Biomedical Applications of Electrospun Nanofibers: Drug and Nanoparticle Delivery
Abstract
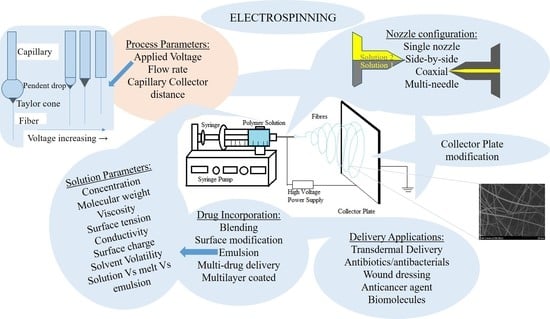
:1. Electrospinning and Its History
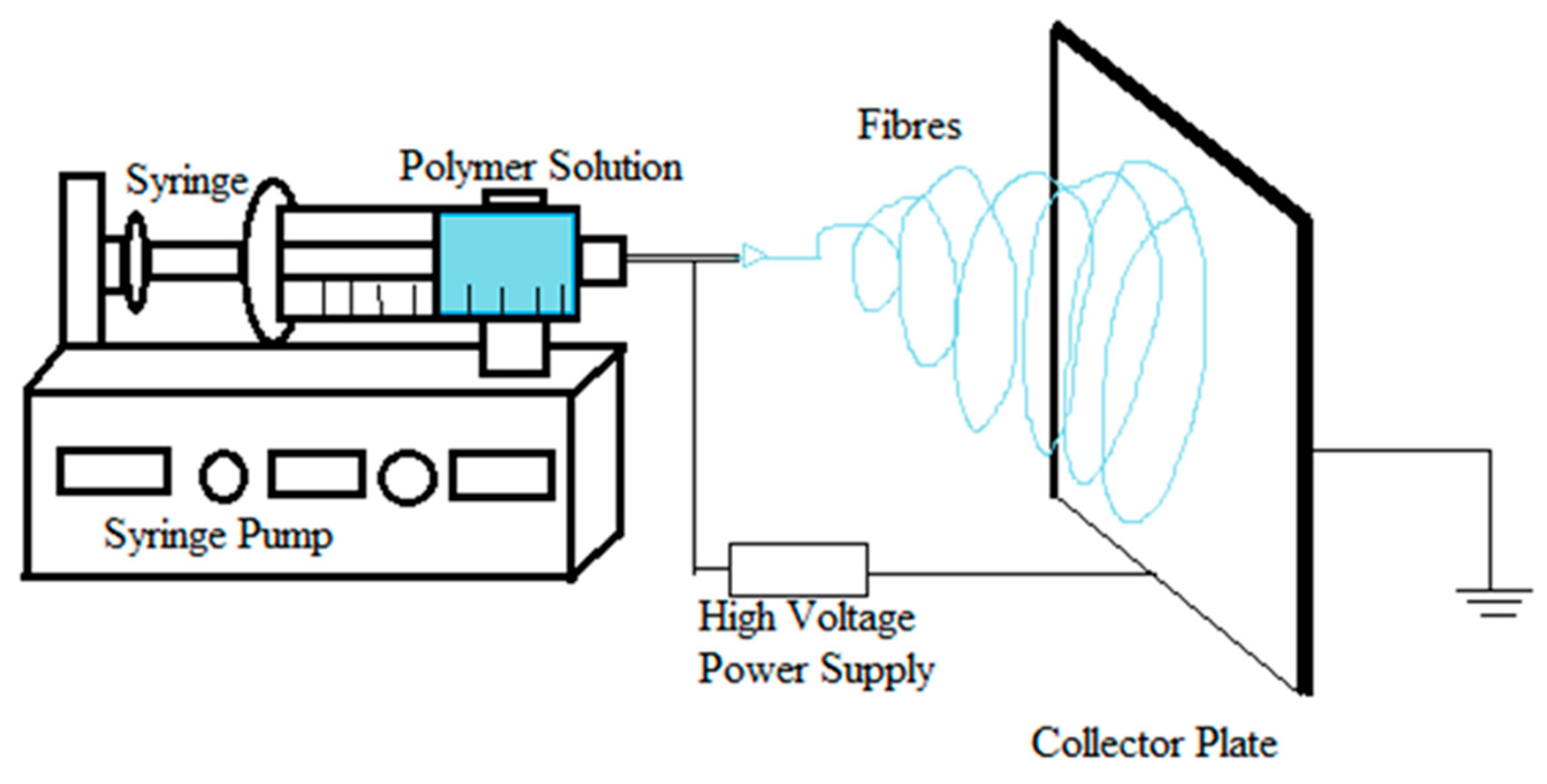
1.1. Process of Electrospinning
1.2. Physics of Electrospinning
2. Parameters of Electrospinning
2.1. Process-Related Parameters of Electrospinning
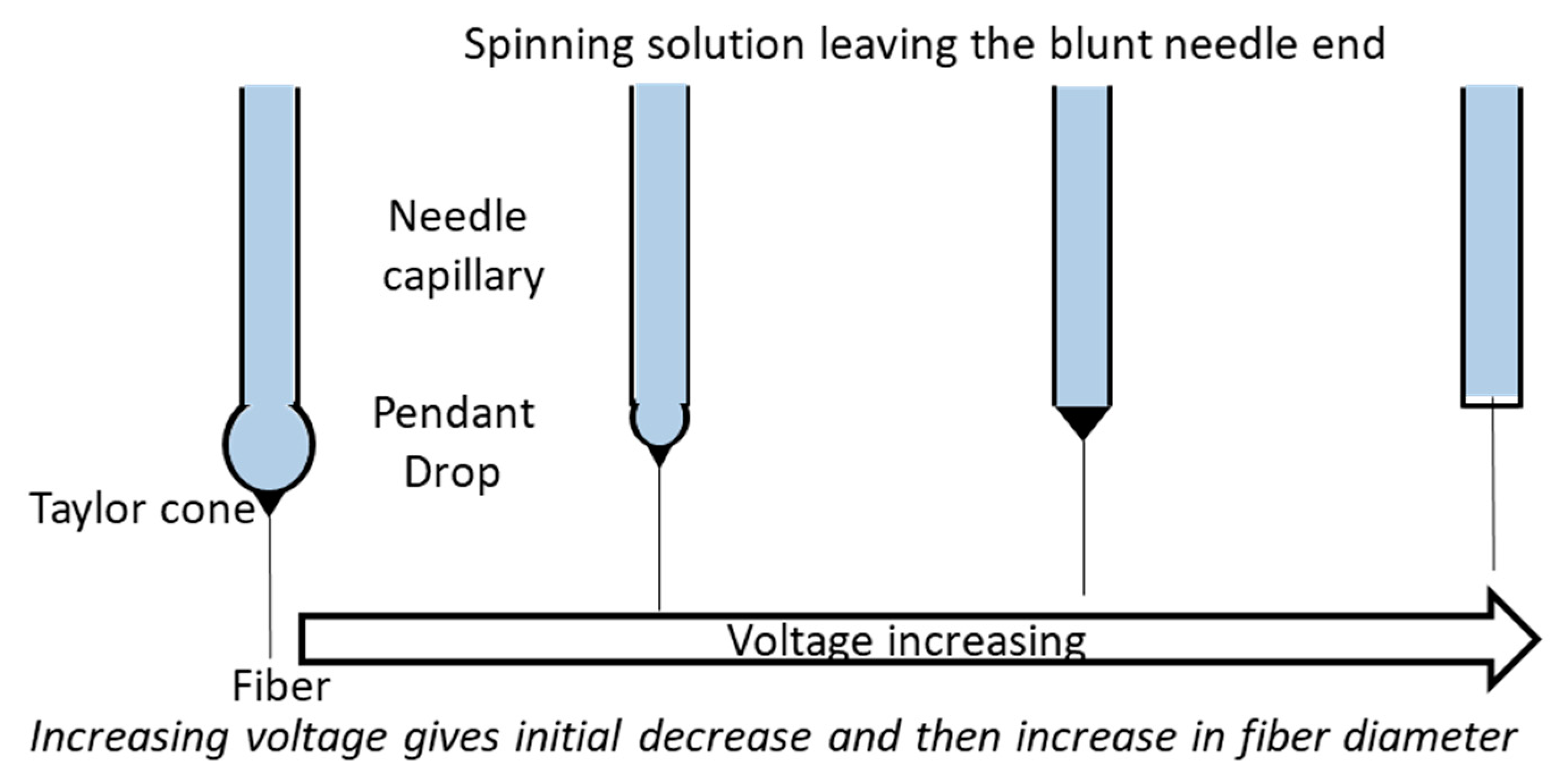
2.1.1. Applied Voltage
2.1.2. Flow Rate
2.1.3. Capillary–Collector Distance
2.2. Solution-Related Parameters of Electrospinning
2.2.1. Concentration of Solution
2.2.2. Molecular Weight
2.2.3. Solution Viscosity
2.2.4. Surface Tension of the Solution
2.2.5. Conductivity and Surface Charge Density
2.2.6. Solvent Volatility
3. Types of Electrospinning
3.1. Solution vs. Melt vs. Emulsion Electrospinning
3.2. Nozzle Configuration
3.3. Collector Modification
4. Methods of Incorporating Drugs
4.1. Blending
4.2. Surface Modification
4.3. Emulsion
4.4. Multi-Drug Delivery
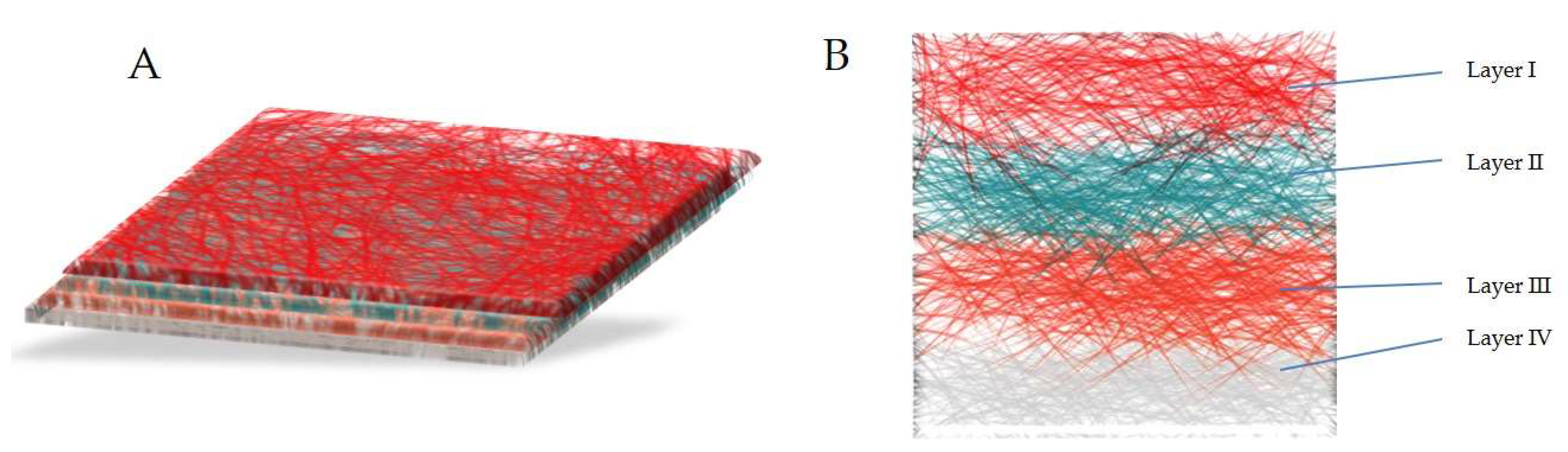
4.5. Multilayer Coated
5. Electrospun Nanofibers in Drug Delivery
5.1. Vitamins, NSAIDS and Natural Products
5.2. Antibiotics/Antibacterial Agents and Wound Dressing
5.3. Delivery of Anticancer Agents
5.4. DNA, RNA, Protein and Growth Factor Delivery
5.5. Nanoparticle Impregnated Nanofibers
6. Commercialization Challenges of Electrospinning
7. Conclusions
Funding
Conflicts of Interest
References
- Nayak, R.; Padhye, R.; Kyratzis, I.L.; Truong, Y.; Arnold, L. Recent advances in nanofibre fabrication techniques. Text. Res. J. 2011. [Google Scholar] [CrossRef]
- Greiner, A.; Wendorff, J.H. Electrospinning: A fascinating method for the preparation of ultrathin fibers. Angew. Chem. Int. Ed. 2007, 46, 5670–5703. [Google Scholar] [CrossRef] [PubMed]
- Reneker, D.H.; Yarin, A.L. Electrospinning jets and polymer nanofibers. Polymer 2008, 49, 2387–2425. [Google Scholar] [CrossRef]
- Kiyohiko, H. Process for Manufacturing Artificial Silk and Other Filaments by Applying Electric Current. U.S. Patents No. US1699615A, 22 January 1929. [Google Scholar]
- Huang, Z.-M.; Zhang, Y.-Z.; Kotaki, M.; Ramakrishna, S. A review on polymer nanofibers by electrospinning and their applications in nanocomposites. Compos. Sci. Technol. 2003, 63, 2223–2253. [Google Scholar] [CrossRef]
- Sill, T.J.; von Recum, H.A. Electrospinning: Applications in drug delivery and tissue engineering. Biomaterials 2008, 29, 1989–2006. [Google Scholar] [CrossRef] [PubMed]
- Taylor, G. Electrically driven jets. Proc. R. Soc. Lond. A Math. Phys. Eng. Sci. 1969, 313, 453–475. [Google Scholar] [CrossRef]
- Yarin, A.; Koombhongse, S.; Reneker, D.H. Bending instability in electrospinning of nanofibers. J. Appl. Phys. 2001, 89, 3018–3026. [Google Scholar] [CrossRef]
- Doshi, J.; Reneker, D.H. Electrospinning process and applications of electrospun fibers. J. Electrost. 1995, 35, 154–160. [Google Scholar] [CrossRef]
- Yang, Q.; Li, Z.; Hong, Y.; Zhao, Y.; Qiu, S.; Wang, C.; Wei, Y. Influence of solvents on the formation of ultrathin uniform poly(vinyl pyrrolidone) nanofibers with electrospinning. J. Polym. Sci. Part B Polym. Phys. 2004, 42, 3721–3726. [Google Scholar] [CrossRef]
- Shin, Y.; Hohman, M.; Brenner, M.; Rutledge, G. Electrospinning: A whipping fluid jet generates submicron polymer fibers. Appl. Phys. Lett. 2001, 78, 1149–1151. [Google Scholar] [CrossRef]
- Jaeger, R.; Bergshoef, M.M.; Batlle, C.M.I.; Schönherr, H.; Julius Vancso, G. Electrospinning of ultra-thin polymer fibers. Macromol. Symp. 1998, 127, 141–150. [Google Scholar] [CrossRef] [Green Version]
- Carson, R.; Hendricks, C.; Hogan, J.; Schneider, J. Factors influencing electrically sprayed liquids. AIAA J. 1964, 2, 1460–1461. [Google Scholar] [CrossRef]
- Buchko, C.J.; Chen, L.C.; Shen, Y.; Martin, D.C. Processing and microstructural characterization of porous biocompatible protein polymer thin films. Polymer 1999, 40, 7397–7407. [Google Scholar] [CrossRef]
- Deitzel, J.; Kleinmeyer, J.; Harris, D.; Tan, N.B. The effect of processing variables on the morphology of electrospun nanofibers and textiles. Polymer 2001, 42, 261–272. [Google Scholar] [CrossRef]
- Reneker, D.H.; Chun, I. Nanometre diameter fibres of polymer, produced by electrospinning. Nanotechnology 1996, 7, 216. [Google Scholar] [CrossRef]
- Zhang, C.; Yuan, X.; Wu, L.; Han, Y.; Sheng, J. Study on morphology of electrospun poly(vinyl alcohol) mats. Eur. Polym. J. 2005, 41, 423–432. [Google Scholar] [CrossRef]
- Li, Z.; Wang, C. Effects of working parameters on electrospinning. In One-Dimensional Nanostructures; Springer: Berlin, Germany, 2013; pp. 15–28. [Google Scholar]
- Megelski, S.; Stephens, J.S.; Chase, D.B.; Rabolt, J.F. Micro-and nanostructured surface morphology on electrospun polymer fibers. Macromolecules 2002, 35, 8456–8466. [Google Scholar] [CrossRef]
- Kilic, A.; Oruc, F.; Demir, A. Effects of polarity on electrospinning process. Text. Res. J. 2008, 78, 532–539. [Google Scholar] [CrossRef]
- Varesano, A.; Carletto, R.A.; Mazzuchetti, G. Experimental investigations on the multi-jet electrospinning process. J. Mater. Process. Technol. 2009, 209, 5178–5185. [Google Scholar] [CrossRef]
- Zargham, S.; Bazgir, S.; Tavakoli, A.; Rashidi, A.S.; Damerchely, R. The effect of flow rate on morphology and deposition area of electrospun nylon 6 nanofiber. J. Eng. Fibers Fabr. 2012, 7, 42–49. [Google Scholar]
- Bhardwaj, N.; Kundu, S.C. Electrospinning: A fascinating fiber fabrication technique. Biotechnol. Adv. 2010, 28, 325–347. [Google Scholar] [CrossRef] [PubMed]
- Nurwaha, D.; Han, W.; Wang, X. Investigation of a new needleless electrospinning method for the production of nanofibers. J. Eng. Fibers Fabr. 2013, 8, 42–49. [Google Scholar]
- Sukigara, S.; Gandhi, M.; Ayutsede, J.; Micklus, M.; Ko, F. Regeneration of Bombyx mori silk by electrospinning—Part 1: Processing parameters and geometric properties. Polymer 2003, 44, 5721–5727. [Google Scholar] [CrossRef]
- Fong, H.; Chun, I.; Reneker, D. Beaded nanofibers formed during electrospinning. Polymer 1999, 40, 4585–4592. [Google Scholar] [CrossRef]
