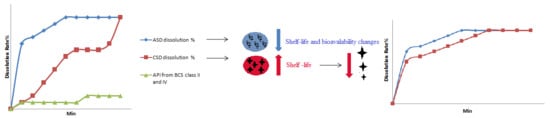
Engineering of Nanofibrous Amorphous and Crystalline Solid Dispersions for Oral Drug Delivery
Abstract
:1. Introduction
2. Nanofibrous Amorphous Solid Dispersions
2.1. Electrospinning
2.1.1. Mono-Axial Electrospinning
2.1.2. Coaxial and Multi-Axial Electrospinning
2.1.3. Others
3. Nanofibrous Micro/Nano-Crystalline Solid Dispersions
4. Conclusions
Funding
Conflicts of Interest
References
- Vo, C.L.N.; Park, C.; Lee, B.J. Current trends and future perspectives of solid dispersions containing poorly water-soluble drugs. Eur. J. Pharm. Biopharm. 2013, 85, 799–813. [Google Scholar] [CrossRef] [PubMed]
- Demetzos, C. Pharmaceutical Nanotechnology; Springer Singapore: Singapore, 2016; ISBN 978-981-10-0790-3. [Google Scholar]
- Serajuddln, A.T.M. Solid dispersion of poorly water-soluble drugs: Early promises, subsequent problems, and recent breakthroughs. J. Pharm. Sci. 1999, 88, 1058–1066. [Google Scholar] [CrossRef]
- Loh, Z.H.; Samanta, A.K.; Sia Heng, P.W. Overview of milling techniques for improving the solubility of poorly water-soluble drugs. Asian J. Pharm. Sci. 2015, 10, 255–274. [Google Scholar] [CrossRef]
- Paudel, A.; Worku, Z.A.; Meeus, J.; Guns, S.; Van den Mooter, G. Manufacturing of solid dispersions of poorly water soluble drugs by spray drying: Formulation and process considerations. Int. J. Pharm. 2013, 453, 253–284. [Google Scholar] [CrossRef] [PubMed]
- Shah, N.; Iyer, R.M.; Mair, H.-J.; Choi, D.; Tian, H.; Diodone, R.; Fahnrich, K.; Pabst-Ravot, A.; Tang, K.; Scheubel, E.; et al. Improved human bioavailability of vemurafenib, a practically insoluble drug, using an amorphous polymer-stabilized solid dispersion prepared by a solvent-controlled coprecipitation process. J. Pharm. Sci. 2013, 102, 967–981. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Dai, W.-G. Fundamental aspects of solid dispersion technology for poorly soluble drugs. Acta Pharm. Sin. B. 2014, 4, 18–25. [Google Scholar] [CrossRef]
- Sekiguchi, K.; Obi, N. Studies on absorption of eutectic mixture I. A comparison of the behaviour of eutectic mixture of sulfathiazole and that of ordinary sulfathiazole in man. Chem. Pharm. Bull. 1961, 9, 866–872. [Google Scholar] [CrossRef]
- Chiou, W.L.; Riegelman, S. Pharmaceutical applications of solid dispersion systems. J. Pharm. Sci. 1971, 60, 1281–1302. [Google Scholar] [CrossRef]
- Leuner, C.; Dressman, J. Improving drug solubility for oral delivery using solid dispersions. Eur. J. Pharm. Biopharm. 2000, 50, 47–60. [Google Scholar] [CrossRef]
- Craig, D.Q.M. The mechanisms of drug release from solid dispersions in water-soluble polymers. Int. J. Pharm. 2002, 231, 131–144. [Google Scholar] [CrossRef]
- Marano, S.; Barker, S.A.; Raimi-Abraham, B.T.; Missaghi, S.; Rajabi-Siahboomi, A.; Aliev, A.E.; Craig, D.Q.M. Microfibrous solid dispersions of poorly water-soluble drugs produced via centrifugal spinning: unexpected dissolution behavior on recrystallization. Mol. Pharm. 2017, 14, 1666–1680. [Google Scholar] [CrossRef] [PubMed]
