Qualification and Application of a Liquid Chromatography-Quadrupole Time-of-Flight Mass Spectrometric Method for the Determination of Adalimumab in Rat Plasma
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Stocks, Standard (STD) and Quality Control (QC) Samples
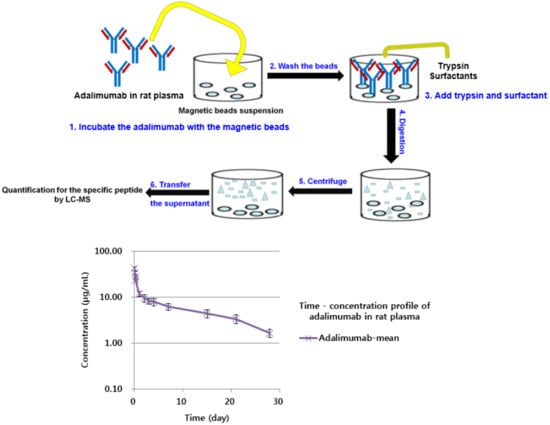
2.3. Preparation of Sample Digests for Quantification
2.4. Liquid Chromatography–Mass Spectrometry
2.5. Method Qualification and Sample Analysis Procedure
2.5.1. Calibration Curve, Accuracy and Precision
2.5.2. Species-Dependent Matrix Effect
2.5.3. Freeze and Thaw Stability
2.6. Software
2.7. Application for a Pharmacokinetic Study in Rat
3. Results
3.1. Method Development
3.1.1. Sample Preparation Method
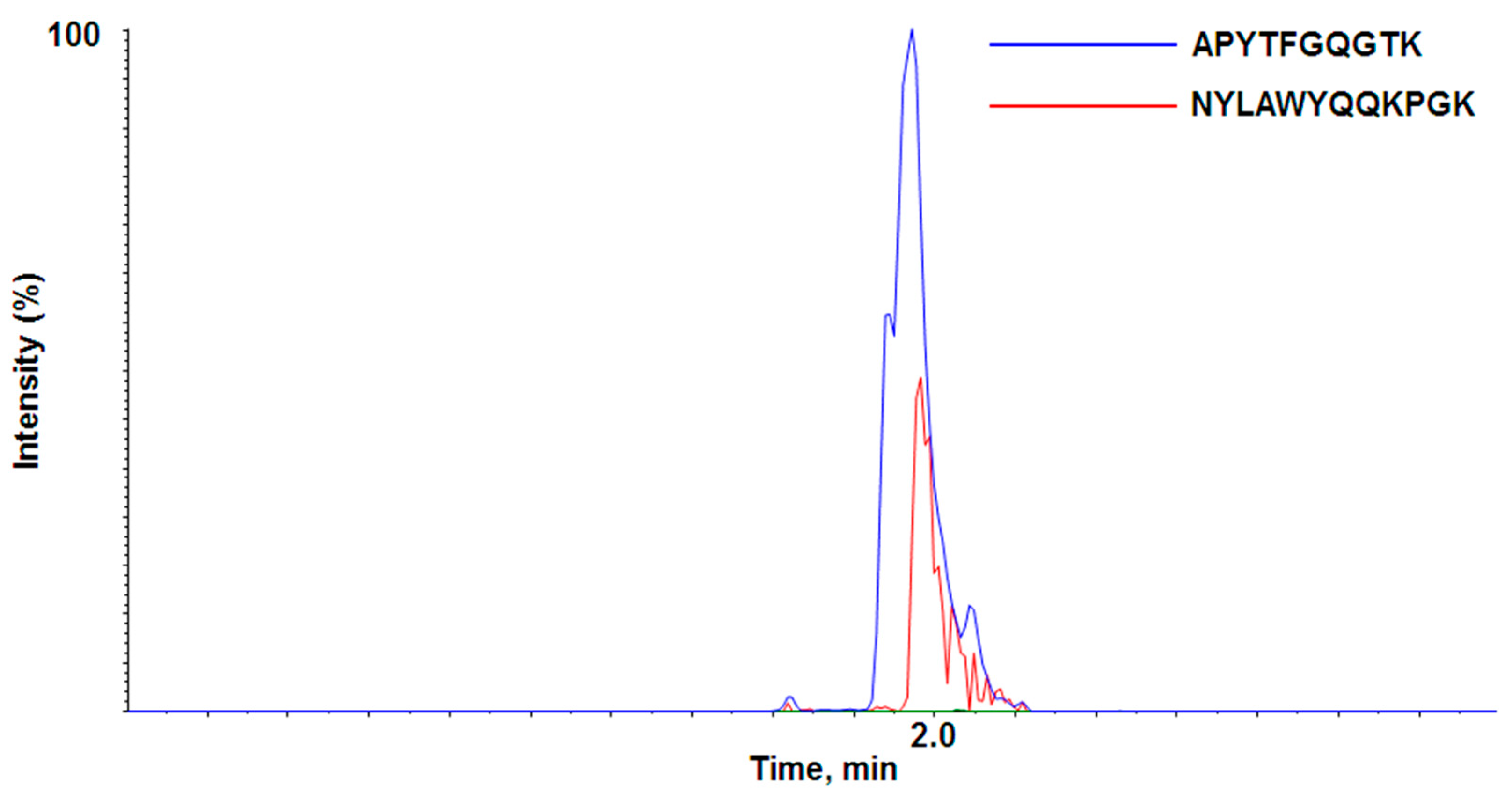
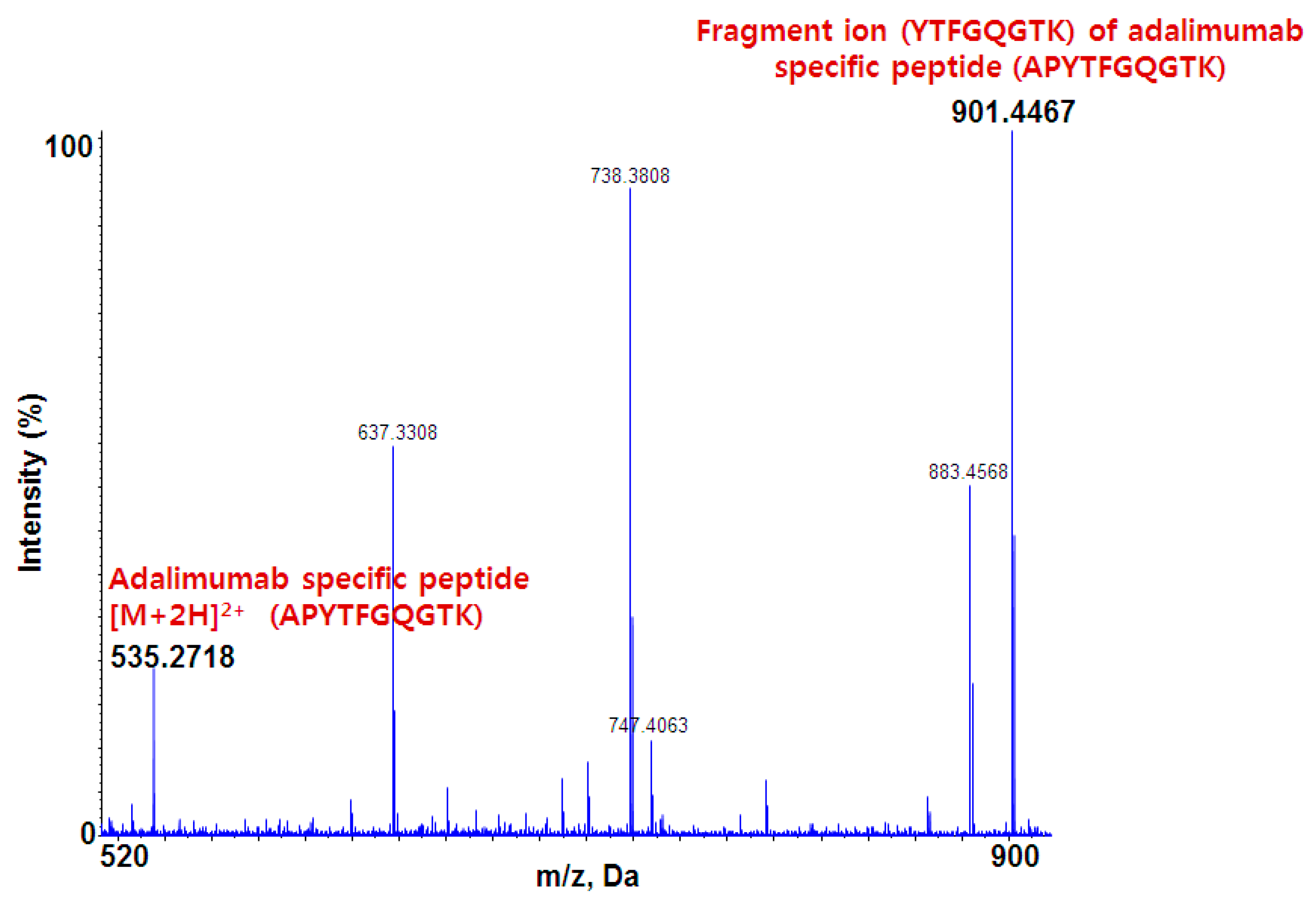
3.1.2. Selection of Target-Specific Signature Peptide
3.1.3. Liquid Chromatography–Mass Spectrometry Analysis Using Quadrupole Time-of-Flight mass spectrometer
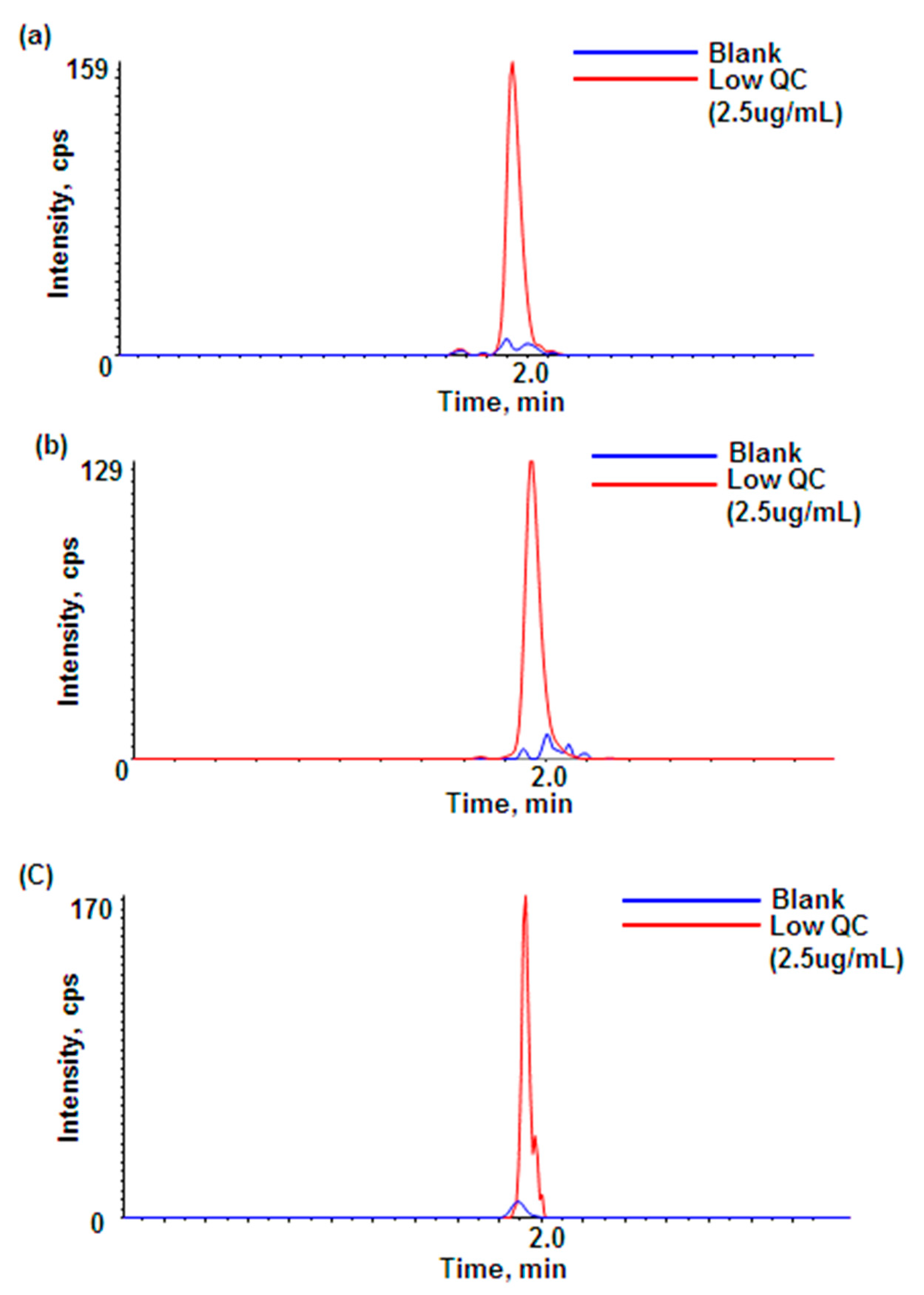
3.2. Method Qualification
3.2.1. Calibration Curve, Linearity and Sensitivity
3.2.2. Accuracy, Precision and Species-Dependent Matrix Effect
3.2.3. Freeze and Thaw Stability
3.2.4. Application to a Pharmacokinetic Study in Rats
4. Discussion
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Ecker, D.M.; Jones, S.D.; Levine, H.L. The therapeutic monoclonal antibody market. MAbs 2015, 7, 9–14. [Google Scholar] [CrossRef] [PubMed]