- Baumgarten, P.K. Electrostatic spinning of acrylic microfibers. J. Colloid Interface Sci. 1971, 36, 71–79. [Google Scholar] [CrossRef]
- Meechaisue, C.; Dubin, R.; Supaphol, P.; Hoven, V.P.; Kohn, J. Electrospun mat of tyrosine-derived polycarbonate fibers for potential use as tissue scaffolding material. J. Biomater. Sci. Polym. Ed. 2006, 17, 1039–1056. [Google Scholar] [CrossRef] [PubMed]
- Koski, A.; Yim, K.; Shivkumar, S. Effect of molecular weight on fibrous PVA produced by electrospinning. Mater. Lett. 2004, 58, 493–497. [Google Scholar] [CrossRef]
- Zhao, Y.; Yang, Q.; Lu, X.-F.; Wang, C.; Wei, Y. Study on correlation of morphology of electrospun products of polyacrylamide with ultrahigh molecular weight. J. Polym. Sci. Part B Polym. Phys. 2005, 43, 2190–2195. [Google Scholar] [CrossRef]
- Lyons, J.; Li, C.; Ko, F. Melt-electrospinning part I: Processing parameters and geometric properties. Polymer 2004, 45, 7597–7603. [Google Scholar] [CrossRef]
- Larrondo, L.; St John Manley, R. Electrostatic fiber spinning from polymer melts. I. Experimental observations on fiber formation and properties. J. Polym. Sci. Polym. Phys. Ed. 1981, 19, 909–920. [Google Scholar] [CrossRef]
- Ding, B.; Kim, H.Y.; Lee, S.C.; Shao, C.L.; Lee, D.R.; Park, S.J.; Kwag, G.B.; Choi, K.J. Preparation and characterization of a nanoscale poly(vinyl alcohol) fiber aggregate produced by an electrospinning method. J. Polym. Sci. Part B Polym. Phys. 2002, 40, 1261–1268. [Google Scholar] [CrossRef]
- Inai, R.; Kotaki, M.; Ramakrishna, S. Structure and properties of electrospun PLLA single nanofibres. Nanotechnology 2005, 16, 208. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.-H.; Jeong, L.; Park, H.-N.; Shin, S.-Y.; Park, W.-H.; Lee, S.-C.; Kim, T.-I.; Park, Y.-J.; Seol, Y.-J.; Lee, Y.-M. Biological efficacy of silk fibroin nanofiber membranes for guided bone regeneration. J. Biotechnol. 2005, 120, 327–339. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.S.; Choi, K.H.; Ghim, H.D.; Kim, S.S.; Chun, D.H.; Kim, H.Y.; Lyoo, W.S. Role of molecular weight of atactic poly(vinyl alcohol)(PVA) in the structure and properties of PVA nanofabric prepared by electrospinning. J. Appl. Polym. Sci. 2004, 93, 1638–1646. [Google Scholar] [CrossRef]
- Pillay, V.; Dott, C.; Choonara, Y.E.; Tyagi, C.; Tomar, L.; Kumar, P.; du Toit, L.C.; Ndesendo, V.M. A review of the effect of processing variables on the fabrication of electrospun nanofibers for drug delivery applications. J. Nanomater. 2013, 2013. [Google Scholar] [CrossRef]
- Zong, X.; Kim, K.; Fang, D.; Ran, S.; Hsiao, B.S.; Chu, B. Structure and process relationship of electrospun bioabsorbable nanofiber membranes. Polymer 2002, 43, 4403–4412. [Google Scholar] [CrossRef]
- Hayati, I.; Bailey, A.; Tadros, T.F. Investigations into the mechanisms of electrohydrodynamic spraying of liquids: I. Effect of electric field and the environment on pendant drops and factors affecting the formation of stable jets and atomization. J. Colloid Interface Sci. 1987, 117, 205–221. [Google Scholar] [CrossRef]
- Huang, C.; Chen, S.; Lai, C.; Reneker, D.H.; Qiu, H.; Ye, Y.; Hou, H. Electrospun polymer nanofibres with small diameters. Nanotechnology 2006, 17, 1558. [Google Scholar] [CrossRef] [PubMed]
- Gupta, B.; Revagade, N.; Hilborn, J. Poly(lactic acid) fiber: An overview. Prog. Polym. Sci. 2007, 32, 455–482. [Google Scholar] [CrossRef]
- Cicero, J.A.; Dorgan, J.R.; Garrett, J.; Runt, J.; Lin, J. Effects of molecular architecture on two-step, melt-spun poly (lactic acid) fibers. J. Appl. Polym. Sci. 2002, 86, 2839–2846. [Google Scholar] [CrossRef]
- Kim, M.S.; Kim, J.C.; Kim, Y.H. Effects of take-up speed on the structure and properties of melt-spun poly(l-lactic acid) fibers. Polym. Adv. Technol. 2008, 19, 748–755. [Google Scholar] [CrossRef]
- Postema, A.; Luiten, A.; Pennings, A. High-strength poly(l-lactide) fibers by a dry-spinning/hot-drawing process. I. Influence of the ambient temperature on the dry-spinning process. J. Appl. Polym. Sci. 1990, 39, 1265–1274. [Google Scholar] [CrossRef]
- Persano, L.; Camposeo, A.; Tekmen, C.; Pisignano, D. Industrial upscaling of electrospinning and applications of polymer nanofibers: A review. Macromol. Mater. Eng. 2013, 298, 504–520. [Google Scholar] [CrossRef]
- Tan, E.; Ng, S.; Lim, C. Tensile testing of a single ultrafine polymeric fiber. Biomaterials 2005, 26, 1453–1456. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Shen, Z.; Lee, S.-H.; Marquez, M.; McHugh, M.A. Electrospinning in compressed carbon dioxide: Hollow or open-cell fiber formation with a single nozzle configuration. J. Supercrit. Fluids 2010, 53, 142–150. [Google Scholar] [CrossRef]
- Stitzel, J.; Liu, J.; Lee, S.J.; Komura, M.; Berry, J.; Soker, S.; Lim, G.; Van Dyke, M.; Czerw, R.; Yoo, J.J. Controlled fabrication of a biological vascular substitute. Biomaterials 2006, 27, 1088–1094. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Freitag, M.; Hone, J.; Staii, C.; Johnson, A., Jr.; Pinto, N.J.; MacDiarmid, A. Fabrication and electrical characterization of polyaniline-based nanofibers with diameter below 30 nm. Appl. Phys. Lett. 2003, 83, 3800–3802. [Google Scholar] [CrossRef]
- Agarwal, S.; Burgard, M.; Greiner, A.; Wendorff, J. Electrospinning: A Practical Guide to Nanofibers; Walter de Gruyter GmbH & Co KG: Berlin, Germany, 2016. [Google Scholar]
- Gupta, P.; Wilkes, G.L. Some investigations on the fiber formation by utilizing a side-by-side bicomponent electrospinning approach. Polymer 2003, 44, 6353–6359. [Google Scholar] [CrossRef]
- Townsend-Nicholson, A.; Jayasinghe, S.N. Cell electrospinning: A unique biotechnique for encapsulating living organisms for generating active biological microthreads/scaffolds. Biomacromolecules 2006, 7, 3364–3369. [Google Scholar] [CrossRef] [PubMed]
