Solvation and Aggregation of Meta-Aminobenzoic Acid in Water: Density Functional Theory and Molecular Dynamics Study
Abstract
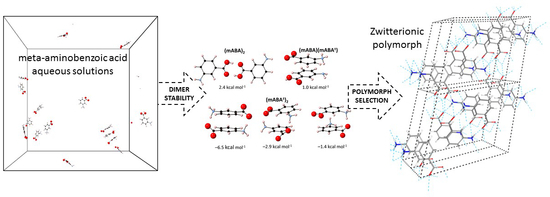
:1. Introduction
2. Computational Methods
2.1. Density Functional Theory Calculations
2.2. Molecular Dynamics Simulations
3. Results
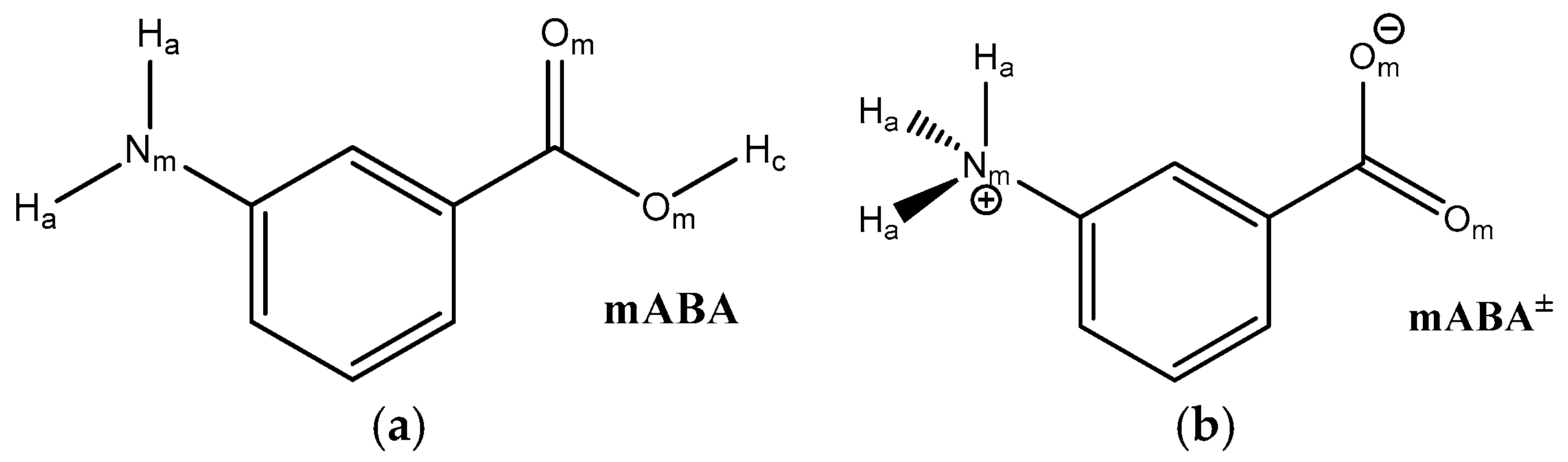
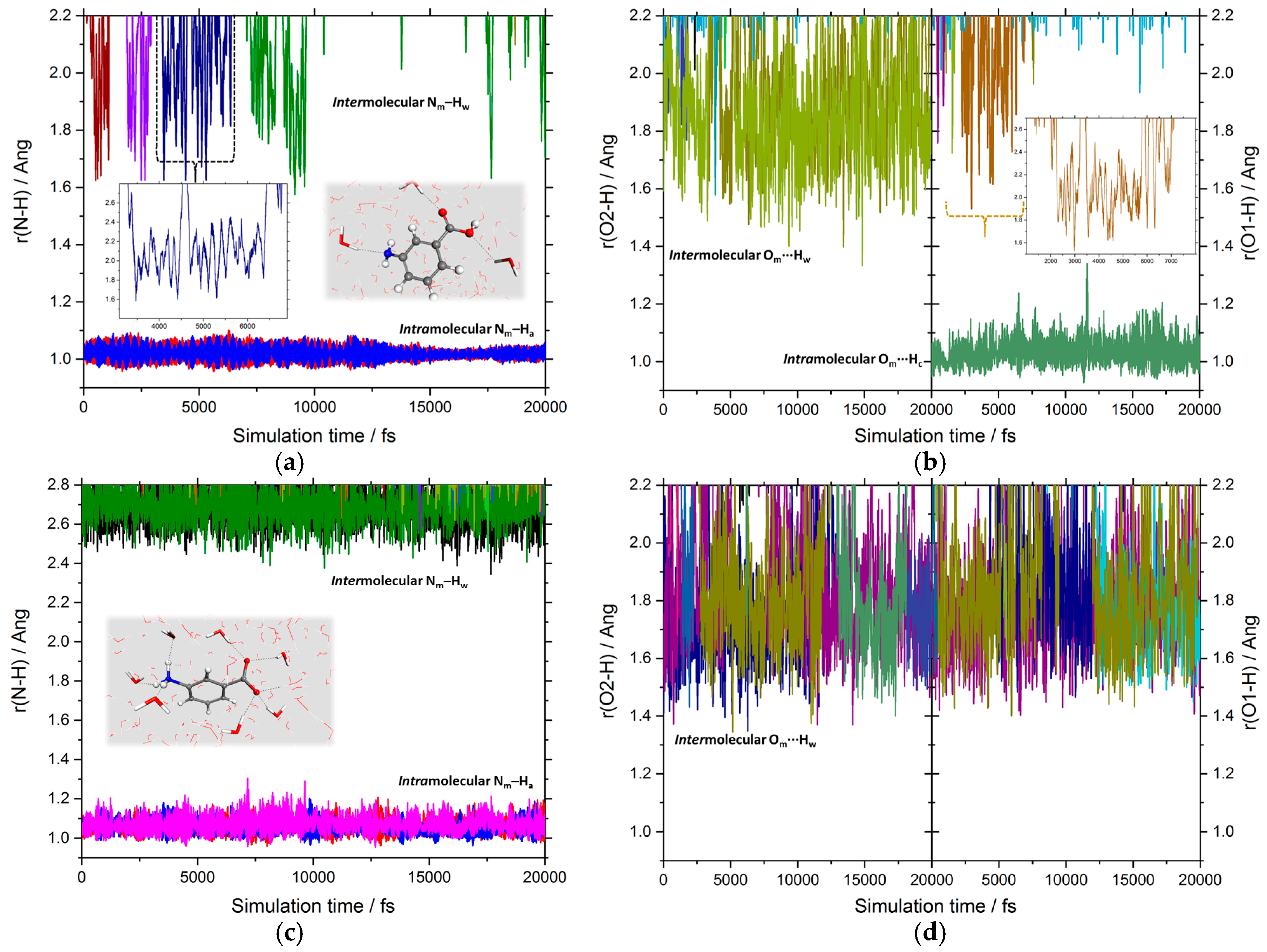
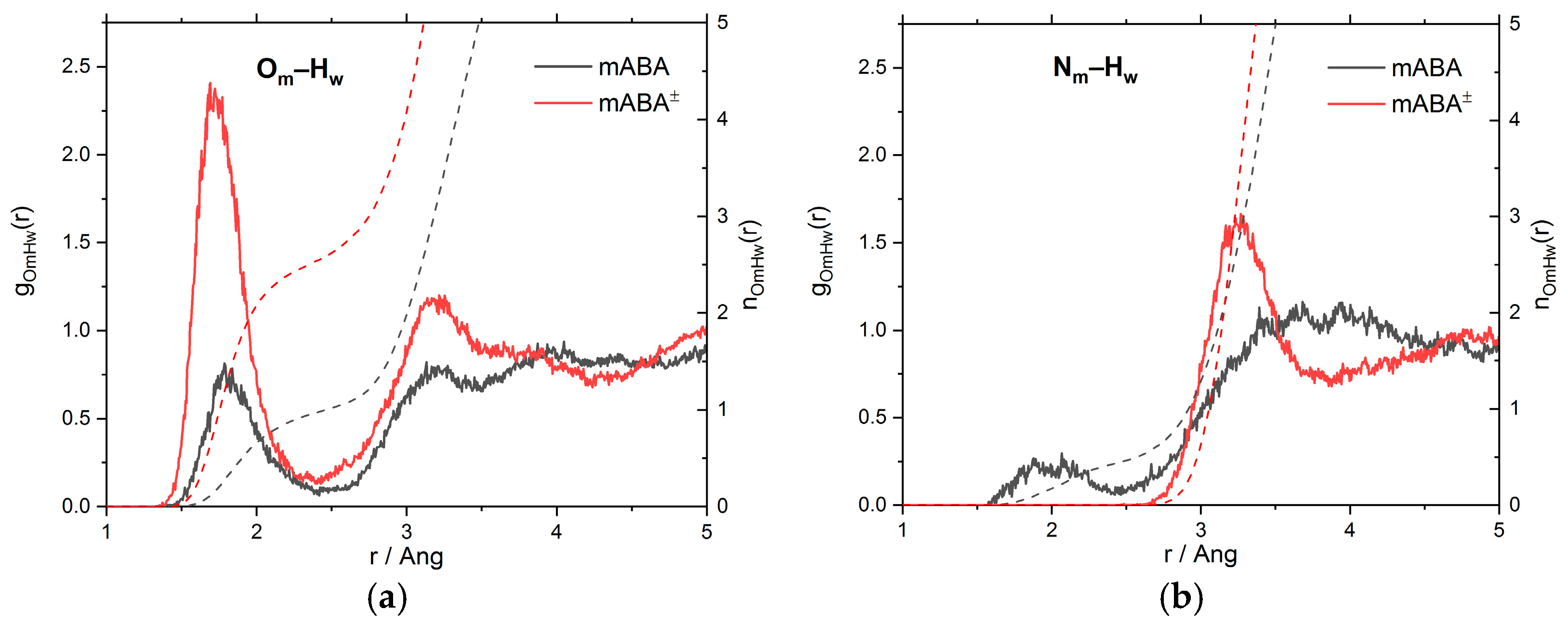
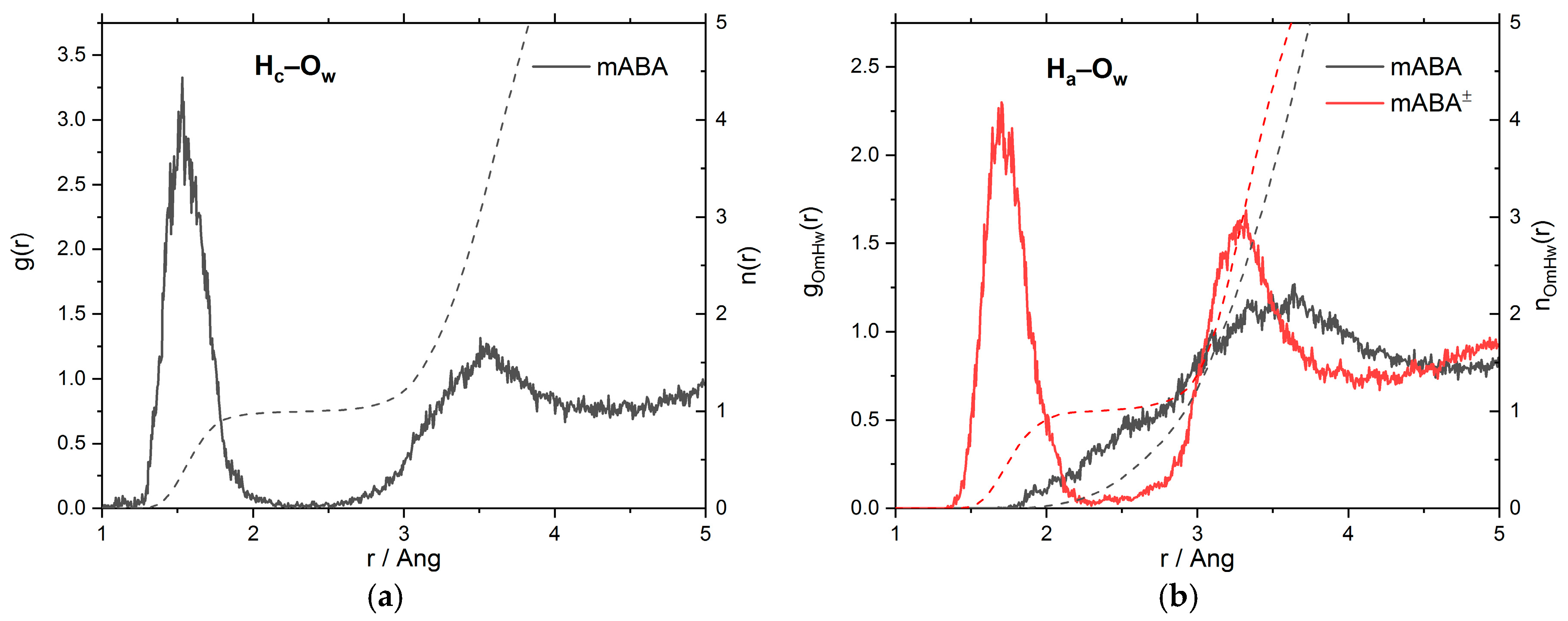
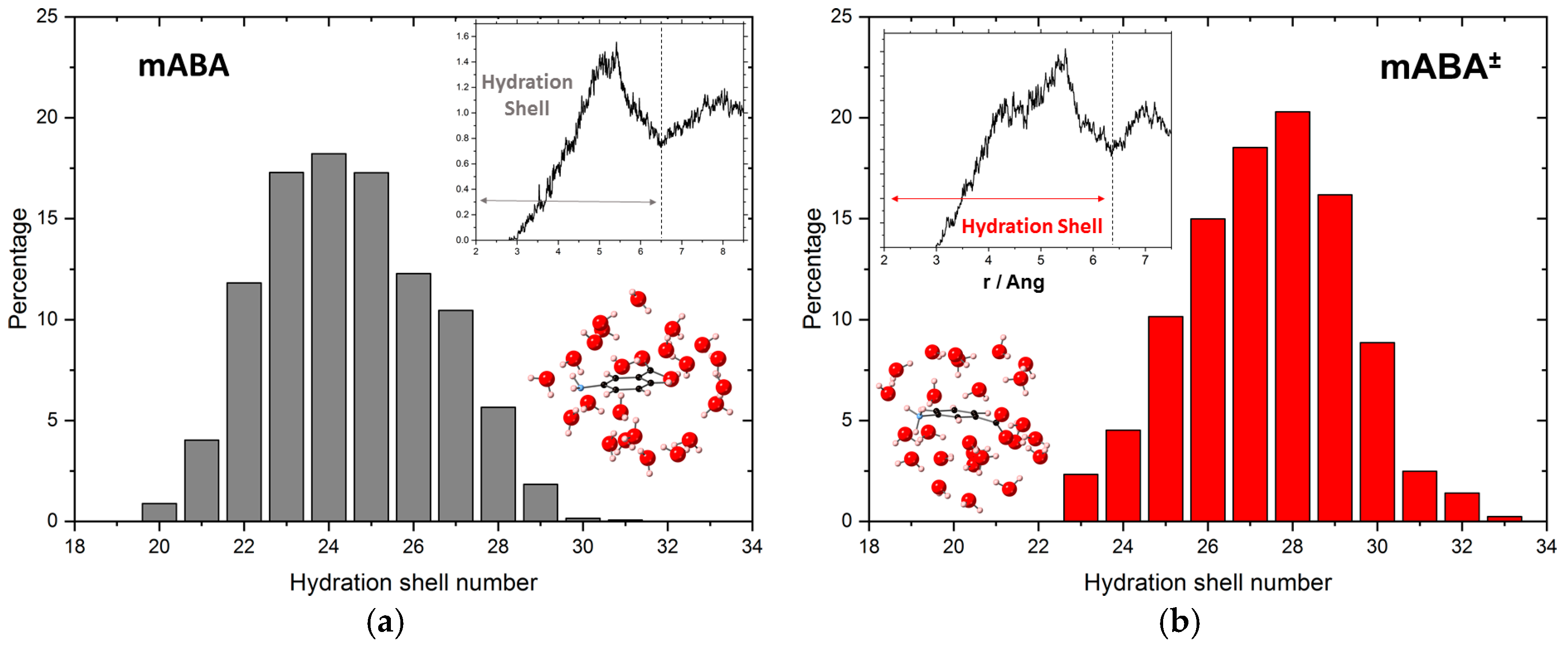
3.1. Intermolecular Properties and Hydration Structure
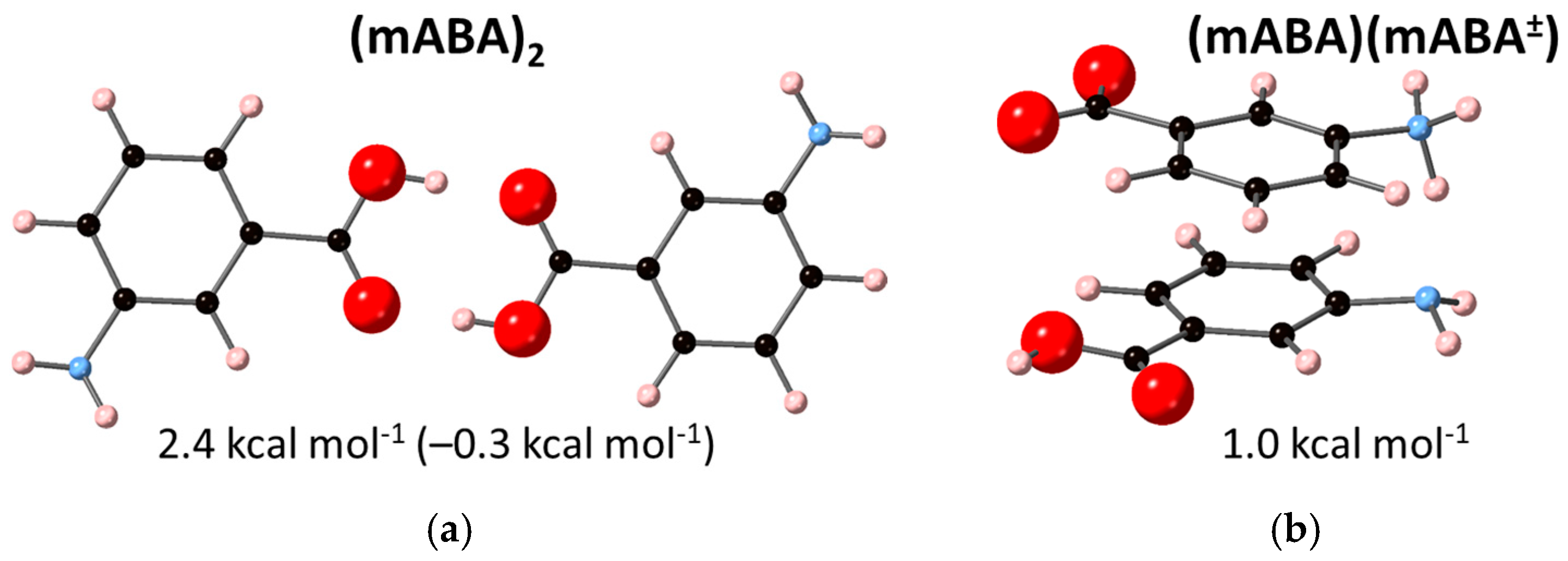
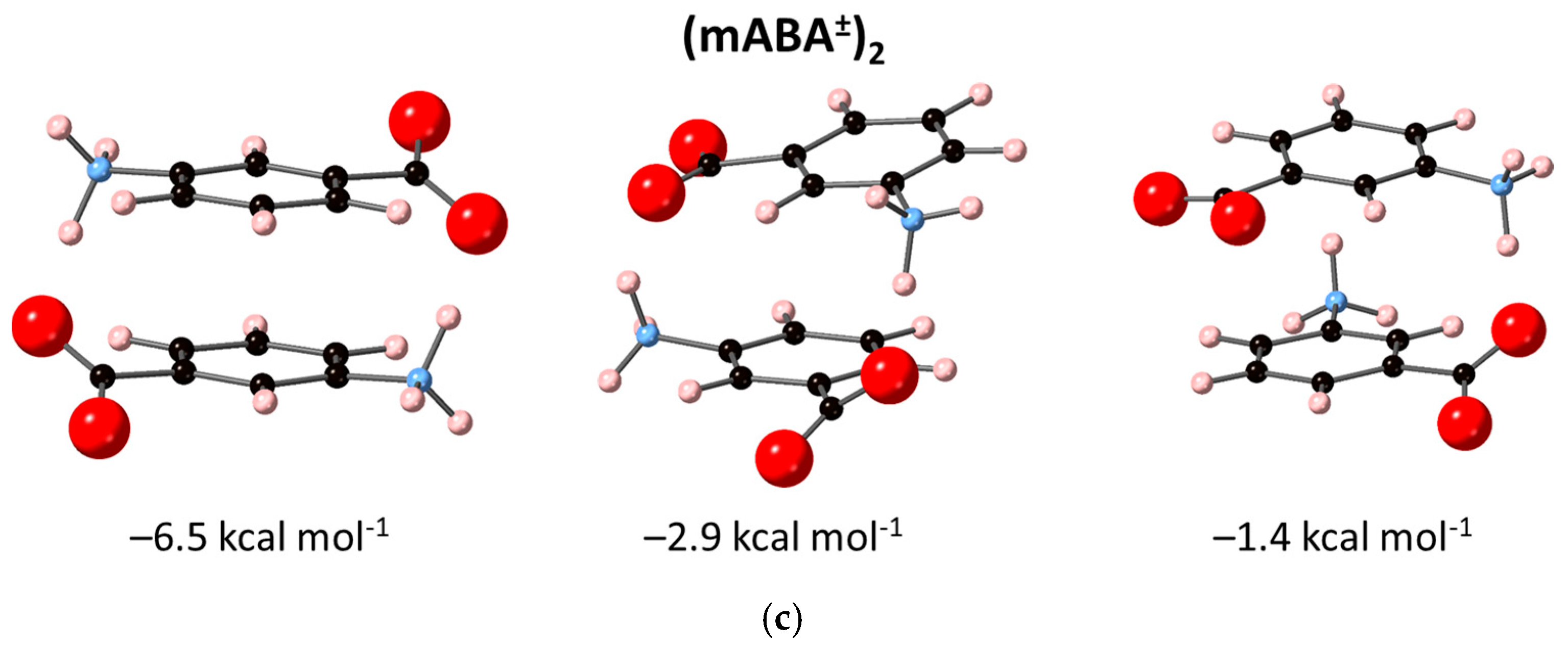
3.2. Dimerization of Meta-Aminobenzoic Acid
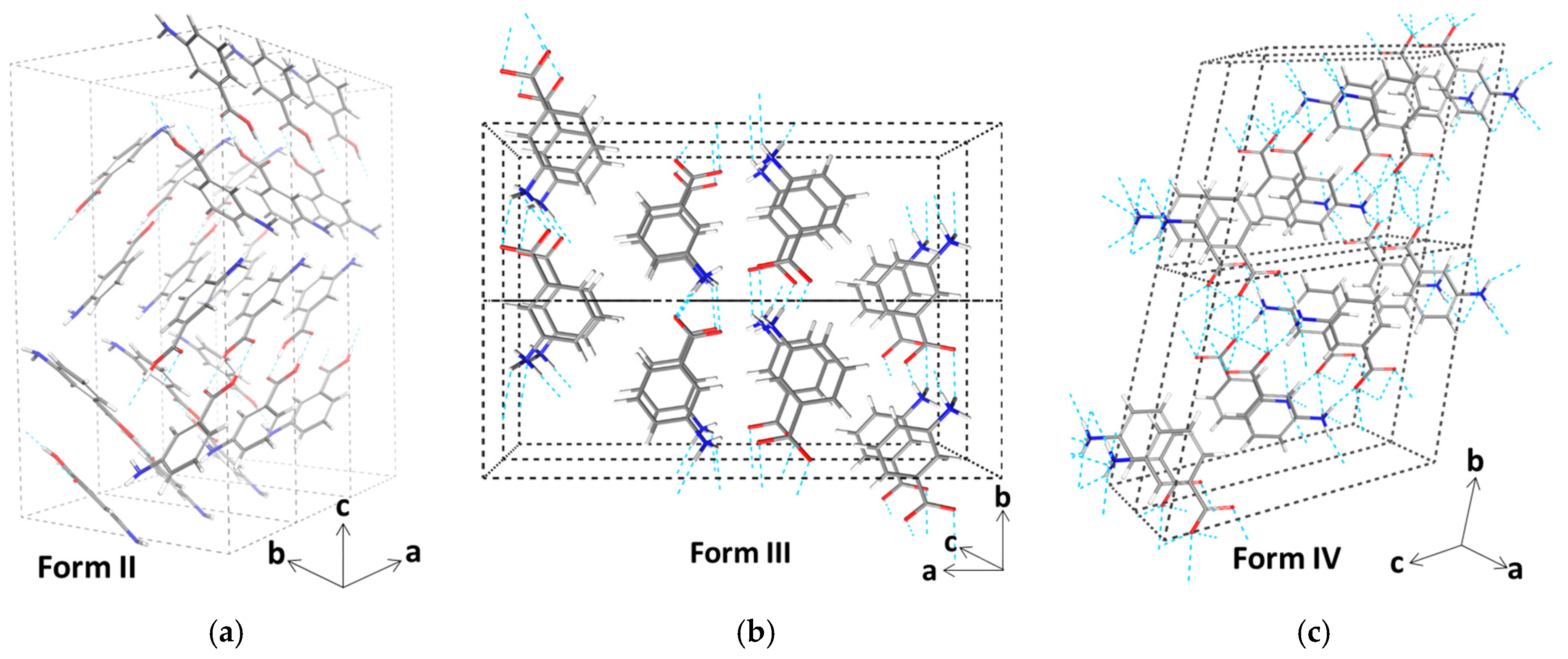
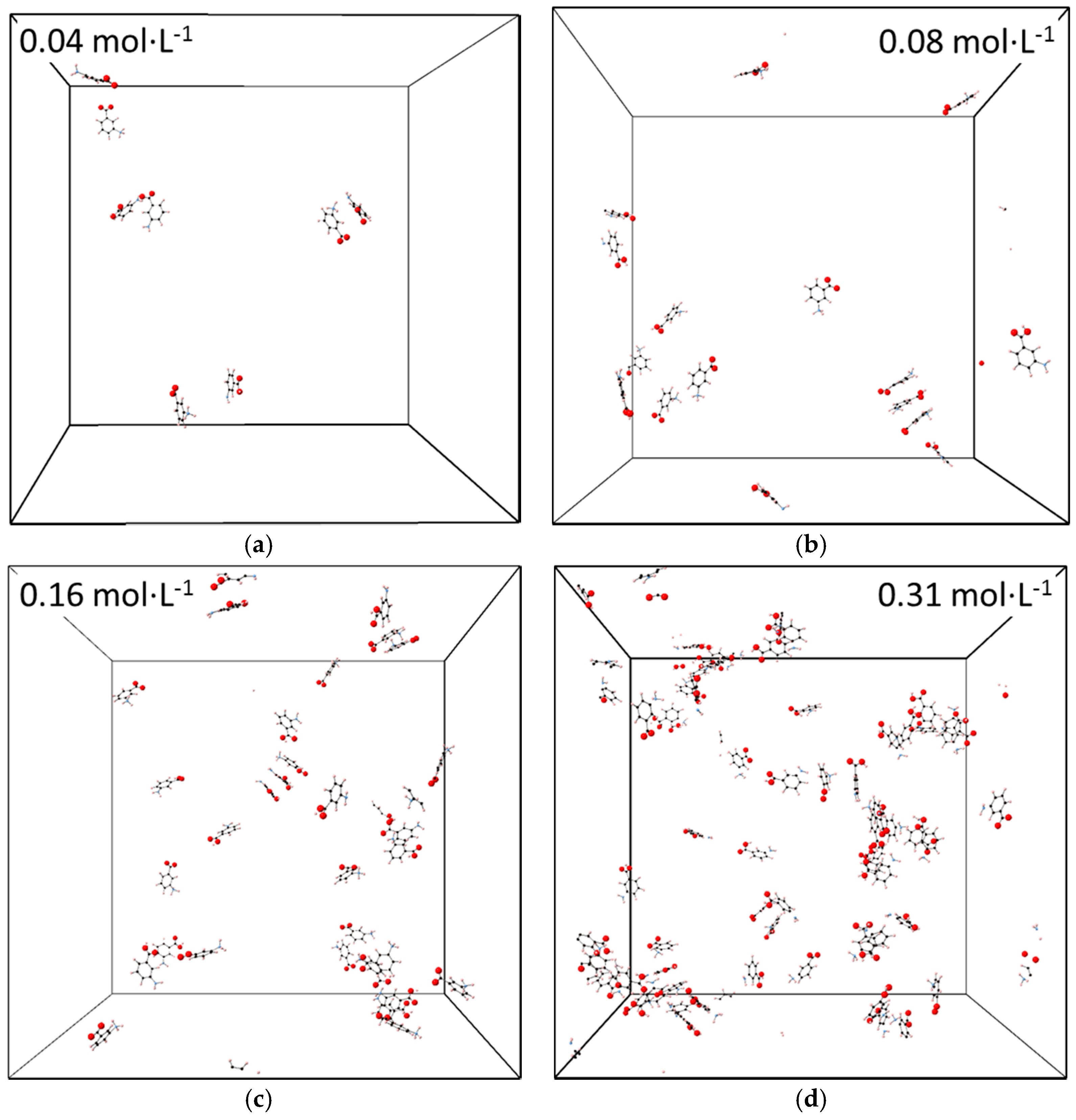
3.3. Molecular Aggregation in Mixed mABA–mABA± Aqueous Solutions
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Palafox, M.A.; Gill, M.; Núñez, J.L. Meta-aminobenzoic acid: Structures and spectral characteristics. Spectrosc. Lett. 1996, 29, 609–629. [Google Scholar] [CrossRef]
- Williams, P.A.; Hughes, C.E.; Lim, G.K.; Kariuki, B.M.; Harris, K.D. Discovery of a new system exhibiting abundant polymorphism: M-aminobenzoic acid. Cryst. Growth Des. 2012, 12, 3104–3113. [Google Scholar] [CrossRef]
