1. Introduction
Curcumin (Cur) is the main active component in rhizomes of
Curcuma longa, which is an ancient Chinese spice that is extremely appreciated in traditional medicine remedies [
1]. It has been recently discovered that this compound has multiple applications as an anti-inflammatory, anti-cancer, antioxidant, anti-viral and cytoprotective agent [
2]. As a polyphenol, Cur is capable of forming hydrogen bonds with other Cur molecules and not with water, which reduces its solubility in this medium [
3]. Due to this problem, Cur exhibits a low water solubility, which implies low bioavailability [
2,
4,
5] To overcome these drawbacks, numerous strategies have been proposed, such as cyclodextrin complexation [
4,
6], or entrapment in liposomes [
7,
8], micelles [
9,
10], dendrimers [
11], etc.
Cyclodextrins (CD) are cyclic oligosaccharides containing a different number of units of (α-1,4)-linked-
d-glucopyranoside. Modified CD, which is derived from native molecules (α-CD, β-CD, and γ-CD), was designed to increase its solubility. Some of these derivatives are 2-hydroxypropyl-α/β/γ-CD (HPβCD), methylated-β-CD (MβCD), sulfobutylether-β-CD, etc. [
12,
13]. Polymeric CD consists of native CD linked with one (dimeric) or more (polymeric) spacers. These structures allow improved drug-binding abilities [
14]. The truncated cone shape of CD with an internal hydrophobic cavity and external hydrophilic surface [
15] makes the formation of inclusion complexes with hydrophobic compounds possible, such as curcumin [
4,
6], budesonide [
16], prilocaine [
17], etc., increasing their water solubility and bioavailability. Besides, CD can also improve thermal and physical stability and reduce drug toxicity [
15]. Despite these advantages, in some cases, the amount of CD that is required to dissolve the drug is high, and can trigger toxic effects [
14].
To date, liposomes continue to be among the lipid nanocarriers that are most used for the drug delivery of hydrophilic and lipophilic compounds [
18]. Among the different types of these lipid vesicles, those containing a single bilayer (unilamellar vesicles or ULV) are ideal to encapsulate hydrophilic drugs, while those containing multiple bilayers (multilamellar vesicles or MLV) are more suitable for lipophilic drugs [
19].
Since 1994, drug-in-cyclodextrins-in-liposomes (DCL) has been employed as a drug delivery strategy to increase the loading capacity of hydrophobic drugs into the liposomes. The addition of the lipophilic molecule into the lipid bilayer and the inclusion complex drug CD into the aqueous compartment of liposome, by double-loading (DL), gives rise to DCL–DL. This last approach has several advantages such as, in particular, the prevention of the rapid clearance of the drug, when injected, due to the affinity of other blood components with CD [
12]. In addition, the combination of both systems (CD and liposome) is able to improve the encapsulation efficiency of drugs [
15]. The coupling of both delivery systems, by encapsulating a drug CD inclusion complex into liposomes, is proposed to circumvent the drawbacks of each separate system. A very interesting review about these nanoparticulate systems has been reported [
15]. Our research group has wide experience in the use of this combined approach in order to increase the therapeutic efficacy of anesthetics and anti-inflammatory compounds [
20,
21,
22]. Moreover, DCL–DL enhanced the amount of the drug in stratum corneum and deeper skin layers compared to conventional liposomes (CL). From the studies, we concluded that liposomes in the presence of CD may be a promising carrier for the effective cutaneous delivery of drugs [
20,
21]. In the last years, many studies have been published with the aim of improving the water solubility, chemical stability, and bioavailability of drugs [
23]. This is the case of using this combination strategy for paclitaxel to improve loading into liposomes and pharmacokinetics due to the increased aqueous solubility of paclitaxel complexed in DMβCD as compared to pure drug solubility [
24]. Wang et al. have used this strategy for increasing risperidone stability, achieving a second release phase of the combined delivery system that is slightly slower, after an initial burst release, and potentially suitable as a long-acting injection formulation [
25].