- Park, M.H.; Lee, M.W.; Shin, Y.G. Qualification and application of a liquid chromatography-quadrupole time-of-flight mass spectrometric method for the determination of trastuzumab in rat plasma. Biomed. Chromatogr. 2016, 30, 625–631. [Google Scholar] [CrossRef] [PubMed]
- Breedveld, F.C. Therapeutic monoclonal antibodies. Lancet 2000, 355, 735–740. [Google Scholar] [CrossRef]
- Catapano, A.L.; Papadopoulos, N. The safety of therapeutic monoclonal antibodies: Implications for cardiovascular disease and targeting the pcsk9 pathway. Atherosclerosis 2013, 228, 18–28. [Google Scholar] [CrossRef] [PubMed]
- Voller, A.; Bartlett, A.; Bidwell, D.E. Enzyme immunoassays with special reference to elisa techniques. J. Clin. Pathol. 1978, 31, 507–520. [Google Scholar] [CrossRef] [PubMed]
- Balkwill, F. Tnf-alpha in promotion and progression of cancer. Cancer Metastasis Rev. 2006, 25, 409–416. [Google Scholar] [CrossRef] [PubMed]
- Azevedo, V.F.; Troiano, L.D.C.; Galli, N.B.; Kleinfelder, A.; Catolino, N.M.; Martins, P.C.U. Adalimumab: A review of the reference product and biosimilars. Biosimilars 2016, 6, 29–44. [Google Scholar] [CrossRef]
- Sandborn, W.J.; Hanauer, S.B.; Rutgeerts, P.; Fedorak, R.N.; Lukas, M.; MacIntosh, D.G.; Panaccione, R.; Wolf, D.; Kent, J.D.; Bittle, B.; et al. Adalimumab for maintenance treatment of crohn’s disease: Results of the classic ii trial. Gut 2007, 56, 1232–1239. [Google Scholar] [CrossRef] [PubMed]