- Théorêt, A. Structure moléculaire et zwitterion des acides aminés—I. Spectres infrarouges des acides o, m et p-aminobenzoïques dans différentes formes cristallines. Spectrochim. Acta Part A Mol. Spectrosc. 1971, 27, 11–18. [Google Scholar] [CrossRef]
- Svärd, M.; Nordström, F.L.; Jasnobulka, T.; Rasmuson, Å.C. Thermodynamics and nucleation kinetics of m-aminobenzoic acid polymorphs. Cryst. Growth Des. 2010, 10, 195–204. [Google Scholar] [CrossRef]
- Kumar, S.S.; Nangia, A. A solubility comparison of neutral and zwitterionic polymorphs. Cryst. Growth Des. 2014, 14, 1865–1881. [Google Scholar] [CrossRef]
- Lahav, M.; Leiserowitz, L. The effect of solvent on crystal growth and morphology. Chem. Eng. Sci. 2001, 56, 2245–2253. [Google Scholar] [CrossRef]
- Ter Horst, J.H.; Geertman, R.M.; van Rosmalen, G.M. The effect of solvent on crystal morphology. J. Cryst. Growth 2001, 230, 277–284. [Google Scholar] [CrossRef]
- Gaines, E.; Maisuria, K.; Di Tommaso, D. The role of solvent in the self-assembly of m-aminobenzoic acid: A density functional theory and molecular dynamics study. CrystEngComm 2016, 18, 2937–2948. [Google Scholar] [CrossRef]
- Blagden, N.; Davey, R.J. Polymorph selection: Challenges for the future? Cryst. Growth Des. 2003, 3, 873–885. [Google Scholar] [CrossRef]
- Musumeci, D.; Hunter, C.A.; McCabe, J.F. Solvent effects on acridine polymorphism. Cryst. Growth Des. 2010, 10, 1661–1664. [Google Scholar] [CrossRef]