DCL–DL is also being investigated for loading substances to be used as additives for providing the photoprotection and preservation of essential oils [
26] or food nutraceuticals [
27,
28].
Although many studies are being realized with this combined approach, most of them have centered the research in how the presence of cyclodextrin affects the drug solubility, the physicochemical properties of resulted vesicles, the stability, and the in vitro release. Other authors make reference to the use of drug delivery systems combining the advantages of cyclodextrin inclusion complexes and those of deformable liposomes that are mainly targeted for skin delivery, with the purpose of increasing the diffusion of the encapsulated drug [
29]. The enhanced therapeutic efficacy of drug-in-cyclodextrin-in deformable liposomes has been confirmed by our studies, achieving favorable effects of drug CD complexation and allowing a significant enhancement of intensity and duration of anesthetic effect with respect to those that are single-loaded [
22].
Taking into account the scarce aqueous solubility of Cur, it is predictable that this molecule is placed on the lipid moiety of the liposome, specifically near the acyl region. In addition, it is known to interact with the bilayer, ordering or disordering it as a function of the presence of cholesterol [
30]. Some years ago, Jaruga et al. [
31] and Jaruga et al. [
32] demonstrated that Cur highly impacts the permeability of membranes, in the sense of increasing it [
31], and provoking changes in membrane fluidity [
31,
32]. However, only a few articles make reference to the effects of the presence of CD in Cur double-loaded into liposomes on the lipid bilayer, drug entrapment, and drug release. Therefore, in an attempt to minimize the effects of Cur on the bilayer, and at the same time, increase the drug entrapment efficiency, Cur-loaded DCL–DL were formulated. Firstly, we aimed to optimize the lipid bilayer composition of this combined formulation and secondly, the analysis of the effects of the CD and Cur over some bilayer properties, drug solubility, and drug stability, was carried out. Finally, the presence of CD into lipid vesicles on Cur release behavior was also investigated.
2. Materials and Methods
2.1. Materials
Sigma-Aldrich Co (Barcelone, Spain) supplied Curcumin (Cur), didodecyldimethylammonium bromide (DDAB), cholesterol (Cho), cholesteryl hemisuccinate (Chems), l-α-Phosphatidylcholine from egg yolk (EPC) and 1,2-Dipalmitoyl-sn-glycero-3-phosphocholine (DPPC). Hydroxypropyl)-β-cyclodextrin (HPβCD) was purchased from Roquette (Lestrem, France). Other chemicals were high-quality analytical. Solvents were HPLC quality.
2.2. Quantification of Curcumin
Cur concentration was measured by HPLC (Hitachi Elite LaChrom, San Jose, CA, USA). The analytical method was optimized by using a column Agilent Zorbax SB C-18 4.6 × 150 mm, 3.5 µm, by following a method previously proposed by Musfiroh et al. [
33]. The mobile phase consisted of acetonitrile:acetic acid 2% (50:50
v/
v). The flow rate was fixed at 1.2 mL/min, and had an injection volume of 20 µL. Absorbance was measured at 420 nm. With these conditions, peak areas were measured, and HPLC analysis was conducted at room temperature.
UV/VIS spectrophotometry was used in formulations containing HPβCD. Cur content was measured by using an Agilent 8453 UV-visible spectrophotometer (Agilent Technologies, Budapest, Hungary). A 200-µL sample were diluted up to 5 mL with an acetonitrile-acetic acid 2% 1:1 v/v mixture, and absorbance was measured at 425 nm.
2.3. Phase Solubility Studies
These studies were performed as Higuchi and Connors described [
34]. This approach examines the effect of HPβCD (ligand) on the Cur being solubilized (substrate). Briefly, increasing concentrations of HPβCD (0 mM, 5 mM, 10 mM, 15 mM, 20 mM, and 25 mM) were dissolved in MilliQ water in closed vials. To each vial, 25 mg of Cur was added to reach saturation. Vials were shaken over seven days at room temperature and were protected from light. Samples were filtered through 0.45-µm nylon filters and measured by UV/VIS. All of the samples were performed in duplicate.