- Ravichandran, R.; Venugopal, J.R.; Sundarrajan, S.; Mukherjee, S.; Sridhar, R.; Ramakrishna, S. Expression of cardiac proteins in neonatal cardiomyocytes on PGS/fibrinogen core/shell substrate for Cardiac tissue engineering. Int. J. Cardiol. 2013, 167, 1461–1468. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Yuan, Y.; Huang, X.; Yue, T. Controlled release of protein from core–shell nanofibers prepared by emulsion electrospinning based on green chemical. J. Appl. Polym. Sci. 2015, 132. [Google Scholar] [CrossRef]
- Su, Y.; Su, Q.; Liu, W.; Lim, M.; Venugopal, J.R.; Mo, X.; Ramakrishna, S.; Al-Deyab, S.S.; El-Newehy, M. Controlled release of bone morphogenetic protein 2 and dexamethasone loaded in core–shell PLLACL–collagen fibers for use in bone tissue engineering. Acta Biomater. 2012, 8, 763–771. [Google Scholar] [CrossRef] [PubMed]
- Rubert, M.; Dehli, J.; Li, Y.-F.; Taskin, M.B.; Xu, R.; Besenbacher, F.; Chen, M. Electrospun PCL/PEO coaxial fibers for basic fibroblast growth factor delivery. J. Mater. Chem. B 2014, 2, 8538–8546. [Google Scholar] [CrossRef]
- Liao, I.; Chew, S.; Leong, K. Aligned core–shell nanofibers delivering bioactive proteins. Nanomedicine 2006, 1, 465–471. [Google Scholar] [CrossRef] [PubMed]
- Tian, L.; Prabhakaran, M.P.; Ding, X.; Kai, D.; Ramakrishna, S. Emulsion electrospun vascular endothelial growth factor encapsulated poly(l-lactic acid-co-ε-caprolactone) nanofibers for sustained release in cardiac tissue engineering. J. Mater. Sci. 2012, 47, 3272–3281. [Google Scholar] [CrossRef]
- Choi, J.S.; Choi, S.H.; Yoo, H.S. Coaxial electrospun nanofibers for treatment of diabetic ulcers with binary release of multiple growth factors. J. Mater. Chem. 2011, 21, 5258–5267. [Google Scholar] [CrossRef]
- Liao, I.-C.; Chen, S.; Liu, J.B.; Leong, K.W. Sustained viral gene delivery through core-shell fibers. J. Control. Release 2009, 139, 48–55. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, C.; Yan, K.-W.; Lin, Y.-D.; Hsieh, P.C. Biodegradable core/shell fibers by coaxial electrospinning: Processing, fiber characterization, and its application in sustained drug release. Macromolecules 2010, 43, 6389–6397. [Google Scholar] [CrossRef]
- Saraf, A.; Baggett, L.S.; Raphael, R.M.; Kasper, F.K.; Mikos, A.G. Regulated non-viral gene delivery from coaxial electrospun fiber mesh scaffolds. J. Control. Release 2010, 143, 95–103. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liao, I.-C.; Leong, K.W. Efficacy of engineered FVIII-producing skeletal muscle enhanced by growth factor-releasing co-axial electrospun fibers. Biomaterials 2011, 32, 1669–1677. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, W.; Zou, T.; Li, S.; Jing, J.; Xia, X.; Liu, X. Drug-loaded zein nanofibers prepared using a modified coaxial electrospinning process. AAPS PharmSciTech 2013, 14, 675–681. [Google Scholar] [CrossRef] [PubMed]
- Zhu, T.; Chen, S.; Li, W.; Lou, J.; Wang, J. Flurbiprofen axetil loaded coaxial electrospun poly (vinyl pyrrolidone)–nanopoly(lactic-co-glycolic acid) core–shell composite nanofibers: Preparation, characterization, and anti-adhesion activity. J. Appl. Polym. Sci. 2015, 132. [Google Scholar] [CrossRef]
- Ignatova, М.; Rashkov, I.; Manolova, N. Drug-loaded electrospun materials in wound-dressing applications and in local cancer treatment. Expert Opin. Drug Deliv. 2013, 10, 469–483. [Google Scholar] [CrossRef] [PubMed]
- Ding, B.; Kimura, E.; Sato, T.; Fujita, S.; Shiratori, S. Fabrication of blend biodegradable nanofibrous nonwoven mats via multi-jet electrospinning. Polymer 2004, 45, 1895–1902. [Google Scholar] [CrossRef]
- Theron, S.; Yarin, A.; Zussman, E.; Kroll, E. Multiple jets in electrospinning: Experiment and modeling. Polymer 2005, 46, 2889–2899. [Google Scholar] [CrossRef]
- Teo, W.; Ramakrishna, S. Electrospun fibre bundle made of aligned nanofibres over two fixed points. Nanotechnology 2005, 16, 1878. [Google Scholar] [CrossRef]
- Kim, G.; Cho, Y.-S.; Kim, W.D. Stability analysis for multi-jets electrospinning process modified with a cylindrical electrode. Eur. Polym. J. 2006, 42, 2031–2038. [Google Scholar] [CrossRef]
- Hong, Y.; Fujimoto, K.; Hashizume, R.; Guan, J.; Stankus, J.J.; Tobita, K.; Wagner, W.R. Generating elastic, biodegradable polyurethane/poly(lactide-co-glycolide) fibrous sheets with controlled antibiotic release via two-stream electrospinning. Biomacromolecules 2008, 9, 1200–1207. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Um, I.C.; Fang, D.; Okamoto, A.; Hsiao, B.S.; Chu, B. Formation of water-resistant hyaluronic acid nanofibers by blowing-assisted electro-spinning and non-toxic post treatments. Polymer 2005, 46, 4853–4867. [Google Scholar] [CrossRef]
- Sundaray, B.; Subramanian, V.; Natarajan, T.; Xiang, R.-Z.; Chang, C.-C.; Fann, W.-S. Electrospinning of continuous aligned polymer fibers. Appl. Phys. Lett. 2004, 84, 1222–1224. [Google Scholar] [CrossRef]
- Li, D.; Wang, Y.; Xia, Y. Electrospinning nanofibers as uniaxially aligned arrays and layer-by-layer stacked films. Adv. Mater. 2004, 16, 361–366. [Google Scholar] [CrossRef]
- Ki, C.S.; Kim, J.W.; Hyun, J.H.; Lee, K.H.; Hattori, M.; Rah, D.K.; Park, Y.H. Electrospun three-dimensional silk fibroin nanofibrous scaffold. J. Appl. Polym. Sci. 2007, 106, 3922–3928. [Google Scholar] [CrossRef]
- Smit, E.; Bűttner, U.; Sanderson, R.D. Continuous yarns from electrospun fibers. Polymer 2005, 46, 2419–2423. [Google Scholar] [CrossRef]
- Xu, C.; Inai, R.; Kotaki, M.; Ramakrishna, S. Aligned biodegradable nanofibrous structure: A potential scaffold for blood vessel engineering. Biomaterials 2004, 25, 877–886. [Google Scholar] [CrossRef]
- Bazilevsky, A.V.; Yarin, A.L.; Megaridis, C.M. Co-electrospinning of core-shell fibers using a single-nozzle technique. Langmuir 2007, 23, 2311–2314. [Google Scholar] [CrossRef] [PubMed]
- Bhattarai, N.; Edmondson, D.; Veiseh, O.; Matsen, F.A.; Zhang, M. Electrospun chitosan-based nanofibers and their cellular compatibility. Biomaterials 2005, 26, 6176–6184. [Google Scholar] [CrossRef] [PubMed]
- Theron, A.; Zussman, E.; Yarin, A. Electrostatic field-assisted alignment of electrospun nanofibres. Nanotechnology 2001, 12, 384. [Google Scholar] [CrossRef]