- Kitamura, M.; Umeda, E.; Miki, K. Mechanism of solvent effect in polymorphic crystallization of BPT. Ind. Eng. Chem. Res. 2012, 51, 12814–12820. [Google Scholar] [CrossRef]
- Hughes, C.E.; Williams, P.A.; Harris, K.D.M. “CLASSIC NMR”: An In-Situ NMR Strategy for Mapping the Time-Evolution of Crystallization Processes by Combined Liquid-State and Solid-State Measurements. Angew. Chem. Int. Ed. 2014, 53, 8939–8943. [Google Scholar] [CrossRef] [PubMed]
- Kumler, W.D. Acidic and basic dissociation constants and structure. J. Org. Chem. 1955, 20, 700–706. [Google Scholar] [CrossRef]
- Cohn, E.J.; Edsall, J.T. Proteins, Amino Acids and Peptides; Reinhold Publishing Corporation: New York, NY, USA, 1943. [Google Scholar]
- Bjerrun, N.Z. Die Konstitution der Ampholyte, besonders der Aminosäuren, und ihre Dissoziationskonstanten. Phys. Chem. 1923, 104, 147–173. [Google Scholar]
- Valiev, M.; Bylaska, E.J.; Govind, N.; Kowalski, K.; Straatsma, T.P.; Van Dam, H.J.; Wang, D.; Nieplocha, J.; Apra, E.; Windus, T.L.; et al. NWChem: A comprehensive and scalable open-source solution for large scale molecular simulations. Comput. Phys. Commun. 2010, 181, 1477–1489. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A.; et al. Gaussian Software; Gaussian, Inc.: Wallingford, CT, USA, 2009. [Google Scholar]