- Marano, S.; Barker, S.A.; Raimi-Abraham, B.T.; Missaghi, S.; Rajabi-Siahboomi, A.; Craig, D.Q.M. Development of micro-fibrous solid dispersions of poorly water-soluble drugs in sucrose using temperature-controlled centrifugal spinning. Eur. J. Pharm. Biopharm. 2016, 103, 84–94. [Google Scholar] [CrossRef]
- Yan, H.-X.; Zhang, S.-S.; He, J.-H.; Liu, J.-P. Application of ethyl cellulose, microcrystalline cellulose and octadecanol for wax based floating solid dispersion pellets. Carbohydr. Polym. 2016, 148, 143–152. [Google Scholar] [CrossRef] [PubMed]
- Corrigan, O.I. Retardation of polymeric carrier dissolution by dispersed drugs: factors influencing the dissolution of solid dispersions containing polyethlene glycols. Drug Dev. Ind. Pharm. 1986, 12, 1777–1793. [Google Scholar] [CrossRef]
- Leonardi, D.; Salomon, C.J. Unexpected performance of physical mixtures over solid dispersions on the dissolution behavior of benznidazole from tablets. J. Pharm. Sci. 2013, 102, 1016–1023. [Google Scholar] [CrossRef] [PubMed]
- Sharma, P.K.; Panda, A.; Pradhan, A.; Zhang, J.; Thakkar, R.; Whang, C.-H.; Repka, M.A.; Murthy, S.N. Solid-state stability issues of drugs in transdermal patch formulations. AAPS PharmSciTech 2017, 19. [Google Scholar] [CrossRef] [PubMed]
- Vasconcelos, T.; Sarmento, B.; Costa, P. Solid dispersions as strategy to improve oral bioavailability of poor water soluble drugs. Drug Discov. Today 2007, 12, 1068–1075. [Google Scholar] [CrossRef]
- van Drooge, D.J.; Braeckmans, K.; Hinrichs, W.L.J.; Remaut, K.; De Smedt, S.C.; Frijlink, H.W. Characterization of the mode of incorporation of lipophilic compounds in solid dispersions at the nanoscale using fluorescence resonance energy transfer (FRET). Macromol. Rapid Commun. 2006, 27, 1149–1155. [Google Scholar] [CrossRef]
- Pouton, C.W. Formulation of poorly water-soluble drugs for oral administration: Physicochemical and physiological issues and the lipid formulation classification system. Eur. J. Pharm. Sci. 2006, 29, 278–287. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.W.; Kotta, S.; Ansari, S.H.; Sharma, R.K.; Ali, J. Enhanced dissolution and bioavailability of grapefruit flavonoid Naringenin by solid dispersion utilizing fourth generation carrier. Drug Dev. Ind. Pharm. 2015, 41, 772–779. [Google Scholar] [CrossRef]
- Shamma, R.N.; Basha, M. Soluplus: A novel polymeric solubilizer for optimization of Carvedilol solid dispersions: Formulation design and effect of method of preparation. Powder Technol. 2013, 237, 406–414. [Google Scholar] [CrossRef]
- Meng, F.; Gala, U.; Chauhan, H. Classification of solid dispersions: correlation to (i) stability and solubility (ii) preparation and characterization techniques. Drug Dev. Ind. Pharm. 2015, 41, 1401–1415. [Google Scholar] [CrossRef] [PubMed]
- Kumar, B. Solid Dispersion—A Review. PharmaTutor 2017, 5, 24–29. [Google Scholar]