Theoretically, we assumed that one Cur molecule may form a complex with
n HPβCD molecules; then, the complexation equilibrium is as follows:
The equilibrium constant
Kc (complexation constant) is expressed as follows:
where [Cur] is the concentration of free drug in the solution and, at saturation, [Cur] is the solubility in water of the free drug
S0:
where
St is the measured solubility in the presence of cyclodextrin solutions.
The n term was obtained from the slope versus . A complexation constant was obtained from the intercept with the y-axis.
Once the drug:cyclodextrin stoichiometric ratio was determined, the apparent stability constant for cur–HPβCD complexes was obtained from the phase-solubility diagrams. In the case of 1:1 ratio (
AL profile),
Ks was calculated as follows:
Data are plotted as the mean ± SD of three independent experiments.
However, for the
Ap-type system and assuming that Cur-HPβCD complexes of 1:1 and 1:2 both coexisted, the stability constants for these two complexes can be estimated from the equation [
35]:
where
K1:1 and
K1:2 are the stability constants of 1:1 and 1:2 inclusion complexes, which can be calculated from the slope and the intercept of the linear curve generated.
The solubility enhancement ratio (ESR) was calculated from the following equation:
Cholesterol (Cho) is one of the main components of liposomes, and it is known to bind well with cyclodextrin, displacing the drug from the hydrophobic core [
12]. To further analyze this replacement, another phase solubility study was performed, adding to each vial 20 mg of Cho and equal molar concentration of Chems. Samples were treated as described previously.
2.4. Preparation of Cur–HPβCD Complex
The complex was formed by dissolving 14.5 mM of HPβCD and 0.4 mM of Cur in citric acid–disodium phosphate buffer at pH 5.4 and stirring the solution over 72 h. This buffer was selected with the aim of maintaining Cur stability. Afterward, the solution was centrifuged at 1000 rpm 10 °C 10 min. The supernatant was collected, and its Cur content was measured.
2.5. Preparation of Liposomes
Liposomes were prepared by thin layer evaporation technique (TLE) as previously described [
20]. In brief, lipid components (EPC, Cho, Cur, and SA or DDAB) were dissolved in a mixture of methanol (3.2 mL) and chloroform (4.8 mL) in a round-bottom flask. The organic solvent was evaporated under vacuum using a rotary evaporator (Büchi R-210 with Heating Bath Büchi B-491, Flawil, Switzerland) until a complete dried lipid film was formed. The fixed temperature in this process was 58 °C, which was above the transition temperature of EPC. Once achieved, three mL of buffer solution (Mellvaine’s citric acid/phosphate buffer pH 5.4) was added, and the film was hydrated until MLV formation. The formulation was rapidly sealed and stored in darkness at 4 °C.
Drug-in-cyclodextrin liposomes by double-loading technique (DCL–DL) were prepared as above. In this case, cholesteryl hemisuccinate (Chems) was added instead of Cho as the lipid component at different molar ratios, as indicated the experimental design. Cur–HPβCD complex (0.04 mM Cur, 3 mL) was incorporated in the aqueous phase of vesicles. The formulation was rapidly sealed and stored in darkness at 4 °C.
As the first objective of this work was to evaluate the effect of the Cur–HPβCD complex on the lipid bilayer of the prepared liposomes, an experimental design was proposed for optimizing the lipid composition of the liposome bilayer in terms of its stability.
Four formulation parameters, named as factors (Chems concentration, DDAB concentration, Cur concentration, and phospholipid type) and three levels of each factor were introduced for generating a L
9 Taguchi orthogonal array. Factors and their levels, as well as the experimental matrix, are shown in
Table 1 and
Table 2. The values of these variables were selected on the basis of our experience as well as from the literature.
Once the experimental data were obtained, the ANOVA test was performed to determine which test parameters were statistically significant for every response variable. The analysis of mean (ANOM) was also realized in order to determine those factors that affect the response and compare the relative strength of the effects.