- Grimme, S. Semiempirical GGA-type density functional constructed with a long-range dispersion correction. J. Comput. Chem. 2006, 27, 1787–1799. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Truhlar, D.G. The M06 suite of density functionals for main group thermochemistry, thermochemical kinetics, noncovalent interactions, excited states, and transition elements: Two new functionals and systematic testing of four M06-class functionals and 12 other functionals. Theor. Chem. Acta 2008, 120, 215–241. [Google Scholar]
- Marenich, A.V.; Cramer, C.J.; Truhlar, D.G. Universal solvation model based on solute electron density and on a continuum model of the solvent defined by the bulk dielectric constant and atomic surface tensions. J.Phys.Chem. B 2009, 113, 6378–6396. [Google Scholar] [CrossRef] [PubMed]
- Ho, J.; Klamt, A.; Coote, M.L. Comment on the correct use of continuum solvent models. J. Phys. Chem. A 2010, 114, 13442–13444. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, R.F.; Marenich, A.V.; Cramer, C.J.; Truhlar, D.G. The solvation, partitioning, hydrogen bonding, and dimerization of nucleotide bases: A multifaceted challenge for quantum chemistry. Phys. Chem. Chem. Phys. 2011, 13, 10908–10922. [Google Scholar] [CrossRef] [PubMed]
- Do, H.; Besley, N.A. Structural optimization of molecular clusters with density functional theory combined with basin hopping. J. Chem. Phys. 2012, 137, 134106. [Google Scholar] [CrossRef] [PubMed]
