sorption: Remodeling the Surface Morphology to Attenuate the Burst Release. Pharm. Res. 2006, 23, 1295–1305. [Google Scholar] [CrossRef]








| Formulation | Inner Water Phase (W1, pH 4.5) | Oil Phase (DCM) | Outer Aqueous Phase (W2) | |||||
|---|---|---|---|---|---|---|---|---|
| Exenatide (mg) | Additives | Volume (mL) | PLGA (mg) | Volume (mL) | PVA (%) | Lysine (M) | Volume (mL) | |
| ELPM1 | 10 | 0.1 | 186 | 2.5 | 1 | 25 | ||
| ELPM2 | 10 | Sucrose (4 mg) | 0.1 | 186 | 2.5 | 1 | 25 | |
| ELPM3 | 10 | Proline (0.1 M) | 0.1 | 186 | 2.5 | 1 | 25 | |
| ELPM4 | 10 | Phenylalanine (0.1 M) | 0.1 | 186 | 2.5 | 1 | 25 | |
| ELPM5 | 10 | 0.1 | 186 | 2.5 | 1 | 0.1 | 25 | |
| ELPM6 | 10 | Sucrose (4 mg) Proline (0.1 M) | 0.1 | 186 | 2.5 | 1 | 0.1 | 25 |
| ELPM7 | 10 | Sucrose (4 mg) Poloxamer188 (4 mg) Proline (0.1 M) | 0.1 | 186 | 2.5 | 1 | 0.1 | 25 |
| Additive | Recovery (%) ± SD (n = 3) | Adsorption (%) to PLGA | |||||
|---|---|---|---|---|---|---|---|
| Type | Name | Added Phase | Solution (pH 4.5) | W/O | Freeze-Thawing | Freeze-Drying | |
| Control | - | - | 34.2 ± 1.7 | 59.4 ± 3.1 | 74.4 ± 3.4 | 60.4 ± 3.9 | 26.8 ± 2.6 |
| Hydrophilic | Sucrose | Water | 64.1 ± 4.3 | 62.1 ± 4.1 | 94.2 ± 4.8 | 92.1 ± 2.8 | 20.3 ± 1.7 |
| Proline | Water | 55.8 ± 3.1 | 69.8 ± 2.6 | 90.8 ± 6.1 | 75.3 ± 4.6 | 5.1 ± 0.6 | |
| Lysine | Water | 49.2 ± 1.3 | 66.0 ± 3.0 | 87.8 ± 5.0 | 89.9 ± 4.6 | 9.1 ± 0.7 | |
| Phenylalanine | Water | 44.8 ± 3.6 | 99.2 ± 2.7 | 79.8 ± 2.9 | 74.3 ± 6.7 | 19.8 ± 1.9 | |
| Amphipathic | Poloxamer 188 | Water | 94.8 ± 5.6 | 70.7 ± 4.5 | 88.9 ± 4.6 | 64.3 ± 4.9 | 3.7 ± 0.4 |
| DCM | 58.3 ± 3.7 | ||||||
| PLGA | 26.5 ± 2.0 | ||||||
| DMPC | DCM | 70.9 ± 3.3 | |||||
| PLGA | 43.8 ± 3.4 | ||||||
| Formulation | VMD 1 (um) | Span 2 | LC 3 (%) | EE 4 (%) | IBR 5 (%) |
|---|---|---|---|---|---|
| ELPM1 | 36.8 ± 3.2 | 2.0 ± 0.3 | 1.54 ± 0.04 | 30.3 ± 0.8 | 23.4 ± 2.1 |
| ELPM2 | 38.8 ± 2.6 | 1.9 ± 0.1 | 2.26 ± 0.04 | 44.2 ± 0.7 | 27.7 ± 1.8 |
| ELPM3 | 33.4 ± 2.1 | 2.0 ± 0.1 | 2.36 ± 0.08 | 46.3 ± 1.5 | 29.2 ± 2.1 |
| ELPM4 | 34.9 ± 1.3 | 1.8 ± 0.1 | 2.68 ± 0.04 | 42.5 ± 0.8 | 21.0 ± 1.6 |
| ELPM5 | 23.5 ± 1.6 | 1.6 ± 0.0 | 2.62 ± 0.06 | 51.3 ± 1.3 | 19.2 ± 1.9 |
| ELPM6 | 23.6 ± 0.7 | 1.6 ± 0.0 | 3.14 ± 0.03 | 61.6 ± 0.6 | 13.3 ± 0.8 |
| ELPM7 | 17.9 ± 0.4 | 1.6 ± 0.0 | 2.44 ± 0.15 | 47.8 ± 2.9 | 36.2 ± 5.5 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, H.; Ha, D.-H.; Ha, E.-S.; Kim, J.-S.; Kim, M.-S.; Hwang, S.-J. Effect of Stabilizers on Encapsulation Efficiency and Release Behavior of Exenatide-Loaded PLGA Microsphere Prepared by the W/O/W Solvent Evaporation Method. Pharmaceutics 2019, 11, 627. https://doi.org/10.3390/pharmaceutics11120627
Park H, Ha D-H, Ha E-S, Kim J-S, Kim M-S, Hwang S-J. Effect of Stabilizers on Encapsulation Efficiency and Release Behavior of Exenatide-Loaded PLGA Microsphere Prepared by the W/O/W Solvent Evaporation Method. Pharmaceutics. 2019; 11(12):627. https://doi.org/10.3390/pharmaceutics11120627
Chicago/Turabian StylePark, Heejun, Dong-Hyun Ha, Eun-Sol Ha, Jeong-Soo Kim, Min-Soo Kim, and Sung-Joo Hwang. 2019. "Effect of Stabilizers on Encapsulation Efficiency and Release Behavior of Exenatide-Loaded PLGA Microsphere Prepared by the W/O/W Solvent Evaporation Method" Pharmaceutics 11, no. 12: 627. https://doi.org/10.3390/pharmaceutics11120627