The effect of each variable on the different responses was calculated by using the following equation:
where
E(Xi) is the effect of levels of the tested variables,
Y(+)i and
Y(−)i are the response variables from the experimental runs in which the variables being tested are added at their maximum and minimum levels, respectively, and L is the number of experiments that are carried out. When the value of effect (
E(Xi)) of the tested variable is positive (>0), the influence of the variable is greater at the high level, while when it is negative (<0), the influence of the variable is greater at the low level [
36].
The Pareto chart shows the absolute values of the standardized effects. On the basis of these charts and analysis of variance (ANOVA) results, we established the variables that exhibited significant main effects on the selected responses.
Finally, optimization techniques have been applied in order to obtain a predictable response as a function of the desired properties. In this work, minimizing the vesicle size and polydispersity index (PdI), and maximizing the PDE and zeta potential, were desirable. Regression analysis has been tested as a mathematical tool in this stage.
2.6. Liposome Characterization
2.6.1. Liposome Size, Polydispersity Index, and Zeta Potential
Liposome size and PdI were determined by dynamic light scattering technique by using Zetasizer Nano-S equipment (Malvern Instruments, Malvern, UK). Results of size were expressed as the average liposomal hydrodynamic diameter (nm). Values of the dimensionless parameter PdI less than 0.5 indicated a homogeneous and monodisperse population.
The surface charge of vesicles was determined by correlation spectroscopy from electrophoretic mobility (μ) measurements, by using the same equipment as above. Results were expressed as zeta potential (Z, mV) after conversion of μ to Z by the Smoluchowski equation: Z = μη/ε, where η is the viscosity, and ε is the permittivity of the solution.
Both measurements were made at room temperature, and 200 µL of samples were diluted with citric-phosphate buffer solution (1/20).
2.6.2. Encapsulation Efficacy
In order to determine the amount of Cur entrapped, encapsulation efficacy (EE) was measured. An aliquot of sample was centrifuged at 10000 rpm 4 °C for one hour. Afterwards, the resulting pellet was treated with sodium lauryl sulfate followed by three cycles of sonication (10 min) and vortex (one min) to disrupt the vesicles. Absorbance of the samples was obtained as mentioned in
Section 2.1.
2.7. Antioxidant Activity
The aim of this assay was to measure the antioxidant activity of Cur due to its ability to scavenge free radicals generated in aqueous and lipophilic phases [
37]. ABTS (2,2′-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid) was used as the oxidizing agent in an electron transfer-based reaction, as was reported by Pisoschi and Negulescu [
38]. This oxidizing radical (ABTS
•+) was obtained after an electron from the nitrogen atom of ABTS was lost. For this, 2.98 mM of ABTS and 0.98 mM of K
2S
2O
8 (potassium persulfate) in purified water were mixed. This solution, which has a dark blue color, is reduced by an antioxidant into colorless ABTS because the nitrogen atom quenches the hydrogen atom, yielding the solution decolorization. The decrease in the absorbance values was monitored, and Trolox (1.05 mg/mL in ethanol absolute) was chosen as the standard antioxidant.
The antioxidant activity of Cur was calculated to the total amount or Cur added to the sample after destroying it with sodium lauryl sulfate, and to the supernatant that resulted after the centrifugation process.
Afterwards, working solutions were prepared into 96-well plates, adding serial dilutions of each sample and placing 10 µL of each of them with 90 μL of ethanol to react with the fresh ABTS solution (100 μL). Trolox standard stock solution was also prepared in ethanol and was equally diluted. Then, the absorbance at 734 nm was measured six minutes after initial mixing (Synergy HT Plate Reader, Winooski, VT, USA). All of the measurements were performed in triplicate.
The antioxidant activity was defined as EC50, which was indicative of the equivalent concentration of antioxidant to decrease the initial concentration of ABTS in 50%. Finally, this parameter was expressed as EC50 compound/EC50 Trolox [
39].