- Montero, L.A.; Esteva, A.M.; Molina, J.; Zapardiel, A.; Herna, L.; Màrquez, H.; Acosta, A. A theoretical approach to analytical properties of 2, 4-diamino-5-phenylthiazole in water solution. Tautomerism and dependence on pH. J. Am. Chem. Soc. 1998, 120, 12023–12033. [Google Scholar] [CrossRef]
- Sanchez-Garcia, E.; Studentkowski, M.; Montero Luis, A.; Sander, W. Noncovalent Complexes between Dimethyl Ether and Formic Acid—An Ab Initio and Matrix Isolation Study. ChemPhysChem 2005, 6, 618–624. [Google Scholar] [CrossRef] [PubMed]
- Hutter, J.; Iannuzzi, M.; Schiffmann, F.; VandeVondele, J. CP2K: Atomistic simulations of condensed matter systems. Wiley Interdiscip. Rev. Comput. Mol. Sci. 2014, 4, 15–25. [Google Scholar] [CrossRef] [Green Version]
- Perdew, J.; Burke, K.; Ernzerhof, M. Generalized gradient approximation made simple. Phys. Rev. Lett. 1996, 77, 3865–3868. [Google Scholar] [CrossRef] [PubMed]
- Goedecker, S.; Teter, M.; Hutter, J. Separable dual-space Gaussian pseudopotentials. Phys. Rev. B 1996, 54, 1703–1710. [Google Scholar] [CrossRef]
- Van Der Spoel, D.; Lindahl, E.; Hess, B.; Groenhof, G.; Mark, A.E.; Berendsen, H.J.C. GROMACS: Fast, flexible, and free. J. Comput. Chem. 2005, 26, 1701–1718. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Wolf, R.M.; Caldwell, J.W.; Kollman, P.A.; Case, D.A. Development and testing of a general amber force field. J. Comput. Chem. 2004, 25, 1157–1174. [Google Scholar] [CrossRef] [PubMed]
