An Adenovirus-Vectored Influenza Vaccine Induces Durable Cross-Protective Hemagglutinin Stalk Antibody Responses in Mice
Abstract
:1. Introduction
2. Materials and Methods
2.1. Viruses
2.2. Recombinant Adenoviral Vectored Vaccine
2.3. Animals
2.4. Cell Lines
2.5. Protein, Recombinant Chimeric HA Protein and Mouse Monoclonal Antibodies (mAbs)
2.6. Generation of Convalescent Sera for Vaccine Candidates
2.7. Vaccination/Challenge Experiments
2.8. Passive Immunization
2.9. Mouse Immunoglobulin Isotyping Magnetic Bead Panel
2.10. Enzyme Linked Immunosorbent Assay (ELISA)
2.11. Magnetic Luminex Screening Assay
2.12. Hemagglutination Inhibition (HI) Assay
2.13. Microneutralization (MN) Assay
2.14. Statistics
3. Results
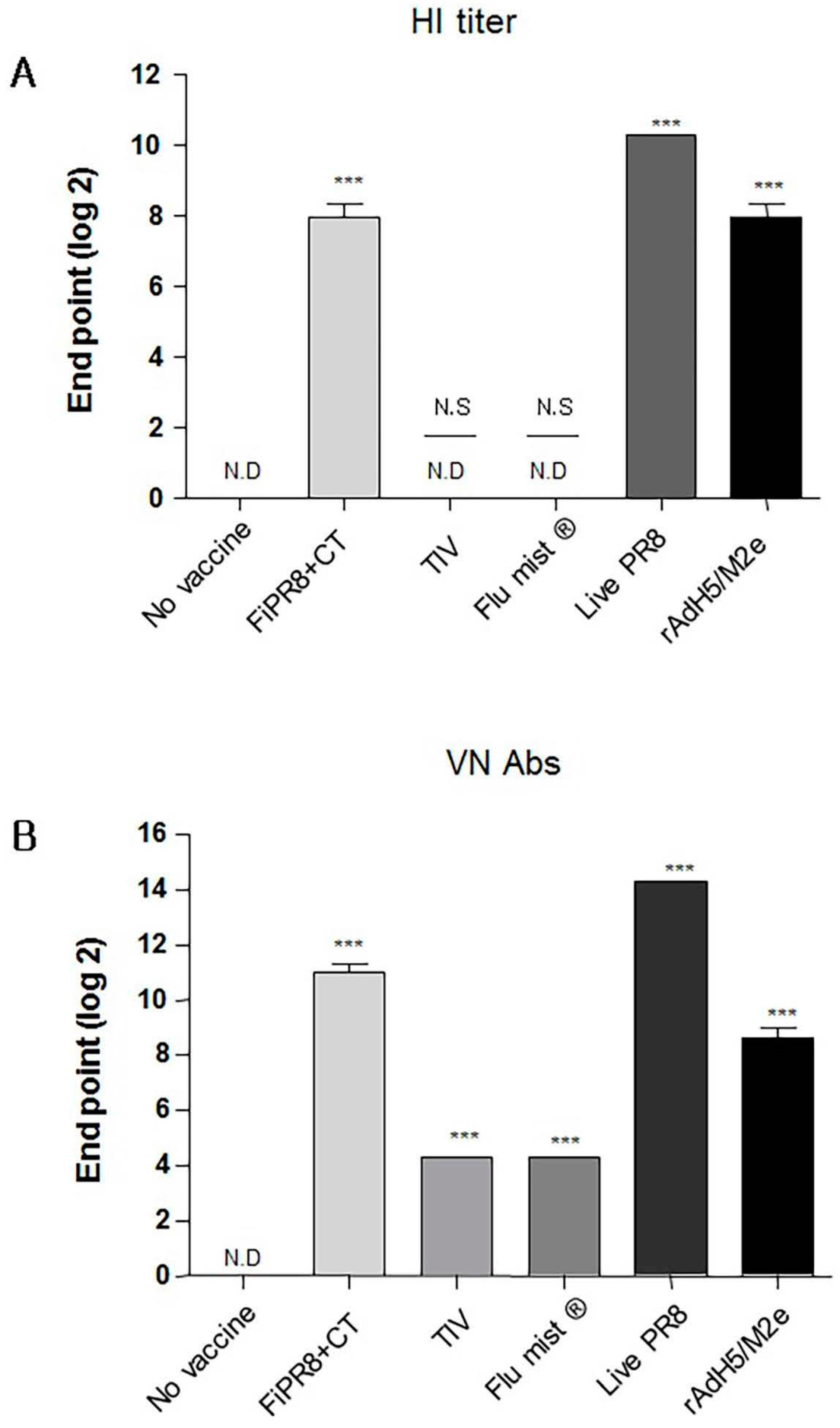
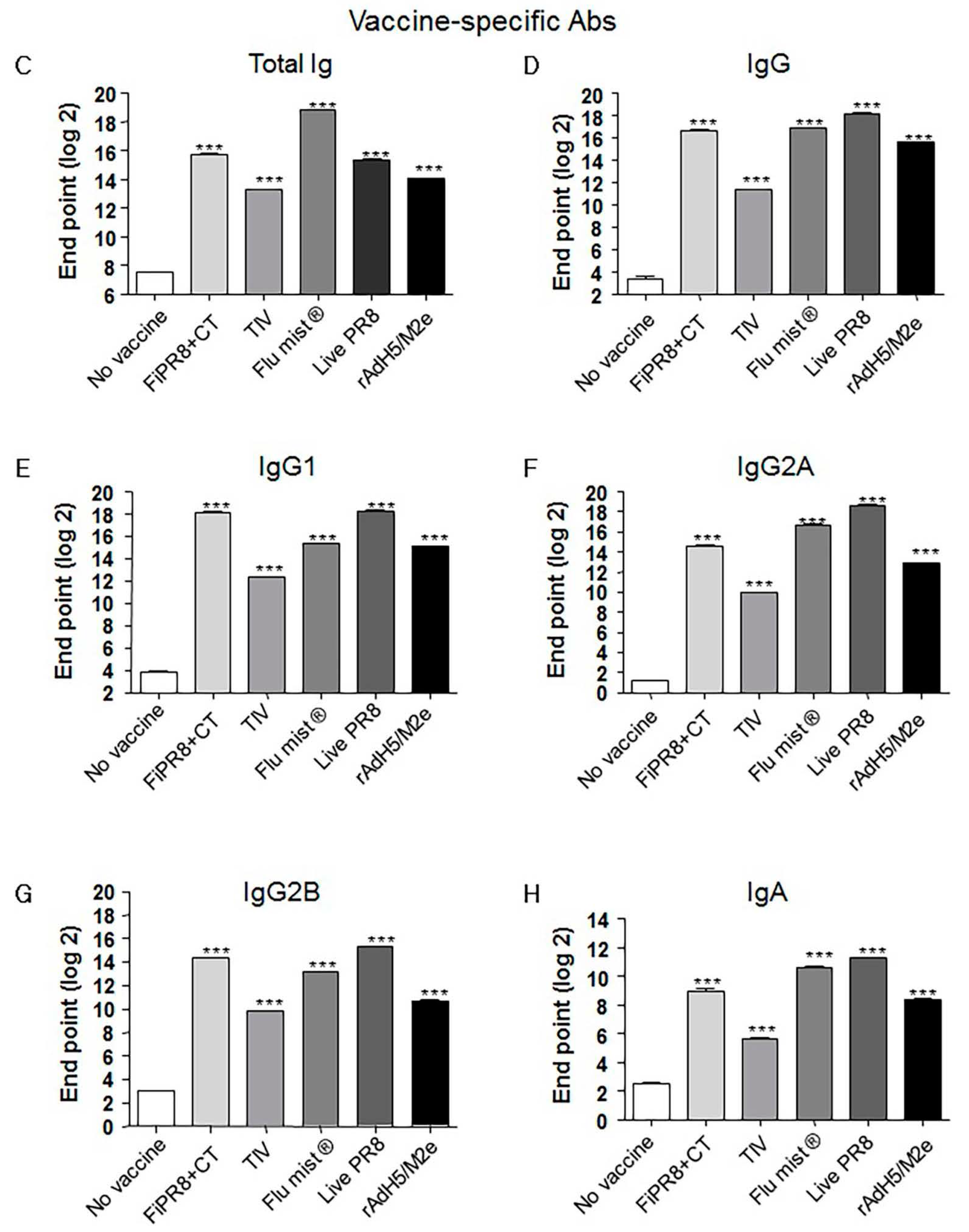