- Pina, M.F.; Zhao, M.; Pinto, J.F.; Sousa, J.J.; Craig, D.Q.M. The influence of drug physical state on the dissolution enhancement of solid dispersions prepared via hot-melt extrusion: A case study using olanzapine. J. Pharm. Sci. 2014, 103, 1214–1223. [Google Scholar] [CrossRef] [PubMed]
- Qi, S.; Craig, D. Recent developments in micro- and nanofabrication techniques for the preparation of amorphous pharmaceutical dosage forms. Adv. Drug Deliv. Rev. 2016, 100, 67–84. [Google Scholar] [CrossRef] [PubMed]
- Alhusein, N.; Blagbrough, I.S.; Beeton, M.L.; Bolhuis, A.; De Bank, P.A. Electrospun Zein/PCL fibrous matrices release tetracycline in a controlled manner, killing Staphylococcus aureus both in biofilms and ex vivo on pig skin, and are compatible with human skin cells. Pharm. Res. 2016, 33, 237–246. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Konecke, S.; Harich, K.; Wegiel, L.; Taylor, L.S.; Edgar, K.J. Solid dispersion of quercetin in cellulose derivative matrices influences both solubility and stability. Carbohydr. Polym. 2013, 92, 2033–2040. [Google Scholar] [CrossRef]
- Zidan, A.S.; Rahman, Z.; Sayeed, V.; Raw, A.; Yu, L.; Khan, M.A. Crystallinity evaluation of tacrolimus solid dispersions by chemometric analysis. Int. J. Pharm. 2012, 423, 341–350. [Google Scholar] [CrossRef]
- Alhusein, N.; De Bank, P.A.; Blagbrough, I.S.; Bolhuis, A. Killing bacteria within biofilms by sustained release of tetracycline from triple-layered electrospun micro/nanofibre matrices of polycaprolactone and poly (ethylene-co-vinyl acetate). Drug Deliv. Transl Res. 2013, 3, 531–541. [Google Scholar] [CrossRef]
- Alshahrani, S.M.; Lu, W.; Park, J.-B.; Morott, J.T.; Alsulays, B.B.; Majumdar, S.; Langley, N.; Kolter, K.; Gryczke, A.; Repka, M.A. Stability-enhanced hot-melt extruded amorphous solid dispersions via combinations of soluplus® and hpmcas-hf. AAPS PharmSciTech 2015, 16, 824–834. [Google Scholar] [CrossRef]
- Modica de Mohac, L.; de Fátima Pina, M.; Raimi-Abraham, B.T. Solid microcrystalline dispersion films as a new strategy to improve the dissolution rate of poorly water soluble drugs: A case study using olanzapine. Int. J. Pharm. 2016, 508, 42–50. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Caro, V.; Ajovalasit, A.; Sutera, F.M.; Murgia, D.; Sabatino, M.A.; Dispenza, C. Development and characterization of an amorphous solid dispersion of furosemide in the form of a sublingual bioadhesive film to enhance bioavailability. Pharmaceutics 2017, 9, 22. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Pang, H.; Guo, Z.; Lin, L.; Dong, Y.; Li, G.; Lu, M.; Wu, C. Interactions between drugs and polymers influencing hot melt extrusion. J. Pharm. Pharmacol. 2014, 66, 148–166. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Yu, H.; Luo, Q.; Yang, S.; Lin, X.; Zhang, Y.; Tian, B.; Tang, X. Increased dissolution and oral absorption of itraconazole/Soluplus extrudate compared with itraconazole nanosuspension. Eur. J. Pharm. Biopharm. 2013, 85, 1285–1292. [Google Scholar] [CrossRef] [PubMed]