- Salvalaglio, M.; Perego, C.; Giberti, F.; Mazzotti, M.; Parrinello, M. Molecular-dynamics simulations of urea nucleation from aqueous solution. Proc. Natl. Acad. Sci. USA 2014, 112, E6–E14. [Google Scholar] [CrossRef] [PubMed]
- Salvalaglio, M.; Giberti, F.; Parrinello, M. 1,3,5-Tris (4-bromophenyl) benzene prenucleation clusters from metadynamics. Acta Crystallogr. Sect. C Struct. Chem. 2014, 70, 132–136. [Google Scholar] [CrossRef] [PubMed]
- Toroz, D.; Hammond, R.B.; Roberts, K.J.; Harris, S.; Ridley, T. Molecular dynamics simulations of organic crystal dissolution: The lifetime and stability of the polymorphic forms of para-amino benzoic acid in aqueous environment. J. Cryst. Growth 2014, 401, 38–43. [Google Scholar] [CrossRef]
- Berendsen, H.J.C.; Grigera, J.R.; Straatsma, T.P. The missing term in effective pair potentials. J. Phys. Chem. 1987, 91, 6269–6271. [Google Scholar] [CrossRef]
- Gaigeot, M.-P.; Sprik, M. Ab initio molecular dynamics study of uracil in aqueous solution. J. Phys. Chem. B 2004, 108, 7458–7467. [Google Scholar] [CrossRef]
- Tang, E.; Di Tommaso, D.; de Leeuw, N.H. Hydrogen transfer and hydration properties of HnPO4 3 − n (n = 0–3) in water studied by first principles molecular dynamics simulations. J. Chem. Phys. 2009, 130, 234502. [Google Scholar] [CrossRef] [PubMed]