- Zeng, J.; Yang, L.; Liang, Q.; Zhang, X.; Guan, H.; Xu, X.; Chen, X.; Jing, X. Influence of the drug compatibility with polymer solution on the release kinetics of electrospun fiber formulation. J. Control. Release 2005, 105, 43–51. [Google Scholar] [CrossRef] [PubMed]
- Meng, Z.; Xu, X.; Zheng, W.; Zhou, H.; Li, L.; Zheng, Y.; Lou, X. Preparation and characterization of electrospun PLGA/gelatin nanofibers as a potential drug delivery system. Colloids Surf. B Biointerfaces 2011, 84, 97–102. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.; Luu, Y.K.; Chang, C.; Fang, D.; Hsiao, B.S.; Chu, B.; Hadjiargyrou, M. Incorporation and controlled release of a hydrophilic antibiotic using poly(lactide-co-glycolide)-based electrospun nanofibrous scaffolds. J. Control. Release 2004, 98, 47–56. [Google Scholar] [CrossRef] [PubMed]
- Jannesari, M.; Varshosaz, J.; Morshed, M.; Zamani, M. Composite poly (vinyl alcohol)/poly (vinyl acetate) electrospun nanofibrous mats as a novel wound dressing matrix for controlled release of drugs. Int. J. Nanomed. 2011, 6, 993–1003. [Google Scholar]
- Nair, R.; Arunkumar, K.; Vishnu Priya, K.; Sevukarajan, M. Recent advances in solid lipid nanoparticle based drug delivery systems. J. Biomed. Sci. Res. 2011, 3, 368–384. [Google Scholar]
- Mickova, A.; Buzgo, M.; Benada, O.; Rampichova, M.; Fisar, Z.; Filova, E.; Tesarova, M.; Lukas, D.; Amler, E. Core/shell nanofibers with embedded liposomes as a drug delivery system. Biomacromolecules 2012, 13, 952–962. [Google Scholar] [CrossRef] [PubMed]
- Volpato, F.Z.; Almodóvar, J.; Erickson, K.; Popat, K.C.; Migliaresi, C.; Kipper, M.J. Preservation of FGF-2 bioactivity using heparin-based nanoparticles, and their delivery from electrospun chitosan fibers. Acta Biomater. 2012, 8, 1551–1559. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.S.; Yoo, H.S. MMPs-responsive release of DNA from electrospun nanofibrous matrix for local gene therapy: In vitro and in vivo evaluation. J. Control. Release 2010, 145, 264–271. [Google Scholar] [CrossRef] [PubMed]
- Mottaghitalab, F.; Farokhi, M.; Mottaghitalab, V.; Ziabari, M.; Divsalar, A.; Shokrgozar, M.A. Enhancement of neural cell lines proliferation using nano-structured chitosan/poly(vinyl alcohol) scaffolds conjugated with nerve growth factor. Carbohydr. Polym. 2011, 86, 526–535. [Google Scholar] [CrossRef]
- Choi, J.S.; Leong, K.W.; Yoo, H.S. In vivo wound healing of diabetic ulcers using electrospun nanofibers immobilized with human epidermal growth factor (EGF). Biomaterials 2008, 29, 587–596. [Google Scholar] [CrossRef] [PubMed]
- Im, J.S.; Yun, J.; Lim, Y.-M.; Kim, H.-I.; Lee, Y.-S. Fluorination of electrospun hydrogel fibers for a controlled release drug delivery system. Acta Biomater. 2010, 6, 102–109. [Google Scholar] [CrossRef] [PubMed]
- Yun, J.; Im, J.S.; Lee, Y.-S.; Kim, H.-I. Electro-responsive transdermal drug delivery behavior of PVA/PAA/MWCNT nanofibers. Eur. Polym. J. 2011, 47, 1893–1902. [Google Scholar] [CrossRef]
- Kim, H.; Yoo, H. Matrix metalloproteinase-inspired suicidal treatments of diabetic ulcers with siRNA-decorated nanofibrous meshes. Gene Ther. 2013, 20, 378–385. [Google Scholar] [CrossRef] [PubMed]
- Luu, Y.; Kim, K.; Hsiao, B.; Chu, B.; Hadjiargyrou, M. Development of a nanostructured DNA delivery scaffold via electrospinning of PLGA and PLA–PEG block copolymers. J. Control. Release 2003, 89, 341–353. [Google Scholar] [CrossRef]
- Zamani, M.; Prabhakaran, M.P.; Ramakrishna, S. Advances in drug delivery via electrospun and electrosprayed nanomaterials. Int. J. Nanomed. 2013, 8, 2997–3017. [Google Scholar]
- Xu, X.; Yang, L.; Xu, X.; Wang, X.; Chen, X.; Liang, Q.; Zeng, J.; Jing, X. Ultrafine medicated fibers electrospun from W/O emulsions. J. Control. Release 2005, 108, 33–42. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Li, X.; Qi, M.; Zhou, S.; Weng, J. Release pattern and structural integrity of lysozyme encapsulated in core–sheath structured poly(dl-lactide) ultrafine fibers prepared by emulsion electrospinning. Eur. J. Pharm. Biopharm. 2008, 69, 106–116. [Google Scholar] [CrossRef] [PubMed]
- He, S.; Xia, T.; Wang, H.; Wei, L.; Luo, X.; Li, X. Multiple release of polyplexes of plasmids VEGF and bFGF from electrospun fibrous scaffolds towards regeneration of mature blood vessels. Acta Biomater. 2012, 8, 2659–2669. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Li, X.; Cheng, L.; He, S.; Zou, J.; Chen, F.; Zhang, Z. Core–sheath structured fibers with pDNA polyplex loadings for the optimal release profile and transfection efficiency as potential tissue engineering scaffolds. Acta Biomater. 2011, 7, 2533–2543. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Qiao, W.; Yin, T. A novel controlled release drug delivery system for multiple drugs based on electrospun nanofibers containing nanoparticles. J. Pharm. Sci. 2010, 99, 4805–4811. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Jiao, Y.; Shao, X.; Zhou, C. Controlled dual release of hydrophobic and hydrophilic drugs from electrospun poly(l-lactic acid) fiber mats loaded with chitosan microspheres. Mater. Lett. 2011, 65, 2800–2803. [Google Scholar] [CrossRef]
- Okuda, T.; Tominaga, K.; Kidoaki, S. Time-programmed dual release formulation by multilayered drug-loaded nanofiber meshes. J. Control. Release 2010, 143, 258–264. [Google Scholar] [CrossRef] [PubMed]
- Chunder, A.; Sarkar, S.; Yu, Y.; Zhai, L. Fabrication of ultrathin polyelectrolyte fibers and their controlled release properties. Colloids Surf. B Biointerfaces 2007, 58, 172–179. [Google Scholar] [CrossRef] [PubMed]
- Im, J.S.; Lee, S.K.; Bai, B.C.; Lee, Y.-S. Prediction and characterization of drug release in a multi-drug release system. J. Ind. Eng. Chem. 2012, 18, 325–330. [Google Scholar] [CrossRef]
- Prausnitz, M.R.; Langer, R. Transdermal drug delivery. Nat. Biotechnol. 2008, 26, 1261–1268. [Google Scholar] [CrossRef] [PubMed]