3.1. Influenza Vaccines Induce Neutralizing Th1/Th2 Vaccine-Specific Antibody Responses
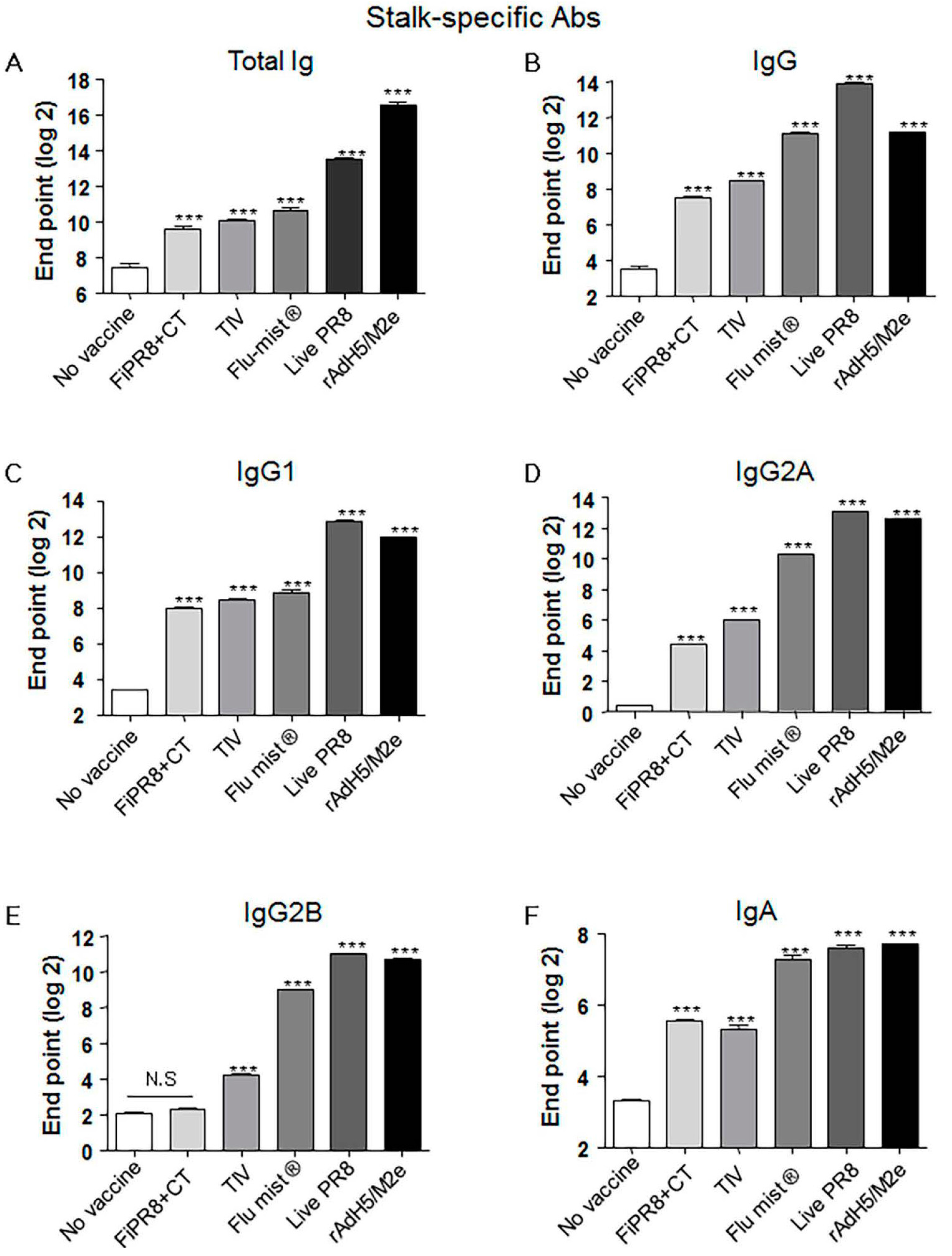
3.2. Adenovirus-Vectored Vaccine Induce HA Stalk-Specific Antibodies
3.3. Adenovirus-Vectored Influenza Vaccine Skews the Th1/Th2 Balance towards a Th2 Cytokine Response