- Davey, R.J.; Dent, G.; Mughal, R.K.; Parveen, S. Concerning the relationship between structural and growth synthons in crystal nucleation: Solution and crystal chemistry of carboxylic acids as revealed through IR spectroscopy. Cryst. Growth Des. 2006, 6, 1788–1796. [Google Scholar] [CrossRef]
- Du, W.; Cruz-Cabeza, A.J.; Woutersen, S.; Davey, R.J.; Yin, Q. Can the study of self-assembly in solution lead to a good model for the nucleation pathway? The case of tolfenamic acid. Chem. Sci. 2015, 6, 3515–3524. [Google Scholar] [CrossRef]
- Khamar, D.; Zeglinski, J.; Mealey, D.; Rasmuson, Å.C. Investigating the Role of Solvent–Solute Interaction in Crystal Nucleation of Salicylic Acid from Organic Solvents. J. Am. Chem. Soc. 2014, 136, 11664–11673. [Google Scholar] [CrossRef] [PubMed]

| mABA | mABA± | |
|---|---|---|
| 1.79 | 1.72 | |
| 0.81 | 2.38 | |
| 2.50 | 2.52 | |
| 0.09 | 0.17 | |
| 9.00 | 14.00 | |
| 1.0 | 2.6 | |
| 1.88 | - | |
| 0.27 | - | |
| 2.46 | - | |
| 0.06 | - | |
| 4.50 | - | |
| 0.5 | 0 |
| mABA | mABA± | |
|---|---|---|
| 1.51 | - | |
| 3.06 | - | |
| 2.31 | - | |
| 0.01 | - | |
| 306.00 | - | |
| 1.0 | - | |
| - | 1.77 | |
| - | 2.15 | |
| - | 2.23 | |
| - | 0.03 | |
| - | 71.7 | |
| 0 | 1.0 |
| Reaction | |||
|---|---|---|---|
| 2 mABA → (mABA)2 | −18.3 | –6.6 | –0.1 1 |