- Keating, A.V.; Soto, J.; Tuleu, C.; Forbes, C.; Zhao, M.; Craig, D.Q.M. Solid state characterisation and taste masking efficiency evaluation of polymer based extrudates of isoniazid for paediatric administration. Int. J. Pharm. 2018, 536, 536–546. [Google Scholar] [CrossRef] [PubMed]
- Davis, M.T.; Potter, C.B.; Mohammadpour, M.; Albadarin, A.B.; Walker, G.M. Design of spray dried ternary solid dispersions comprising itraconazole, soluplus and HPMCP: Effect of constituent compositions. Int. J. Pharm. 2017, 519, 365–372. [Google Scholar] [CrossRef] [PubMed]
- Qi, S.; Moffat, J.G.; Yang, Z. Early stage phase separation in pharmaceutical solid dispersion thin films under high humidity: improved spatial understanding using probe based thermal and spectroscopic nano-characterisation methods. Mol. Pharm. 2013, 10, 918–930. [Google Scholar] [CrossRef]
- Ng, Y.C.; Yang, Z.; McAuley, W.J.; Qi, S. Stabilisation of amorphous drugs under high humidity using pharmaceutical thin films. Eur. J. Pharm. Biopharm. 2013, 84, 555–565. [Google Scholar] [CrossRef]
- Luo, C.J.; Stoyanov, S.D.; Stride, E.; Pelan, E.; Edirisinghe, M. Electrospinning versus fibre production methods: from specifics to technological convergence. Chem. Soc. Rev. 2012, 41, 4708–4735. [Google Scholar] [CrossRef]
- Illangakoon, U.E.; Nazir, T.; Williams, G.R.; Chatterton, N.P. Mebeverine-loaded electrospun nanofibers: physicochemical characterization and dissolution studies. J. Pharm. Sci. 2014, 103, 283–292. [Google Scholar] [CrossRef]
- Raimi-Abraham, B.T.; Mahalingam, S.; Davies, P.J.; Edirisinghe, M.; Craig, D.Q.M. Development and characterization of amorphous nanofiber drug dispersions prepared using pressurized gyration. Mol. Pharm. 2015, 12, 3851–3861. [Google Scholar] [CrossRef] [PubMed]
- Bhut, V.Z.; Prajapati, A.B.; Patel, K.N.; Patel, B.A.; Patel, P.A. Solid dispersion as a strategy to enhance solubility: A review article. IJPRS 2012, 5, 490–498. [Google Scholar]
- Thakral, S.; Thakral, N.K. Prediction of drug–polymer miscibility through the use of solubility parameter based flory–huggins interaction parameter and the experimental validation: PEG as model polymer. J. Pharm. Sci. 2013, 102, 2254–2263. [Google Scholar] [CrossRef] [PubMed]
- Son, Y.J.; Kim, W.J.; Yoo, H.S. Therapeutic applications of electrospun nanofibers for drug delivery systems. Arch. Pharm. Res. 2013, 37, 69–78. [Google Scholar] [CrossRef]
- Liu, M.; Duan, X.-P.; Li, Y.-M.; Yang, D.-P.; Long, Y.-Z. Electrospun nanofibers for wound healing. Mater. Sci. Eng. C 2017, 76, 1413–1423. [Google Scholar] [CrossRef] [PubMed]
- Jun, I.; Han, H.S.; Edwards, J.R.; Jeon, H. Electrospun Fibrous Scaffolds for Tissue Engineering: Viewpoints on Architecture and Fabrication. Int. J. Mol. Sci. 2018, 19, 745. [Google Scholar] [CrossRef] [PubMed]
- Cooley, J.F. Improved methods of and apparatus for electrically separating the relatively volatile liquid component from the component of relatively fixed substances of composite fluids. United Kingdom Patent 1900, 6385, 19. [Google Scholar]