- Suwantong, O.; Ruktanonchai, U.; Supaphol, P. Electrospun cellulose acetate fiber mats containing asiaticoside or Centella asiatica crude extract and the release characteristics of asiaticoside. Polymer 2008, 49, 4239–4247. [Google Scholar] [CrossRef]
- Taepaiboon, P.; Rungsardthong, U.; Supaphol, P. Drug-loaded electrospun mats of poly (vinyl alcohol) fibres and their release characteristics of four model drugs. Nanotechnology 2006, 17, 2317. [Google Scholar] [CrossRef]
- Suwantong, O.; Opanasopit, P.; Ruktanonchai, U.; Supaphol, P. Electrospun cellulose acetate fiber mats containing curcumin and release characteristic of the herbal substance. Polymer 2007, 48, 7546–7557. [Google Scholar] [CrossRef]
- Im, J.S.; Bai, B.C.; Lee, Y.-S. The effect of carbon nanotubes on drug delivery in an electro-sensitive transdermal drug delivery system. Biomaterials 2010, 31, 1414–1419. [Google Scholar] [CrossRef] [PubMed]
- Taepaiboon, P.; Rungsardthong, U.; Supaphol, P. Vitamin-loaded electrospun cellulose acetate nanofiber mats as transdermal and dermal therapeutic agents of vitamin A acid and vitamin E. Eur. J. Pharm. Biopharm. 2007, 67, 387–397. [Google Scholar] [CrossRef] [PubMed]
- Ngawhirunpat, T.; Opanasopit, P.; Rojanarata, T.; Akkaramongkolporn, P.; Ruktanonchai, U.; Supaphol, P. Development of meloxicam-loaded electrospun polyvinyl alcohol mats as a transdermal therapeutic agent. Pharm. Dev. Technol. 2009, 14, 73–82. [Google Scholar] [CrossRef] [PubMed]
- Reda, R.I.; Wen, M.M.; El-Kamel, A.H. Ketoprofen-loaded eudragit electrospun nanofibers for the treatment of oral mucositis. Int. J. Nanomed. 2017, 12, 2335. [Google Scholar] [CrossRef] [PubMed]
- Kenawy, E.-R.; Bowlin, G.L.; Mansfield, K.; Layman, J.; Simpson, D.G.; Sanders, E.H.; Wnek, G.E. Release of tetracycline hydrochloride from electrospun poly(ethylene-co-vinylacetate), poly (lactic acid), and a blend. J. Control. Release 2002, 81, 57–64. [Google Scholar] [CrossRef]
- Alhusein, N.; Blagbrough, I.S.; De Bank, P.A. Electrospun matrices for localised controlled drug delivery: Release of tetracycline hydrochloride from layers of polycaprolactone and poly(ethylene-co-vinyl acetate). Drug Deliv. Transl. Res. 2012, 2, 477–488. [Google Scholar] [CrossRef] [PubMed]
- Alhusein, N.; De Bank, P.A.; Blagbrough, I.S.; Bolhuis, A. Killing bacteria within biofilms by sustained release of tetracycline from triple-layered electrospun micro/nanofibre matrices of polycaprolactone and poly (ethylene-co-vinyl acetate). Drug Deliv. Transl. Res. 2013, 3, 531–541. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Yue, T.; Lee, T.-C. Development of Pleurocidin-poly(vinyl alcohol) electrospun antimicrobial nanofibers to retain antimicrobial activity in food system application. Food Control 2015, 54, 150–157. [Google Scholar] [CrossRef]
- Zhang, Y.; Lim, C.T.; Ramakrishna, S.; Huang, Z.-M. Recent development of polymer nanofibers for biomedical and biotechnological applications. J. Mater. Sci. Mater. Med. 2005, 16, 933–946. [Google Scholar] [CrossRef] [PubMed]
- Boateng, J.S.; Matthews, K.H.; Stevens, H.N.; Eccleston, G.M. Wound healing dressings and drug delivery systems: A review. J. Pharm. Sci. 2008, 97, 2892–2923. [Google Scholar] [CrossRef] [PubMed]
- Said, S.S.; Aloufy, A.K.; El-Halfawy, O.M.; Boraei, N.A.; El-Khordagui, L.K. Antimicrobial PLGA ultrafine fibers: Interaction with wound bacteria. Eur. J. Pharm. Biopharm. 2011, 79, 108–118. [Google Scholar] [CrossRef] [PubMed]
- Thakur, R.; Florek, C.; Kohn, J.; Michniak, B. Electrospun nanofibrous polymeric scaffold with targeted drug release profiles for potential application as wound dressing. Int. J. Pharm. 2008, 364, 87–93. [Google Scholar] [CrossRef] [PubMed]
- Wang, A.; Xu, C.; Zhang, C.; Gan, Y.; Wang, B. Experimental investigation of the properties of electrospun nanofibers for potential medical application. J. Nanomater. 2015, 2015, 5. [Google Scholar] [CrossRef]
- GhavamiNejad, A.; Rajan Unnithan, A.; Ramachandra Kurup Sasikala, A.; Samarikhalaj, M.; Thomas, R.G.; Jeong, Y.Y.; Nasseri, S.; Murugesan, P.; Wu, D.; Hee Park, C. Mussel-inspired electrospun nanofibers functionalized with size-controlled silver nanoparticles for wound dressing application. ACS Appl. Mater. Interfaces 2015, 7, 12176–12183. [Google Scholar] [CrossRef] [PubMed]
- Chutipakdeevong, J.; Ruktanonchai, U.; Supaphol, P. Hybrid biomimetic electrospun fibrous mats derived from poly(ε-caprolactone) and silk fibroin protein for wound dressing application. J. Appl. Polym. Sci. 2015, 132, 41653. [Google Scholar] [CrossRef]
- Xu, X.; Chen, X.; Ma, P.; Wang, X.; Jing, X. The release behavior of doxorubicin hydrochloride from medicated fibers prepared by emulsion-electrospinning. Eur. J. Pharm. Biopharm. 2008, 70, 165–170. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Chen, X.; Wang, Z.; Jing, X. Ultrafine PEG–PLA fibers loaded with both paclitaxel and doxorubicin hydrochloride and their in vitro cytotoxicity. Eur. J. Pharm. Biopharm. 2009, 72, 18–25. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.; Tan, R.S.; Wang, C.H. Biodegradable microparticles and fiber fabrics for sustained delivery of cisplatin to treat C6 glioma in vitro. J. Biomed. Mater. Res. Part A 2008, 85, 897–908. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Zhou, G.; Liu, D.; Xie, Z.; Huang, Y.; Wang, X.; Wu, W.; Jing, X. Inhibition of orthotopic secondary hepatic carcinoma in mice by doxorubicin-loaded electrospun polylactide nanofibers. J. Mater. Chem. B 2013, 1, 101–109. [Google Scholar] [CrossRef]
- Luo, X.; Xie, C.; Wang, H.; Liu, C.; Yan, S.; Li, X. Antitumor activities of emulsion electrospun fibers with core loading of hydroxycamptothecin via intratumoral implantation. Int. J. Pharm. 2012, 425, 19–28. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S. Biocompatible microemulsion systems for drug encapsulation and delivery. Curr Sci 2011, 101, 174–188. [Google Scholar]
- Chen, P.; Wu, Q.-S.; Ding, Y.-P.; Chu, M.; Huang, Z.-M.; Hu, W. A controlled release system of titanocene dichloride by electrospun fiber and its antitumor activity in vitro. Eur. J. Pharm. Biopharm. 2010, 76, 413–420. [Google Scholar] [CrossRef] [PubMed]