| 2.4 2 | |||
| mABA + mABA± → (mABA)(mABA±) | 1.3 2 | ||
| 2 mABA± → (mABA±)2 | – | – | –5.8 2 |
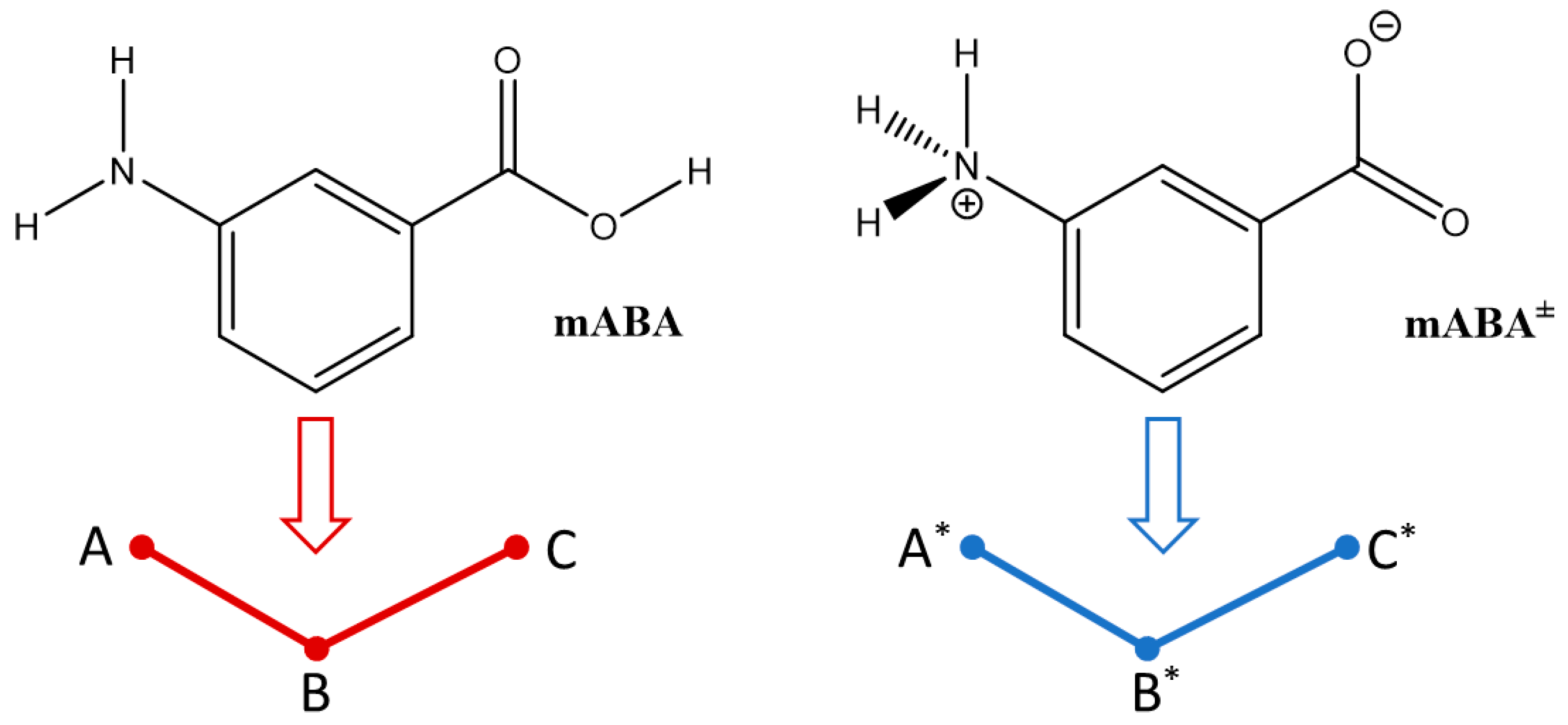
| A* | B* | C* | A | B | C | |
|---|---|---|---|---|---|---|
| A* | 0.2 | 0.6 | 8.7 | 5.3 | 3.4 | 3.5 |
| B* | 9.1 | 2.7 | 2.6 | 10.0 | 7.6 | |
| C* | 0.1 | 3.6 | 2.3 | 3.6 | ||
| A | 4.3 | 4.2 | 5.2 | |||
| B | 6.1 | 10.6 | ||||
| C | 6.5 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gaines, E.; Di Tommaso, D. Solvation and Aggregation of Meta-Aminobenzoic Acid in Water: Density Functional Theory and Molecular Dynamics Study. Pharmaceutics 2018, 10, 12. https://doi.org/10.3390/pharmaceutics10010012
Gaines E, Di Tommaso D. Solvation and Aggregation of Meta-Aminobenzoic Acid in Water: Density Functional Theory and Molecular Dynamics Study. Pharmaceutics. 2018; 10(1):12. https://doi.org/10.3390/pharmaceutics10010012
Chicago/Turabian StyleGaines, Etienne, and Devis Di Tommaso. 2018. "Solvation and Aggregation of Meta-Aminobenzoic Acid in Water: Density Functional Theory and Molecular Dynamics Study" Pharmaceutics 10, no. 1: 12. https://doi.org/10.3390/pharmaceutics10010012
APA StyleGaines, E., & Di Tommaso, D. (2018). Solvation and Aggregation of Meta-Aminobenzoic Acid in Water: Density Functional Theory and Molecular Dynamics Study. Pharmaceutics, 10(1), 12. https://doi.org/10.3390/pharmaceutics10010012