- Cooley, J.F. Apparatus for Electrically Dispersing Fluids. U.S. Patent Application No. 692,631, 4 February 1902. [Google Scholar]
- Morton, W.J. Method of Dispersing Fluids. U.S. Patent US705691A, 29 July 1902. [Google Scholar]
- Sill, T.J.; von Recum, H.A. Electrospinning: applications in drug delivery and tissue engineering. Biomaterials 2008, 29, 1989–2006. [Google Scholar] [CrossRef] [PubMed]
- Williams, G.R.; Chatterton, N.P.; Nazir, T.; Yu, D.G.; Zhu, L.M.; Branford-White, C.J. Electrospun nanofibers in drug delivery: recent developments and perspectives. Ther. Deliv. 2012, 3, 515–533. [Google Scholar] [CrossRef]
- Cheng, H.; Yang, X.; Che, X.; Yang, M.; Zhai, G. Biomedical application and controlled drug release of electrospun fibrous materials. Mater. Sci. Eng. C 2018, 90, 750–763. [Google Scholar] [CrossRef]
- Vogt, L.; Liverani, L.; Roether, J.; Boccaccini, A. Electrospun Zein Fibers Incorporating Poly(glycerol sebacate) for Soft Tissue Engineering. Nanomaterials 2018, 8, 150. [Google Scholar] [CrossRef]
- Alhusein, N.; Blagbrough, I.S.; De Bank, P.A. Zein/polycaprolactone electrospun matrices for localised controlled delivery of tetracycline. Drug Deliv. Transl. Res. 2013, 3, 542–550. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, H.; Lou, S.; Williams, G.R.; Branford-White, C.; Nie, H.; Quan, J.; Zhu, L.-M. A systematic study of captopril-loaded polyester fiber mats prepared by electrospinning. Int. J. Pharm. 2012, 439, 100–108. [Google Scholar] [CrossRef] [PubMed]
- Qu, H.; Wei, S.; Guo, Z. Coaxial electrospun nanostructures and their applications. J. Mater. Chem. A. 2013, 1, 11513–11518. [Google Scholar] [CrossRef]
- Williams, G.R.; Raimi-Abraham, B.T.; Luo, C.J. Nanofibres in Drug Delivery; UCL Press: London, UK, 2018; ISBN 9781787350182. [Google Scholar]
- Verreck, G.; Chun, I.; Peeters, J.; Rosenblatt, J.; Brewster, M.E. Preparation and characterization of nanofibers containing amorphous drug dispersions generated by electrostatic spinning. Pharm. Res. 2003, 20, 810–817. [Google Scholar] [CrossRef] [PubMed]
- Nagy, Z.K.; Balogh, A.; Vajna, B.; Farkas, A.; Patyi, G.; Kramarics, Á.; Marosi, G. Comparison of electrospun and extruded soluplus®-based solid dosage forms of improved dissolution. J. Pharm. Sci. 2012, 101, 322–332. [Google Scholar] [CrossRef] [PubMed]
- Nagy, Z.K.; Balogh, A.; Démuth, B.; Pataki, H.; Vigh, T.; Szabó, B.; Molnár, K.; Schmidt, B.T.; Horák, P.; Marosi, G.; et al. High speed electrospinning for scaled-up production of amorphous solid dispersion of itraconazole. Int. J. Pharm. 2015, 480, 137–142. [Google Scholar] [CrossRef] [PubMed]
- Yu, D.-G.; Branford-White, C.; White, K.; Li, X.-L.; Zhu, L.-M. dissolution improvement of electrospun nanofiber-based solid dispersions for acetaminophen. AAPS PharmSciTech 2010, 11, 809–817. [Google Scholar] [CrossRef]