- Shao, S.; Li, L.; Yang, G.; Li, J.; Luo, C.; Gong, T.; Zhou, S. Controlled green tea polyphenols release from electrospun PCL/MWCNTs composite nanofibers. Int. J. Pharm. 2011, 421, 310–320. [Google Scholar] [CrossRef] [PubMed]
- Zahedi, P.; Karami, Z.; Rezaeian, I.; Jafari, S.H.; Mahdaviani, P.; Abdolghaffari, A.H.; Abdollahi, M. Preparation and performance evaluation of tetracycline hydrochloride loaded wound dressing mats based on electrospun nanofibrous poly(lactic acid)/poly(ε-caprolactone) blends. J. Appl. Polym. Sci. 2012, 124, 4174–4183. [Google Scholar] [CrossRef]
- He, C.L.; Huang, Z.M.; Han, X.J.; Liu, L.; Zhang, H.S.; Chen, L.S. Coaxial electrospun poly(l-lactic acid) ultrafine fibers for sustained drug delivery. J. Macromol. Sci. Part B 2006, 45, 515–524. [Google Scholar] [CrossRef]
- He, C.L.; Huang, Z.M.; Han, X.J. Fabrication of drug-loaded electrospun aligned fibrous threads for suture applications. J. Biomed. Mater. Res. Part A 2009, 89, 80–95. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gilchrist, S.E.; Lange, D.; Letchford, K.; Bach, H.; Fazli, L.; Burt, H.M. Fusidic acid and rifampicin co-loaded PLGA nanofibers for the prevention of orthopedic implant associated infections. J. Control. Release 2013, 170, 64–73. [Google Scholar] [CrossRef] [PubMed]
- Zamani, M.; Morshed, M.; Varshosaz, J.; Jannesari, M. Controlled release of metronidazole benzoate from poly ε-caprolactone electrospun nanofibers for periodontal diseases. Eur. J. Pharm. Biopharm. 2010, 75, 179–185. [Google Scholar] [CrossRef] [PubMed]
- Toncheva, A.; Paneva, D.; Maximova, V.; Manolova, N.; Rashkov, I. Antibacterial fluoroquinolone antibiotic-containing fibrous materials from poly (l-lactide-co-d, l-lactide) prepared by electrospinning. Eur. J. Pharm. Sci. 2012, 47, 642–651. [Google Scholar] [CrossRef] [PubMed]
- Spasova, M.; Manolova, N.; Paneva, D.; Rashkov, I. Preparation of chitosan-containing nanofibres by electrospinning of chitosan/poly(ethylene oxide) blend solutions. e-Polymers 2004, 4, 624–635. [Google Scholar] [CrossRef]
- Verreck, G.; Chun, I.; Rosenblatt, J.; Peeters, J.; Van Dijck, A.; Mensch, J.; Noppe, M.; Brewster, M.E. Incorporation of drugs in an amorphous state into electrospun nanofibers composed of a water-insoluble, nonbiodegradable polymer. J. Control. Release 2003, 92, 349–360. [Google Scholar] [CrossRef]
- Huang, L.-Y.; Branford-White, C.; Shen, X.-X.; Yu, D.-G.; Zhu, L.-M. Time-engineeringed biphasic drug release by electrospun nanofiber meshes. Int. J. Pharm. 2012, 436, 88–96. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.-N.; Mo, H.-Y.; Yu, D.-G. Electrospun drug-loaded core–sheath PVP/zein nanofibers for biphasic drug release. Int. J. Pharm. 2012, 438, 232–239. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; Fang, D.; Hsiao, B.; Chu, B.; Chen, W. Preparation and characterization of ibuprofen-loaded poly(lactide-co-glycolide)/poly(ethylene glycol)-g-chitosan electrospun membranes. J. Biomater. Sci. Polym. Ed. 2004, 15, 279–296. [Google Scholar] [CrossRef] [PubMed]
- Lu, T.; Jing, X.; Song, X.; Wang, X. Doxorubicin-loaded ultrafine PEG-PLA fiber mats against hepatocarcinoma. J. Appl. Polym. Sci. 2012, 123, 209–217. [Google Scholar] [CrossRef]
- Ranganath, S.H.; Wang, C.-H. Biodegradable microfiber implants delivering paclitaxel for post-surgical chemotherapy against malignant glioma. Biomaterials 2008, 29, 2996–3003. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.; Wang, C.-H. Electrospun micro-and nanofibers for sustained delivery of paclitaxel to treat C6 glioma in vitro. Pharm. Res. 2006, 23, 1817–1826. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Liu, S.; Jing, X.; Li, X.; Li, W.; Huang, Y. Necrosis of cervical carcinoma by dichloroacetate released from electrospun polylactide mats. Biomaterials 2012, 33, 4362–4369. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Zhuang, X.; Chen, X.; Wang, X.; Yang, L.; Jing, X. Preparation of core-sheath composite nanofibers by emulsion electrospinning. Macromol. Rapid Commun. 2006, 27, 1637–1642. [Google Scholar] [CrossRef]
- Chew, S.Y.; Wen, J.; Yim, E.K.; Leong, K.W. Sustained release of proteins from electrospun biodegradable fibers. Biomacromolecules 2005, 6, 2017–2024. [Google Scholar] [CrossRef] [PubMed]
- Rujitanaroj, P.-o.; Wang, Y.-C.; Wang, J.; Chew, S.Y. Nanofiber-mediated controlled release of siRNA complexes for long term gene-silencing applications. Biomaterials 2011, 32, 5915–5923. [Google Scholar] [CrossRef] [PubMed]
- Cao, H.; Jiang, X.; Chai, C.; Chew, S.Y. RNA interference by nanofiber-based siRNA delivery system. J. Control. Release 2010, 144, 203–212. [Google Scholar] [CrossRef] [PubMed]
- Schneider, A.; Wang, X.; Kaplan, D.; Garlick, J.; Egles, C. Biofunctionalized electrospun silk mats as a topical bioactive dressing for accelerated wound healing. Acta Biomater. 2009, 5, 2570–2578. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, X.; Shi, Z.; Fu, W.; Liu, Z.; Fang, Z.; Lu, W.; Wang, Y.; Chen, F. In vitro biocompatibility study of electrospun copolymer ethylene carbonate-ɛ-caprolactone and vascular endothelial growth factor blended nanofibrous scaffolds. Appl. Surf. Sci. 2012, 258, 2301–2306. [Google Scholar] [CrossRef]
- Cho, Y.I.; Choi, J.S.; Jeong, S.Y.; Yoo, H.S. Nerve growth factor (NGF)-conjugated electrospun nanostructures with topographical cues for neuronal differentiation of mesenchymal stem cells. Acta Biomater. 2010, 6, 4725–4733. [Google Scholar] [CrossRef] [PubMed]
- Han, N.; Johnson, J.; Lannutti, J.J.; Winter, J.O. Hydrogel–electrospun fiber composite materials for hydrophilic protein release. J. Control. Release 2012, 158, 165–170. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Lv, Y. Immobilization and application of electrospun nanofiber scaffold-based growth factor in bone tissue engineering. Curr. Pharm. Des. 2015, 21, 1967–1978. [Google Scholar] [CrossRef] [PubMed]