2.8. Differential Scanning Calorimetry (DSC) and Hot Stage Microscopy
Thermal analysis was performed using a DSC (DSC Q20, TA Instruments, New Castle, DE, USA). Samples for DSC were prepared from pure components of the formulation and physical mixtures of all of them with Cur and liposomes. Samples were placed in TA Instruments standard aluminum crucibles with lids of 20-µL capacity. Next, the vessels were tightly closed using a special press. The reference crucible was air in all of the samples except for liposomal formulations, in which the vessel was filled with buffer solution. The scanning was conducted between 25–200 °C at a heating rate of 10 °C/min. The thermal behavior of samples was evaluated by determining the melting temperature (°C), and by calculating the enthalpy (kJ/mol). Thermograms of heat flow versus temperature were depicted.
In addition, different observations were made during heating using a hot stage microscope (HSM Mettler model FP82HT, Greifensee, Switzerland). For this assay, about 10 mg of samples (raw materials and physical mixtures) were placed on glass slides with coverglass and heated at 5 °C/min.
2.9. Transmission Electron Microscopy
Morphological studies were carried out by transmission electron microscopy (TEM) (ZEISS LIBRA 120, Oberkoche, Germany). All samples (10 μL) were previously diluted with one mL of citric/phosphate pH 5.4 solution. Then, a drop of the diluted sample was left to dry on a microscopic copper-coated grid (transmission electron microscopy grid support films of 300-mesh Cu). After drying completely, a drop of an aqueous solution of uranyl acetate (1% w/v) was added for negative staining. Eight minutes later, the excess solution was wiped with filter paper and washed with purified water. Then, the specimen was viewed under the microscope with an accelerating voltage of 75 kV at different magnifications.
2.10. Deformability
The bilayer elasticity of the prepared vesicles was measured by the extrusion method as reported earlier [
40]. Briefly, samples were extruded two times through 800-nm pore sized polycarbonate membrane filters equipped in a Lipex Thermobarrel extruder (Northern Lipids Inc., Burnaby, BC, Canada) under air flow. After samples were homogenized, the recovered volume was measured. The deformability index (DI), which is also named elasticity, was calculated as a relationship between the flux of the sample and the ratio of the measured size and the pore size. To obtain the flux (
Jflux), the recovered liposomes from previous extrusion was extruded through a 100-nm pore size polycarbonate filter (
rp) by applying a pressure of 40 bar for five minutes. The averaged liposome diameter (
rv) was measured by DLS.
The elasticity of the vesicles was calculated from the following equation:
where
Jflux is the rate of penetration through a membrane filter (the volume of sample extruded in one minute);
rv is the vesicle size (after extrusion); and
rp is the size of the membrane pore.
2.11. Phosphorus Content
To further analyze the integrity of the bilayer, the phospholipid recovery after extrusion was also measured. In this case, a Stewart assay was performed to determine the DPPC content before and after extrusion.
Stewart assay is based on the ability of phospholipids to form a complex with ammonium ferrothiocyanate. As an advantage, this protocol avoids interferences with inorganic phosphates, which allows samples to be suspended in any buffer containing phosphate salts [
40]. The protocol was previously described by Zuidam et al. In brief, a standard solution (0.1 M) of ammonium ferrothiocyanate was prepared by adding 27.03 g of ferric chloride and 30.4 g of ammonium thiocyanate into one L of deionized distilled water. At the same time, a standard solution of DPPC (0.1 mg/mL) was prepared in chloroform. For the calibration curve, increasing amounts of lipid standard was added to chloroform up to 2 mL, and the same volume of ferrothiocyanate standard. After vortexing for 15 seconds, samples were centrifuged for five minutes at 1000 rpm and spectrophotometrically quantified at 465 nm. For the liposomes samples, the same procedure was performed before and after extrusion [
41].