- Huang, Z.-M.; Zhang, Y.-Z.; Kotaki, M.; Ramakrishna, S. A review on polymer nanofibers by electrospinning and their applications in nanocomposites. Compos. Sci. Technol. 2003, 63, 2223–2253. [Google Scholar] [CrossRef]
- Li, D.; Xia, Y. Electrospinning of nanofibers: reinventing the wheel? Adv. Mater. 2004, 16, 1151–1170. [Google Scholar] [CrossRef]
- Yu, D.G.; Wang, X.; Li, X.Y.; Chian, W.; Li, Y.; Liao, Y.Z. Electrospun biphasic drug release polyvinylpyrrolidone/ethyl cellulose core/sheath nanofibers. Acta Biomater. 2013, 9, 5665–5672. [Google Scholar] [CrossRef]
- Maincent, J.; Williams, R.O. Sustained-release amorphous solid dispersions. Drug Deliv. Transl. Res. 2018, 8, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Lin, W.C.; Yeh, I.T.; Niyama, E.; Huang, W.R.; Ebara, M.; Wu, C.S. Electrospun poly(ε-caprolactone) nanofibrous mesh for imiquimod delivery in melanoma therapy. Polymers 2018, 10, 231. [Google Scholar] [CrossRef]
- Wei, Q.; Xu, F.; Xu, X.; Geng, X.; Ye, L.; Zhang, A.; Feng, Z. The multifunctional wound dressing with core–shell structured fibers prepared by coaxial electrospinning. Front. Mater. Sci. 2016, 10, 113–121. [Google Scholar] [CrossRef]
- Yang, G.; Li, J.; Yu, D.; He, M.; Yang, J.; Williams, G.R. Nanosized sustained-release drug depots fabricated using modified tri-axial electrospinning. Acta Biomater. 2017, 53, 233–241. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, C.; Yu, D.-G.; Pan, D.; Liu, X.-K.; Wang, X.; Bligh, S.W.A.; Williams, G.R. Electrospun pH-sensitive core–shell polymer nanocomposites fabricated using a tri-axial process. Acta Biomater. 2016, 35, 77–86. [Google Scholar] [CrossRef] [Green Version]
- Lee, B.S.; Jeon, S.Y.; Park, H.; Lee, G.; Yang, H.S.; Yu, W.R. New electrospinning nozzle to reduce jet instability and its application to manufacture of multi-layered nanofibers. Sci. Rep. 2014, 4, 6758. [Google Scholar] [CrossRef] [PubMed]
- Yu, D.-G.; Yang, C.; Jin, M.; Williams, G.R.; Zou, H.; Wang, X.; Bligh, S.W.A. Medicated Janus fibers fabricated using a Teflon-coated side-by-side spinneret. Colloids Surf B Biointerfaces 2016, 138, 110–116. [Google Scholar] [CrossRef] [Green Version]
- Geng, Y.; Zhang, P.; Wang, Q.; Liu, Y.; Pan, K. Novel PAN/PVP Janus ultrafine fiber membrane and its application for biphasic drug release. J. Mater. Chem. B 2017, 5, 5390–5396. [Google Scholar] [CrossRef]
- Van Den Mooter, G. The use of amorphous solid dispersions: A formulation strategy to overcome poor solubility and dissolution rate. Drug Discov. Today Technol. 2012, 9, 79–86. [Google Scholar] [CrossRef]
- Démuth, B.; Farkas, A.; Balogh, A.; Bartosiewicz, K.; Kállai-Szabó, B.; Bertels, J.; Vigh, T.; Mensch, J.; Verreck, G.; Van Assche, I.; et al. Lubricant-induced crystallization of itraconazole from tablets made of electrospun amorphous solid dispersion. J. Pharm. Sci. 2016, 105, 2982–2988. [Google Scholar] [CrossRef]