- Ren, S.; Dong, L.; Zhang, X.; Lei, T.; Ehrenhauser, F.; Song, K.; Li, M.; Sun, X.; Wu, Q. Electrospun Nanofibers Made of Silver Nanoparticles, Cellulose Nanocrystals, and Polyacrylonitrile as Substrates for Surface-Enhanced Raman Scattering. Materials 2017, 10, 68. [Google Scholar] [CrossRef] [PubMed]
- Segala, K.; Nista, S.V.G.; Cordi, L.; Bizarria, M.T.M.; Ávila Júnior, J.D.; Kleinubing, S.A.; Cruz, D.C.; Brocchi, M.; Lona, L.M.F.; Caballero, N.E.D. Silver nanoparticles incorporated into nanostructured biopolymer membranes produced by electrospinning: A study of antimicrobial activity. Braz. J. Pharm. Sci. 2015, 51, 911–921. [Google Scholar] [CrossRef]
- Li, J.; Chen, X.; Ai, N.; Hao, J.; Chen, Q.; Strauf, S.; Shi, Y. Silver nanoparticle doped TiO2 nanofiber dye sensitized solar cells. Chem. Phys. Lett. 2011, 514, 141–145. [Google Scholar] [CrossRef]
- Cui, H.; Yuan, L.; Li, W.; Lin, L. Antioxidant property of SiO2-eugenol liposome loaded nanofibrous membranes on beef. Food Packag. Shelf Life 2017, 11, 49–57. [Google Scholar] [CrossRef]
- Yarin, A.L.; Pourdeyhimi, B.; Ramakrishna, S. Fundamentals and Applications of Micro-and Nanofibers; Cambridge University Press: Cambridge, UK, 2014. [Google Scholar]
- Zou, H.; Lv, P.F.; Wang, X.; Wu, D.; Yu, D.G. Electrospun poly(2-aminothiazole)/cellulose acetate fiber membrane for removing Hg (II) from water. J. Appl. Polym. Sci. 2017, 134. [Google Scholar] [CrossRef]
- Zhou, J.; Zhang, J.; Rehman, A.U.; Kan, K.; Li, L.; Shi, K. Synthesis, characterization, and ammonia gas sensing properties of Co3O4@ CuO nanochains. J. Mater Sci. 2016, 52, 1–14. [Google Scholar] [CrossRef]
- Homaeigohar, S.; Elbahri, M. Nanocomposite electrospun nanofiber membranes for environmental remediation. Materials 2014, 7, 1017–1045. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.J.; Heo, D.N.; Moon, J.-H.; Ko, W.-K.; Lee, J.B.; Bae, M.S.; Park, S.W.; Kim, J.E.; Lee, D.H.; Kim, E.-C. Electrospun chitosan nanofibers with controlled levels of silver nanoparticles. Preparation, characterization and antibacterial activity. Carbohydr. Polym. 2014, 111, 530–537. [Google Scholar] [CrossRef] [PubMed]
- Shi, Q.; Vitchuli, N.; Nowak, J.; Caldwell, J.M.; Breidt, F.; Bourham, M.; Zhang, X.; McCord, M. Durable antibacterial Ag/polyacrylonitrile (Ag/PAN) hybrid nanofibers prepared by atmospheric plasma treatment and electrospinning. Eur. Polym. J. 2011, 47, 1402–1409. [Google Scholar] [CrossRef]
- Castro-Mayorga, J.; Randazzo, W.; Fabra, M.; Lagaron, J.; Aznar, R.; Sánchez, G. Antiviral properties of silver nanoparticles against norovirus surrogates and their efficacy in coated polyhydroxyalkanoates systems. LWT Food Sci. Technol. 2017, 79, 503–510. [Google Scholar] [CrossRef] [Green Version]
- Nie, H.; Wang, C.-H. Fabrication and characterization of PLGA/HAp composite scaffolds for delivery of BMP-2 plasmid DNA. J. Control. Release 2007, 120, 111–121. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, K.; Shiga, T.; Katayama, T. Fabrication of Hydroxyapatite/PLA Composite Nanofiber by Electrospinning. WIT Trans. Built Environ. 2016, 166, 371–379. [Google Scholar]
- Chen, M.; Gao, S.; Dong, M.; Song, J.; Yang, C.; Howard, K.A.; Kjems, J.; Besenbacher, F. Chitosan/siRNA nanoparticles encapsulated in PLGA nanofibers for siRNA delivery. ACS Nano 2012, 6, 4835–4844. [Google Scholar] [CrossRef] [PubMed]
- Chew, S.Y.; Mi, R.; Hoke, A.; Leong, K.W. Aligned Protein–Polymer Composite Fibers Enhance Nerve Regeneration: A Potential Tissue-Engineering Platform. Adv. Funct. Mater. 2007, 17, 1288–1296. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kowalczyk, T.; Nowicka, A.; Elbaum, D.; Kowalewski, T.A. Electrospinning of bovine serum albumin. Optimization and the use for production of biosensors. Biomacromolecules 2008, 9, 2087–2090. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Su, Y.; Liu, S.; Tan, L.; Mo, X.; Ramakrishna, S. Encapsulation of proteins in poly (l-lactide-co-caprolactone) fibers by emulsion electrospinning. Colloids Surf. B Biointerfaces 2010, 75, 418–424. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Apkarian, R.P.; Chaikof, E.L. High-resolution analysis of engineered type I collagen nanofibers by electron microscopy. Scanning 2001, 23, 372–375. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Nagapudi, K.; Apkarian, R.P.; Chaikof, E.L. Engineered collagen–PEO nanofibers and fabrics. J. Biomater. Sci. Polym. Ed. 2001, 12, 979–993. [Google Scholar] [CrossRef] [PubMed]
- Zhang, E.; Zhu, C.; Yang, J.; Sun, H.; Zhang, X.; Li, S.; Wang, Y.; Sun, L.; Yao, F. Electrospun PDLLA/PLGA composite membranes for potential application in guided tissue regeneration. Mater. Sci. Eng. C 2016, 58, 278–285. [Google Scholar] [CrossRef] [PubMed]
- Sudakaran, S.V.; Venugopal, J.R.; Vijayakumar, G.P.; Abisegapriyan, S.; Grace, A.N.; Ramakrishna, S. Sequel of MgO nanoparticles in PLACL nanofibers for anti-cancer therapy in synergy with curcumin/β-cyclodextrin. Mater. Sci. Eng. C 2017, 71, 620–628. [Google Scholar] [CrossRef] [PubMed]
- Khalil, I.S.; Abdel-Kader, R.M.; Gomaa, I.E.; Serag, N.; Klingner, A.; Elwi, M. Targeting of Cancer Cells using Controlled Nanoparticles and Robotics Sperms. Int. J. Adv. Robot. Syst. 2016, 13, 123. [Google Scholar] [CrossRef]
- Sezer, U.A.; Ozturk, K.; Aru, B.; Demirel, G.Y.; Sezer, S.; Bozkurt, M.R. Zero valent zinc nanoparticles promote neuroglial cell proliferation: A biodegradable and conductive filler candidate for nerve regeneration. J. Mater. Sci. Mater. Med. 2017, 28, 19. [Google Scholar] [CrossRef] [PubMed]
- Kuchler-Bopp, S.; Larrea, A.; Petry, L.; Idoux-Gillet, Y.; Sebastian, V.; Ferrandon, A.; Schwinté, P.; Arruebo, M.; Benkirane-Jessel, N. Promoting Bioengineered Tooth Innervation Using Nanostructured and Hybrid Scaffolds. Acta Biomater. 2017, 50, 493–501. [Google Scholar] [CrossRef] [PubMed]
- Teo, W.-E. Addressing the Needs of Nanofiber Mass Production (Limitation of Nozzle System). Available online: http://electrospintech.com/mpchallenge.html#.WK2nq28rLIX (accessed on 27 October 2018).