2.12. In Vitro Release Studies
In vitro release tests of Cur from liposomes were carried out by the dialysis method. In this study, 1.5 mL of liposome dispersion was placed in a dialysis bag (Spectra/Por 4, molecular cut-off 12–14 kD, Califormina, CA, USA), which was previously rinsed and soaked for 30 min, and both borders were sealed with a dialysis clip. The device was then incubated in 500 mL of dialysis solution. This solution was composed by 25% ethanol and 0.5% Tween®-80 (v/v) in a citric/phosphate buffer pH 5.4. The dialysis bags were stirred at 200 rpm at 25 °C (IKA® RT10). At predetermined intervals of 0.5 h, 1 h, 1.5 h, 2 h, 3 h, 4 h, 5 h, 6 h, and 24 h, 50 µL was taken for the dialysis bag and diluted with acetonitrile:acetic acid 2% 1:1 (v/v). Quantification was performed with a spectrophotometer at 425 nm. The assay was performed in the absence of light.
The results were expressed as the percentage of Cur released from liposomes using the following equation:
where Cur
i is the initial concentration of Cur in the dialysis bag, and Cur
t is the concentration of Cur in the dialysis bag at time t.
From the experimental data, three model-dependent approaches were used to compare the Cur dissolution profiles. The model-dependent approaches included the first-order, the Higuchi, and the Korsmeyer-Peppas models.
In addition, model-independent methods were applied in order to generate a single value from a dissolution profile, thus allowing data to be compared directly. In this study, for each sample, the percentage dissolution efficiency (DE%) is calculated as the percentage ratio of the area under the dissolution curve up to time t (AUDC
0t), and that of the area of the rectangle described by 100% dissolution at the same time point (Q
100·t), and is defined as follows:
4. Discussion
In the present work, an attempt has been made to encapsulate the polyphenol Cur-in-HPβCD-in liposomes as a double-loaded system (DCL–DL). This strategy allows Cur to be loaded into both the phospholipid bilayer (free Cur) and the aqueous core of liposomes (Cur–HPβCD). These supramolecular lipid aggregates were investigated for determining the effect of CD in the bilayer containing Cur.
Among the different charged agents for cationic liposomes, SA and DDAB are widely studied [
53,
54]. As a weak base, with three pKa (7.7–8.5, 9.5–10.7, and 8.5–10.4), Cur may be affected by those lipids, since they possess amino moieties that can affect the pH of dispersion. As Cur can change its color with the loss of the first proton [
42], the interaction between those lipids and Cur was studied. These changes in solution were maintained after the evaporation of solvents during the initial step of liposome production. With the aim of discarding a feasible interaction between lipids and the drug, DSC and HSM studies were performed. From the results obtained, we can conclude that Cur got dissolved in the lipid vehicle during the formation of the lipid film, which can improve the assembly of the drug in the bilayer. This behavior might imply an increased stability.
Characterization studies of these formulations enabled select DDAB as a cationic lipid agent. This lipid surfactant is widely used in the study of vesicles and other biomembrane models [
55,
56,
57]. The conical shape that this lipid achieved in aqueous solution provided an increased stability of liposomes, and has also been extensively reported [
58,
59].
Once the Cur behavior in this lipid bilayer was analyzed, the next step was the study of the influence of the HPβCD–Cur complex over a bilayer already containing Cur. A lot of research has been made about the influence of CD on the vesicle bilayer properties [
12,
49,
50]. However, no researchers are investigating the effect of the Cur–HPβCD complex on Cur-loaded liposomes.
The DCL–DL systems are being used by numerous authors with the aim of increasing the loading capacity of poorly-soluble drugs [
20,
21,
22,
24]. However, one of their possible drawbacks is that the presence of cyclodextrins in these lipid systems can give rise to the removal of some of lipid components due to the affinity of CD for lipophilic compounds [
12]. In particular, many studies have evidenced the kidnapping of Cho by these oligosaccharide structures [
49,
60]. We investigated this issue, and a displacement of Cur from the CD cavity by Cho was actually found. In order to prevent any destabilizing effect on the lipid bilayer, we decided to substitute this steroid by a derivative that is widely used in liposome formulations, Chems.