- Darwish, I.A. Immunoassay methods and their applications in pharmaceutical analysis: Basic methodology and recent advances. Int. J. Biomed. Sci. 2006, 2, 217–235. [Google Scholar] [PubMed]
- Liu, H.; Manuilov, A.V.; Chumsae, C.; Babineau, M.L.; Tarcsa, E. Quantitation of a recombinant monoclonal antibody in monkey serum by liquid chromatography-mass spectrometry. Anal. Biochem. 2011, 414, 147–153. [Google Scholar] [CrossRef] [PubMed]
- Becher, F.; Pruvost, A.; Clement, G.; Tabet, J.C.; Ezan, E. Quantification of small therapeutic proteins in plasma by liquid chromatography-tandem mass spectrometry: Application to an elastase inhibitor epi-hne4. Anal. Chem. 2006, 78, 2306–2313. [Google Scholar] [CrossRef] [PubMed]
- Domon, B.; Aebersold, R. Mass spectrometry and protein analysis. Science 2006, 312, 212–217. [Google Scholar] [CrossRef] [PubMed]
- Heudi, O.; Barteau, S.; Zimmer, D.; Schmidt, J.; Bill, K.; Lehmann, N.; Bauer, C.; Kretz, O. Towards absolute quantification of therapeutic monoclonal antibody in serum by lc-ms/ms using isotope-labeled antibody standard and protein cleavage isotope dilution mass spectrometry. Anal. Chem. 2008, 80, 4200–4207. [Google Scholar] [CrossRef] [PubMed]
- Roopenian, D.C.; Akilesh, S. Fcrn: The neonatal fc receptor comes of age. Nat. Rev. Immunol. 2007, 7, 715–725. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Ortiz, R.; Tran, L.; Hall, M.; Spahr, C.; Walker, K.; Laudemann, J.; Miller, S.; Salimi-Moosavi, H.; Lee, J.W. General lc-ms/ms method approach to quantify therapeutic monoclonal antibodies using a common whole antibody internal standard with application to preclinical studies. Anal. Chem. 2012, 84, 1267–1273. [Google Scholar] [CrossRef] [PubMed]