- Démuth, B.; Farkas, A.; Szabó, B.; Balogh, A.; Nagy, B.; Vágó, E.; Vigh, T.; Tinke, A.P.; Kazsu, Z.; Demeter, Á.; et al. Development and tableting of directly compressible powder from electrospun nanofibrous amorphous solid dispersion. Adv. Powder Technol. 2017, 28, 1554–1563. [Google Scholar] [CrossRef]
- Li, M.; Gogos, C.G.; Ioannidis, N. Improving the API dissolution rate during pharmaceutical hot-melt extrusion I: Effect of the API particle size, and the co-rotating, twin-screw extruder screw configuration on the API dissolution rate. Int. J. Pharm. 2015, 478, 103–112. [Google Scholar] [CrossRef] [PubMed]
- Ige, P.P.; Baria, R.K.; Gattani, S.G. Fabrication of fenofibrate nanocrystals by probe sonication method for enhancement of dissolution rate and oral bioavailability. Colloids Surf. B Biointerfaces 2013, 108, 366–373. [Google Scholar] [CrossRef] [PubMed]
- Frizon, F.; de Oliveira Eloy, J.; Donaduzzi, C.M.; Mitsui, M.L.; Marchetti, J.M. Dissolution rate enhancement of loratadine in polyvinylpyrrolidone K-30 solid dispersions by solvent methods. Powder Technol. 2013, 235, 532–539. [Google Scholar] [CrossRef]
- Gao, L.; Liu, G.; Wang, X.; Liu, F.; Xu, Y.; Ma, J. Preparation of a chemically stable quercetin formulation using nanosuspension technology. Int. J. Pharm. 2011, 404, 231–237. [Google Scholar] [CrossRef] [PubMed]
- Rabinow, B.E. Nanosuspensions in drug delivery. Drug Deliv. 2004, 3, 785–796. [Google Scholar] [CrossRef] [PubMed]
- Bodmeier, R.; Oh, K.H.; Chen, H. The effect of the addition of low molecular weight poly(dl-lactide) on drug release from biodegradable poly(dl-lactide) drug delivery systems. Int. J. Pharm. 1989, 51, 1–8. [Google Scholar] [CrossRef]
- Tinke, A.P.; Carnicer, A.; Govoreanu, R.; Scheltjens, G.; Lauwerysen, L.; Mertens, N.; Vanhoutte, K.; Brewster, M.E. Particle shape and orientation in laser diffraction and static image analysis size distribution analysis of micrometer sized rectangular particles. Powder Technol. 2008, 186, 154–167. [Google Scholar] [CrossRef] [Green Version]
- Choi, H.J.; Kim, S.G.; Hyun, Y.H.; Jhon, M.S. Preparation and rheological characteristics of solvent-cast poly (ethylene oxide)/ montmorillonite nanocomposites. Macromol. Rapid Commun. 2001, 22, 320–325. [Google Scholar] [CrossRef]
- Singh, A.; Van den Mooter, G. Spray drying formulation of amorphous solid dispersions. Adv. Drug Deliv. Rev. 2016, 100, 27–50. [Google Scholar] [CrossRef]
- Taylor, M.J.; Tanna, S.; Sahota, T. In vivo study of a polymeric glucose-sensitive insulin delivery system using a rat model. J. Pharm. Sci. 2010, 99, 4215–4227. [Google Scholar] [CrossRef] [PubMed]
 represents dissolved drug, the smaller
represents dissolved drug, the smaller  represent the partially dissolved drug. Grey box represents an aqueous environment.
represent the partially dissolved drug. Grey box represents an aqueous environment.
 represents dissolved drug, the smaller
represents dissolved drug, the smaller  represent the partially dissolved drug. Grey box represents an aqueous environment.