This hemisuccinate derivative is more water-soluble than Cho. Although this steroid has a similar chemical structure to Cho, the loss of one proton from the hemisuccinate moiety gives rise to a lower capacity of packing the lipid bilayer [
61]. A phase solubility diagram containing Chems demonstrated that not only did Chems have a different performance than Cho [
62], it also augmented
K1:2 and Cur solubility. Thus, Chems was selected as a replacement for Cho.
EPC is commonly used as the main lipid in liposome formulation. This phospholipid is known to be prone to oxidation. As Cur is a potent antioxidant agent, it is not desirable to have any component that is sensitive to oxidation in formulation. For this reason, DPPC was selected as the synthetic saturated phospholipid. However, with the aim of evaluating the beneficial selection of this phospholipid compared with EPC, an additional study based on design of experiments was performed, in which the amounts of Chems, DDAB, and Cur were also included.
In order to optimize the formulation, three levels were fixed for each variable. Size, PdI, and zeta potential were evaluated. The results of this study demonstrated that Chems significantly affected the surface charge of vesicles, decreasing it as Chems concentration increased. As was previously mentioned, Chems is a succinic derivative. This provides the molecule with a pKa of 5.5. Some studies have already demonstrated that Chems’ capability of mimicking Cho behavior depends on the ionization of the structure [
61,
62]. In our case, the pH was adjusted to 5.4, which triggered one half of the ratio of Chems to be ionized, decreasing the surface charge of the liposomes. Another factor affecting the surface charge was the concentration of DDAB, as previously reported by González-Rodríguez et al. [
54].
On the other hand, the vesicle size was affected by the Cur amount on the bilayer and the phospholipid that was employed. Nonetheless, the size alteration may be due to the contribution of other components in the bilayer, as reported in the regression equation (r2 adj 0.006). Moreover, TEM images of samples containing CD showed nanosized structures that were different from the liposomes, which could affect the values of the size distribution. This can explain the variability in sizes obtained from DLS analysis and TEM images.
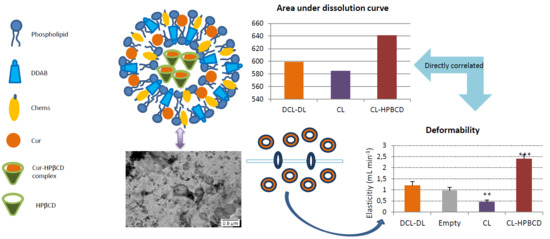
In an attempt to elucidate the reason behind the presence of these nanostructures visualized by TEM, three different samples were prepared, which all contained the same bilayer composition, but had differing aqueous phases: there was one formulation containing only the buffer (CL), a formulation containing the buffer and 14.5 mM of HPβCD (CL–HPβCD) and a formulation that was made after the optimal formulation deduced from the experimental design (DCL–DL). The images clarify that these structures only appear in the presence of CD, independently of the presence of Cur as a complex with CD. Also, images from CL–HPβCD and DCL–DL reported the non-spherical shape of liposomes, which can indicate the presence of deformable liposomes [
63].
To demonstrate this hypothesis, the deformability of liposomes was tested by extrusion, which is a methodology that was previously described by González-Rodríguez et al. [
40]. As mentioned before, empty liposomes possessed a DI close to one mL/min, indicating that the liposomes were extremely flexible. This characteristic was provided by Chems, which is able to increase the elasticity in membranes at a pH above pKa [
61]. The presence of HPBCD solution in the aqueous compartment of the liposome increased the DI 2.5 times compared with empty liposomes. As described before, CD is capable of extracting lipid components to a degree in which holes in the bilayer are created [
49,
64]. It is not clear which component of the bilayer was extracting HPBCD, but it can be concluded from the phase solubility diagram that Chems was not removed due to its lack of interaction with this CD. Regarding DCL–DL, the deformability index was close to that of the empty liposomes, which leads to the conclusion that even though HPBCD increased the DI in liposomes, the Cur condensing effect [
30,
65] counteracted the CD effect. These results are in agreement with those in the literature [
63].