- Shen, H.W.; Yu, A.M. Conference report: New analytical technologies for biological discovery. Bioanalysis 2010, 2, 181–184. [Google Scholar] [CrossRef] [PubMed]
- Ferri, N.; Bellosta, S.; Baldessin, L.; Boccia, D.; Racagni, G.; Corsini, A. Pharmacokinetics interactions of monoclonal antibodies. Pharmacol. Res. 2016, 111, 592–599. [Google Scholar] [CrossRef] [PubMed]
- Cross, T.G.; Hornshaw, M.P. Can lc and lc-ms ever replace immunoassays? J. Appl. Bioanal. 2016, 2, 108–116. [Google Scholar] [CrossRef]
- Wilffert, D.; Bischoff, R.; van de Merbel, N.C. Antibody-free workflows for protein quantification by lc-ms/ms. Bioanalysis 2015, 7, 763–779. [Google Scholar] [CrossRef] [PubMed]
- Kaymakcalan, Z.; Sakorafas, P.; Bose, S.; Scesney, S.; Xiong, L.; Hanzatian, D.K.; Salfeld, J.; Sasso, E.H. Comparisons of affinities, avidities, and complement activation of adalimumab, infliximab, and etanercept in binding to soluble and membrane tumor necrosis factor. Clin. Immunol. 2009, 131, 308–316. [Google Scholar] [CrossRef] [PubMed]


| Run No. | Statistics | QC Low | QC Med | QC High |
|---|---|---|---|---|
| (2.5 µg/mL) | (25 µg/mL) | (50 µg/mL) | ||
| 1 | Mean | 2.53 | 25.3 | 52.3 |
| Precision (%CV) | 13.1 | 1.76 | 3.54 | |
| n | 3 | 3 | 3 | |
| Accuracy (%) | 101 | 101 | 105 | |
| 2 | Mean | 2.73 | 23.9 | 56.3 |