represent the partially dissolved drug. Grey box represents an aqueous environment.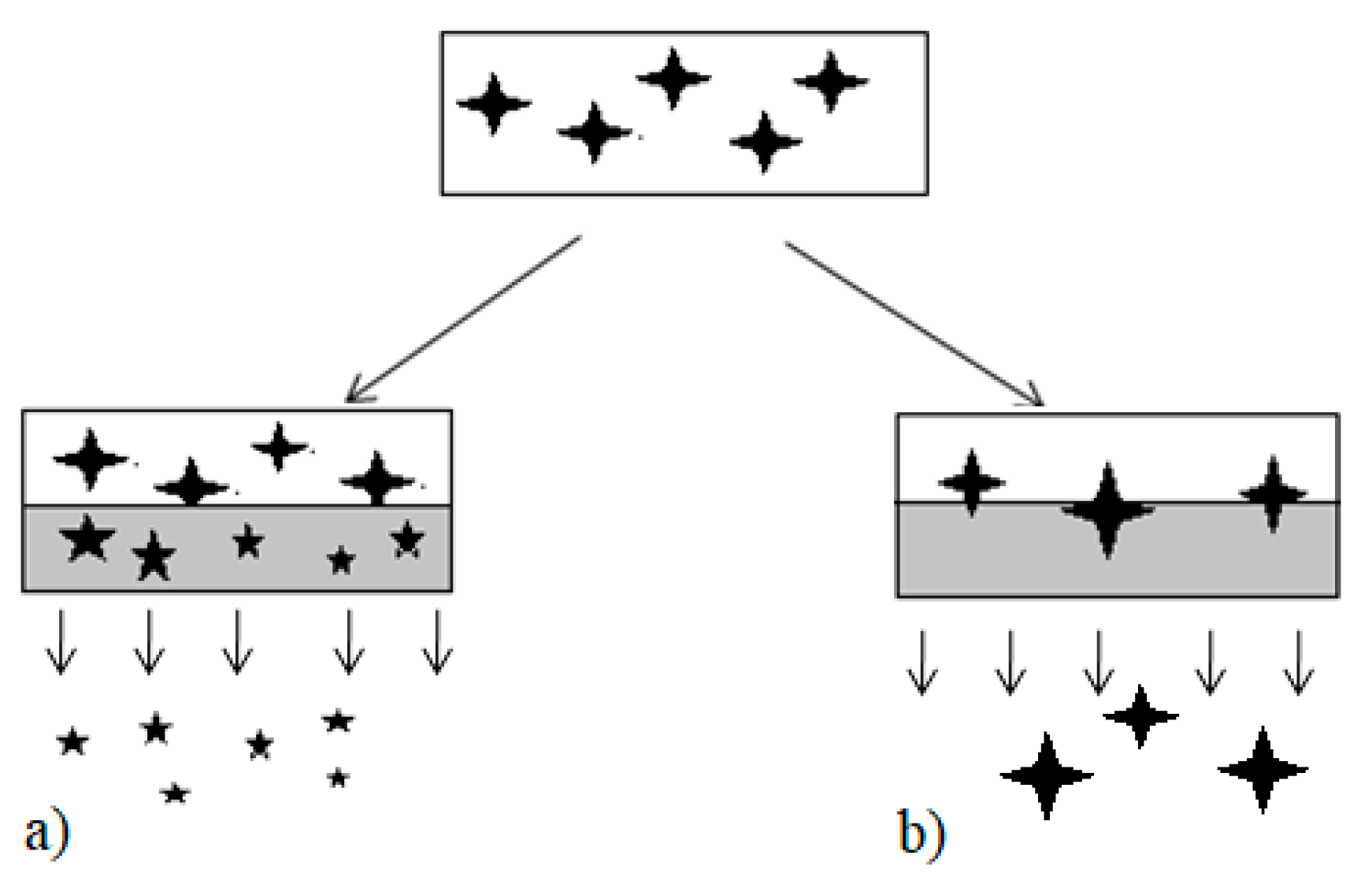
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Modica de Mohac, L.; Keating, A.V.; De Fátima Pina, M.; Raimi-Abraham, B.T. Engineering of Nanofibrous Amorphous and Crystalline Solid Dispersions for Oral Drug Delivery. Pharmaceutics 2019, 11, 7. https://doi.org/10.3390/pharmaceutics11010007
Modica de Mohac L, Keating AV, De Fátima Pina M, Raimi-Abraham BT. Engineering of Nanofibrous Amorphous and Crystalline Solid Dispersions for Oral Drug Delivery. Pharmaceutics. 2019; 11(1):7. https://doi.org/10.3390/pharmaceutics11010007
Chicago/Turabian StyleModica de Mohac, Laura, Alison Veronica Keating, Maria De Fátima Pina, and Bahijja Tolulope Raimi-Abraham. 2019. "Engineering of Nanofibrous Amorphous and Crystalline Solid Dispersions for Oral Drug Delivery" Pharmaceutics 11, no. 1: 7. https://doi.org/10.3390/pharmaceutics11010007