- Li, L.; Frey, M.W.; Green, T.B. Modification of air filter media with nylon-6 nanofibers. J. Eng. Fibers Fabr. 2006, 1, 1–22. [Google Scholar]
- Moon, S.; Gil, M.; Lee, K.J. Syringeless Electrospinning toward Versatile Fabrication of Nanofiber Web. Sci. Rep. 2017, 7, 41424. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, W.; Huang, C.; Jin, X. Electrospinning of grooved polystyrene fibers: Effect of solvent systems. Nanoscale Res. Lett. 2015, 10, 237. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Yan, X.; He, H.-W.; Yu, M.; Ning, X.; Long, Y.-Z. Solvent-free electrospinning: Opportunities and challenges. Polym. Chem. 2017, 8, 333–352. [Google Scholar] [CrossRef]
- Fakirov, S. Nano-Size Polymers: Preparation, Properties, Applications; Springer: Berlin, Germany, 2016. [Google Scholar]
- Niu, H.; Lin, T. Fiber generators in needleless electrospinning. J. Nanomater. 2012, 12. [Google Scholar] [CrossRef]
- Niu, H.; Lin, T.; Wang, X. Needleless electrospinning. I. A comparison of cylinder and disk nozzles. J. Appl. Polym. Sci. 2009, 114, 3524–3530. [Google Scholar] [CrossRef]
- Cengiz, F.; Dao, T.A.; Jirsak, O. Influence of solution properties on the roller electrospinning of poly(vinyl alcohol). Polym. Eng. Sci. 2010, 50, 936–943. [Google Scholar] [CrossRef]
- Niu, H.; Wang, X.; Lin, T. Needleless electrospinning: Influences of fibre generator geometry. J. Text. Inst. 2012, 103, 787–794. [Google Scholar] [CrossRef]
- Liu, Y.; He, J. Bubble electrospinning for mass production of nanofibers. Int. J. Nonlinear Sci. Numer. Simul. 2007, 8, 393. [Google Scholar] [CrossRef]
- Weng, L.; Xie, J. Smart electrospun nanofibers for controlled drug release: Recent advances and new perspectives. Curr. Pharm. Des. 2015, 21, 1944–1959. [Google Scholar] [CrossRef] [PubMed]


| Drug(s) | Polymer(s) | Solvent Composition | Spraying Type |
|---|---|---|---|
| Antibacterial agents | |||
| Tetracycline hydrochloride | PEUU and PLGA [71]; PLA, PEV, PLA/PEVA [113]; PLA/PCL [132] PLLA [133,134]; PCL, PEVA [114] | 1,1,1,3,3,3-Hexafluoro-2-propanol [71]; Chloroform [113]; Chloroform, dimethylformamide [132]; Chloroform: acetone (2:1) [133,134]; chloroform:methanol (9:1) [114] | Single nozzle; Coaxial [133,134] |
| Gentamycin sulfate and Resveratrol (antioxidant) | PCL [133] | Chloroform: ethanol (3:1) | Coaxial |
| Ciprofloxacin Hydrochloride | PVA, Poly(vinyl acetate) [84] | Diluted acetic acid solution | Single nozzle |
| Fusidic acid and rifampicin | PLGA [135] | Tetrahydro Furan/Dimethylformamide | Single nozzle |
| Mefoxin | PLGA [83] | DMF | Single nozzle |
| Metronidazole benzoate | PCL [136] | Dichloromethane (DCM:DMF) | Single nozzle |
| Ciprofloxacin hydrochloride, Levofloxacin hemihydrate, Moxifloxacin hydrochloride | coPLA, coPLA/PEG [137] | DCM:DMSO (3:1) | Single nozzle |
| Lidocaine and mupirocin | PLLA [120] | Hexafluoroisopropanol | Dual spinneret |
| Ornidazole (Biteral®) | PCL | Chloroform and DMF (3:7) | Single nozzle |
| Potassium 5-nitro-8-quinolinolate | Chitosan/PEO [138] | 2% (w/v) acetic acid | Single nozzle |
| Itraconazole and ketanserin | PU [139] | DMF, DMAc | Single nozzle |
| Pleurocidin | PVA [116] | Distilled water | Single nozzle |
| NSAIDS | |||
| Ketoprofen | PVA/PAA/MWCNT [92]; PEO/PETA/MWCNT [109]; EC and PVP [140]; PVP/Zein [141] | Deionized water [92,109] Ethanol-water [140,141] | Single nozzle Coaxial [141] |
| Ibuprofen | PLGA PEG-g-CHN [142] | DMF | Side-by-side |
| Fenbufen | PLGA/Gelatin [82] | 2,2,2-trifluoroethanol | Single nozzle |
| Rhodamine B/Naproxen | Chitosan nanoparticles/PCL composite [100] | Acetic acid/chloroform: methanol (3:1) | Single nozzle yet core/sheath fiber |
| Meloxicam | PVA [111] | Water | Single nozzle |
| Anticancer agents | |||
| Doxorubicin [127] Doxorubicin Hydrochloride | PLLA [127] PEG-PLA [124,143] | Chloroform-methano-DMSO [127] Chloroform [143] w/o emulsion [124] | Single nozzle Emulsion [124] |
| Hydroxycamptothecin | HPCD [128] | DMSO | Emulsion |
| Paclitaxel | Chitosan/PEO/HA [84] PLGA [144,145] | Acetic acid/distilled water [84] DCM and DMF | Single nozzle |
| Cisplatin | PLA/PLGA [126] | DCM | Single nozzle |
| Dichloroacetate | PLA [146] | Chloroform | Single nozzle |
| 1,3-Bis(2-chloroethyl)-1-nitrosourea | PEO and PEG-PLLA [147] | Chloroform | Single nozzle/Emulsion |
| Curcumin | Cellulose acetate [108] | Acetone/dimethylacetamide (2:1) | Single nozzle |
| Green tea polyphenols (GTP) | PCL/MWCNT [131] | Dichloromethane | Single nozzle |
| Titanocene dichloride | PLLA [130] | Dichloromethane | Single nozzle |
| Drug(s) | Polymer(s) | Solvent Composition | Spraying Type |
|---|---|---|---|
| plasmid DNA (pDNA) | PEI-HA [62] | Coaxial | |
| siRNA | PCL [150]; PCLEEP [149] Chitosan/PLGA [169] | 2,2,2-Trifluoroethanol (TFE) [150]; RNase-free water [149]; Hexafluoro-2-isopropanol/water [169] | Single nozzle |
| Human glial cell-derived neurotrophic factor | PCL-co-PCLEEP [170] | Dichloromethane | Single nozzle |
| Human β-nerve growth factor | PCL-co-PCLEEP [148] | Dichloromethane | Single nozzle |
| Endothelial growth factor VEGF | PECCL [152] | - | Single nozzle |
| Bovine Serum Albumin (BSA) | PEO [171] | Deionized water | Single nozzle |
| Lysozyme | PLA [97] | Chloroform | Emulsion |
| Human-nerve growth factor (NGF) | Poly(L-lactide-co-caprolactone) [172] | Chloroform | Emulsion |
| DNA | PLA-PEG and PLGA [94] | DMF | Single nozzle |
| Growth factors (VEGF, PDGF) | Poly(urethane) [63] | Chloroform: ethanol (75:25) | Coaxial |
| Horseradish peroxidase | PVA/PCL [86] | - | Coaxial |
| Drug(s) | Polymer(s) | Solvent Composition | Spraying Type |
|---|---|---|---|
| Wound healing, tissue engineering, hemostatic agent | Collagen-PEO [173,174] | Hydrochloric acid | Single nozzle |
| Adenovirus with gene for green fluorescent protein | Poly(ε-caprolactone) [60] | Chloroform: ethanol (75:25) | Coaxial |
| Guided tissue regeneration | PDLLA/PLGA [175] | Chloroform:DMF (9:1) and THF/DMF (3:1) | Single nozzle |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bhattarai, R.S.; Bachu, R.D.; Boddu, S.H.S.; Bhaduri, S. Biomedical Applications of Electrospun Nanofibers: Drug and Nanoparticle Delivery. Pharmaceutics 2019, 11, 5. https://doi.org/10.3390/pharmaceutics11010005
Bhattarai RS, Bachu RD, Boddu SHS, Bhaduri S. Biomedical Applications of Electrospun Nanofibers: Drug and Nanoparticle Delivery. Pharmaceutics. 2019; 11(1):5. https://doi.org/10.3390/pharmaceutics11010005
Chicago/Turabian StyleBhattarai, Rajan Sharma, Rinda Devi Bachu, Sai H. S. Boddu, and Sarit Bhaduri. 2019. "Biomedical Applications of Electrospun Nanofibers: Drug and Nanoparticle Delivery" Pharmaceutics 11, no. 1: 5. https://doi.org/10.3390/pharmaceutics11010005