| Precision (%CV) | 2.6 | 11.13 | 11.55 | |
| n | 3 | 3 | 3 | |
| Accuracy (%) | 109 | 95 | 113 | |
| 3 | Mean | 2.57 | 25.1 | 47.3 |
| Precision (%CV) | 17.3 | 10.54 | 4.44 | |
| n | 3 | 3 | 3 | |
| Accuracy (%) | 103 | 100 | 95 | |
| Inter-day | Mean | 2.61 | 24.77 | 51.97 |
| Precision (%CV) | 11 | 7.81 | 6.51 | |
| n | 9 | 9 | 9 | |
| Accuracy (%) | 104 | 99 | 104 |
| (a) | |||||
| Mouse | Theoretical Concentration (μg/mL) | Mean Concentration (μg/mL) | Precision (%CV) | n | Accuracy (%) |
| QC low | 2.5 | 2.85 | 13.2 | 3 | 114 |
| QC medium | 25 | 29.4 | 5.74 | 3 | 118 |
| QC high | 50 | 57 | 1.43 | 3 | 114 |
| (b) | |||||
| Monkey | Theoretical Concentration (μg/mL) | Mean Concentration (μg/mL) | Precision (%CV) | n | Accuracy (%) |
| QC low | 2.5 | 2.09 | 4.9 | 3 | 84 |
| QC medium | 25 | 23.4 | 11.19 | 3 | 94 |
| QC high | 50 | 48.1 | 5.12 | 3 | 96 |
| (a) | |||||
| Rat | Theoretical Concentration (μg/mL) | Mean Concentration (μg/mL) | Precision (%) | n | Accuracy (%) |
| QC low | 2.5 | 2.58 | 10.5 | 3 | 103 |
| QC medium | 25 | 25.6 | 13.67 | 3 | 102 |
| QC high | 50 | 57.3 | 8.73 | 3 | 115 |
| (b) | |||||
| Mouse | Theoretical Concentration (μg/mL) | Mean Concentration (μg/mL) | Precision (%) | n | Accuracy (%) |
| QC low | 2.5 | 2.62 | 16.7 | 3 | 105 |
| QC medium | 25 | 28 | 3.26 | 3 | 112 |
| QC high | 50 | 60.2 | 0.94 | 3 | 120 |
| (c) | |||||
| Monkey | Theoretical Concentration (μg/mL) | Mean Concentration (μg/mL) | Precision (%) | n | Accuracy (%) |