Another positive effect of CD over the formulation was the maintenance of the the antioxidant capability of Cur. For this assay, antioxidant capability via an ABTS test was performed over freshly prepared samples and after one week. As can be observed in
Figure 10, liposome formulations exhibited similar antioxidant capability at time 0, making it clear that HPβCD had no effect over this parameter. After one week, the CL formula maintained Cur antioxidant capability, which is in accordance with the literature [
66]; however, CL–HPβCD and DCL–DL ameliorated the antioxidant activity of Cur, which indicates that a stabilization of Cur was occurring with the addition of this CD. This positive effect of CD over the antioxidant capability of the drug was previously reported by Malakzadeh and Alizadeh [
67]. In this study, an amlodipine besylate-γ-CD complex showed an increased capability of scavenging free radicals due to an enfeeblement of the H–O covalent bond in the –OH radical of amlodipine as a consequence of the strong binding between the drug and CD [
67]. In the case of Cur, a 1:2 Cur/CD ratio was observed; this implies that both ends of the molecule are entrapped inside the CD hydrophobic core, leaving the keto-enolic moiety exposed to the media [
4]. Some authors have proposed that this keto–enolic moiety is attacked by free radicals during oxidation processes [
42]. In addition, Cur–HPβCD binding was found to be really strong when Chems was present in the formulation, as previously reported in
Table 3. Thus, it is possible that the Cur–HPβCD association is weakening the O–H bond, which will trigger an increase in the antioxidant activity of Cur.
Regarding drug release, the liposome samples in all cases showed a biphasic release profile characterized by a burst initial release followed by a much slower release, which was probably due to drug diffusion through the bilayer. This performance was also described by Wang et al. (2016) who compared liposomes containing a risperidone–HPβCD complex with conventional liposomes containing the same drug [
25]. As
Figure 11 depicts, DCL–DL and CL samples performed a slower release when compared with ethanolic and HPβCD–Cur complex solutions. On the other hand, CL–HPβCD did not control drug release as much as DCL–DL and CL.
Release behavior, in terms of magnitude of Cur released over time, was quantified by AUDC0t. This parameter was directly correlated with the deformability index of liposomes. In that way, as r is a positive value, an increase in the deformability of the liposome allows Cur to go through the bilayer more easily, and thus, the amount of Cur that can be released from the liposome is higher.
Moreover, the drug release kinetic was studied. Many different models exist for describing drug release, some of them can be applied to nanoformulations, such as Korsmeyer-Peppas, Higuchi, Weibull, etc [
51,
52]. In our case, three different fitting models were performed: the Korsmeyer-Peppas model, Higuchi model, and first-order kinetic model. From the R
2 values presented in
Table 8, it can be deduced that the Korsmeyer-Peppas model was the most accurate one. This model was already described for nanoparticles [
68], nanoparticles containing liposomes [
69], and liposomes [
70]. In addition, kinetic parameters corresponding to this model were extracted, specifically the release rate constant (K) and the release exponent (
η).
As was mentioned in
Section 3.4.4, the
η values that were obtained corresponded to a Fickian diffusion behavior. These values were smaller for CL, CL–HPβCD, and the ethanolic solution of Cur, and higher for the DCL–DL and Cur–HPBCD solutions. The former three samples lacked a Cur–HPβCD complex, even though CL–HPβCD contained CD (however, this CD was not complexed with any Cur). Cur–HPβCD complex is a component of the latter two samples. This indicated that HPβCD has an impact on the diffusion of Cur during drug release when the inclusion complex is formed as a result of the strong binding between the ligand and substrate.
The release rate constant (K) was also affected by the presence of CD in the formulation.
Table 8 depicts that K in the HPβCD–Cur complex solution was lower than K in the ethanolic Cur solution, indicating that CD permits a slight sustained release in the solution. Contrarily, CL–HPβCD and CL showed similar constants, while DCL–DL exhibited a markedly lower one. The presence of the Cur–CD complex and its high affinity when Chems is added in liposome formulations, contribute to a more sustained release of drug.