| QC low | 2.5 | 2.26 | 2.8 | 3 | 91 |
| QC medium | 25 | 19.5 | 12.42 | 3 | 78 |
| QC high | 50 | 40.3 | 8.52 | 3 | 81 |
| PK parameters | ||||||||
|---|---|---|---|---|---|---|---|---|
| Compound | AUC (μg∙Day/mL) | Cl (mL/Day/kg) | Alpha Half Life (Day) | Beta Half Life (Day) | Cmax (μg/mL) | V1 (mL/kg) | Vss (mL/kg) | Compartment Model |
| Adalimumab | 155.29 | 6.68 | 0.2 | 9.82 | 41.64 | 24.1 | 85.83 | 2 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, Y.; Kim, N.; Choi, J.; Park, M.-H.; Lee, B.I.; Shin, S.-H.; Byeon, J.-J.; Shin, Y.G. Qualification and Application of a Liquid Chromatography-Quadrupole Time-of-Flight Mass Spectrometric Method for the Determination of Adalimumab in Rat Plasma. Pharmaceutics 2018, 10, 61. https://doi.org/10.3390/pharmaceutics10020061
Park Y, Kim N, Choi J, Park M-H, Lee BI, Shin S-H, Byeon J-J, Shin YG. Qualification and Application of a Liquid Chromatography-Quadrupole Time-of-Flight Mass Spectrometric Method for the Determination of Adalimumab in Rat Plasma. Pharmaceutics. 2018; 10(2):61. https://doi.org/10.3390/pharmaceutics10020061
Chicago/Turabian StylePark, Yuri, Nahye Kim, Jangmi Choi, Min-Ho Park, Byeong Ill Lee, Seok-Ho Shin, Jin-Ju Byeon, and Young G. Shin. 2018. "Qualification and Application of a Liquid Chromatography-Quadrupole Time-of-Flight Mass Spectrometric Method for the Determination of Adalimumab in Rat Plasma" Pharmaceutics 10, no. 2: 61. https://doi.org/10.3390/pharmaceutics10020061