3.4. Adenovirus-Vectored Vaccine but Not Inactivated Virus Induce Cross-Protective Humoral Immunity
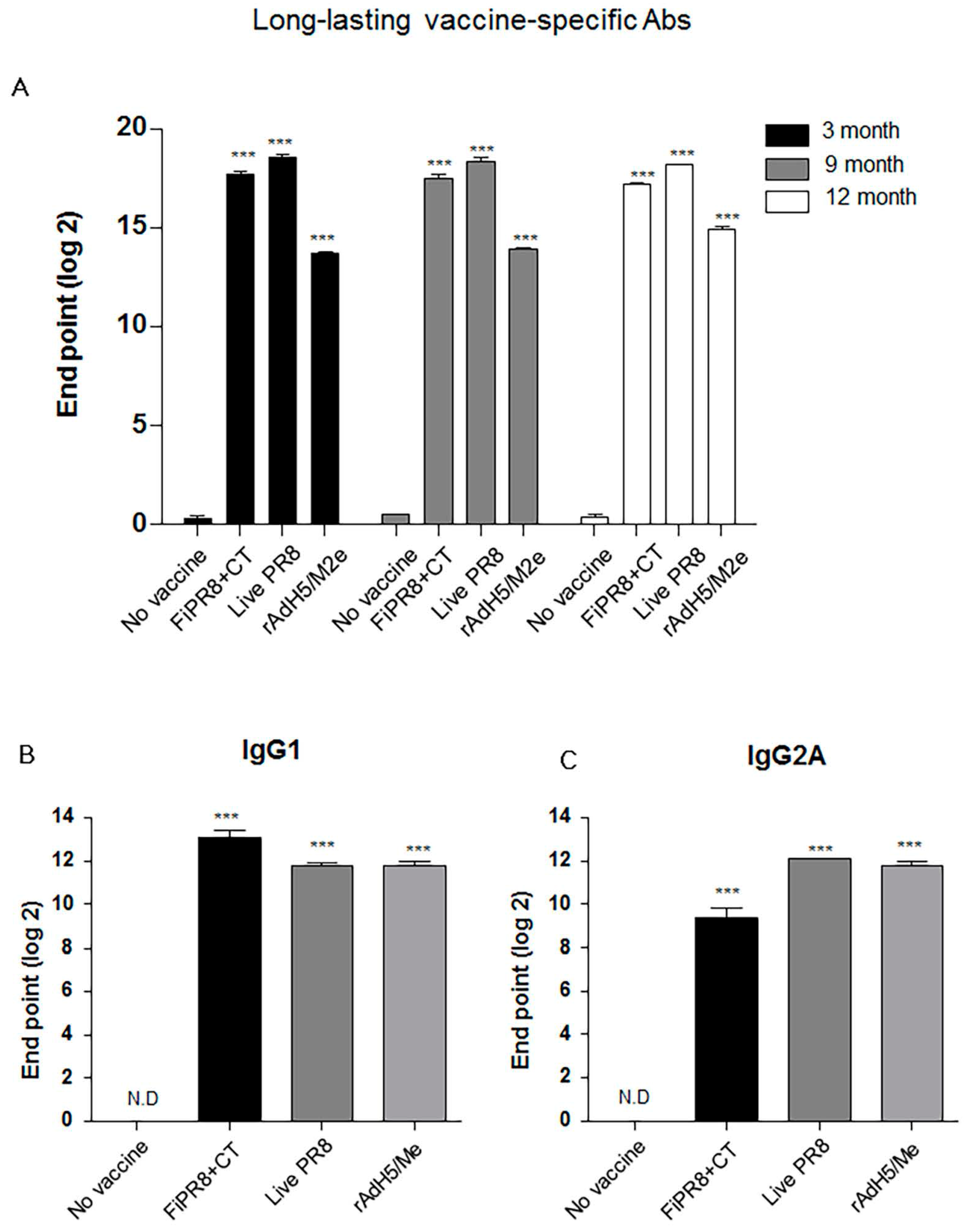
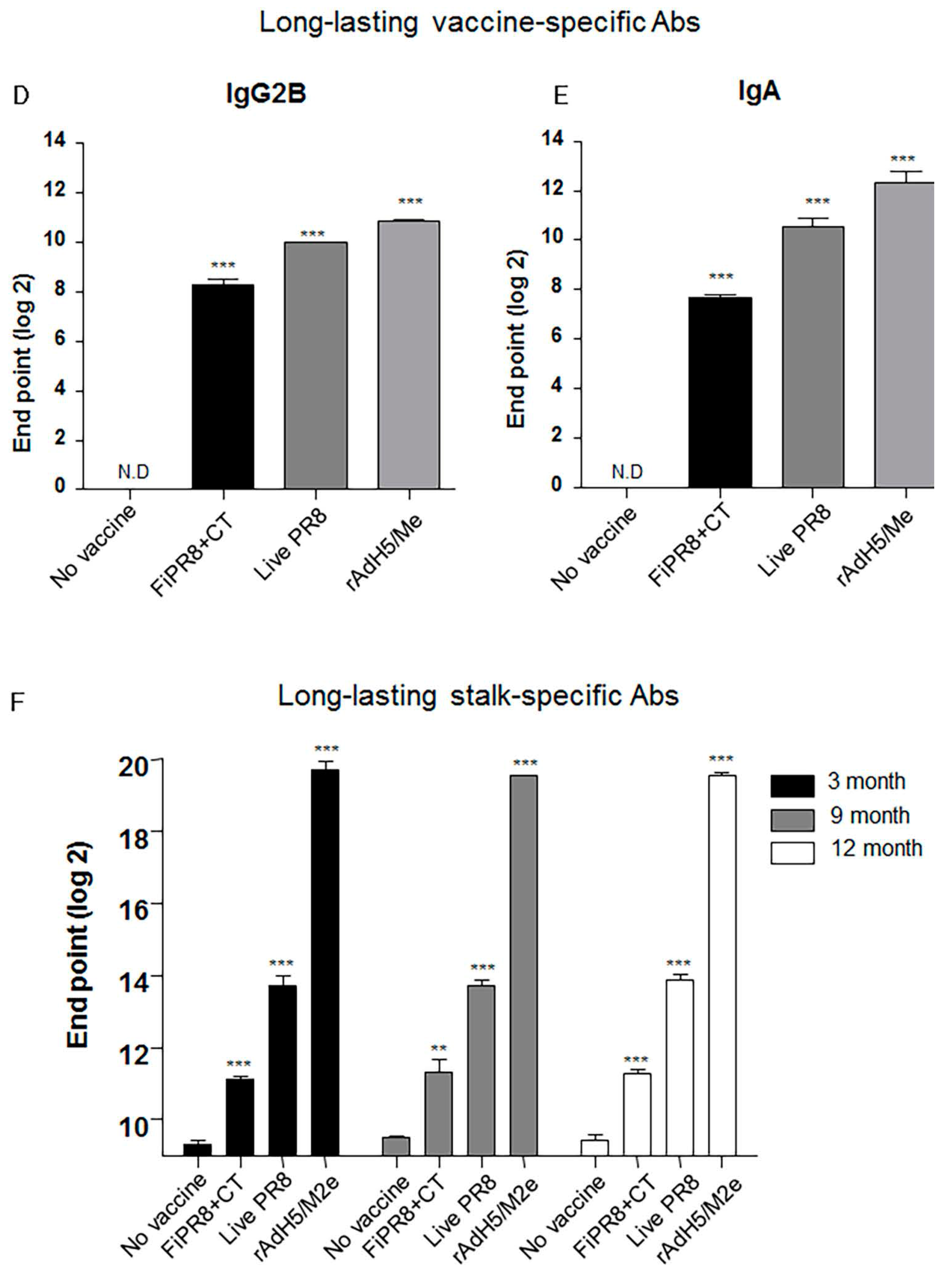
3.5. rAdH5/M2e Vaccination Induced Durable Hemagglutinin Stalk-Specific Antibody Responses
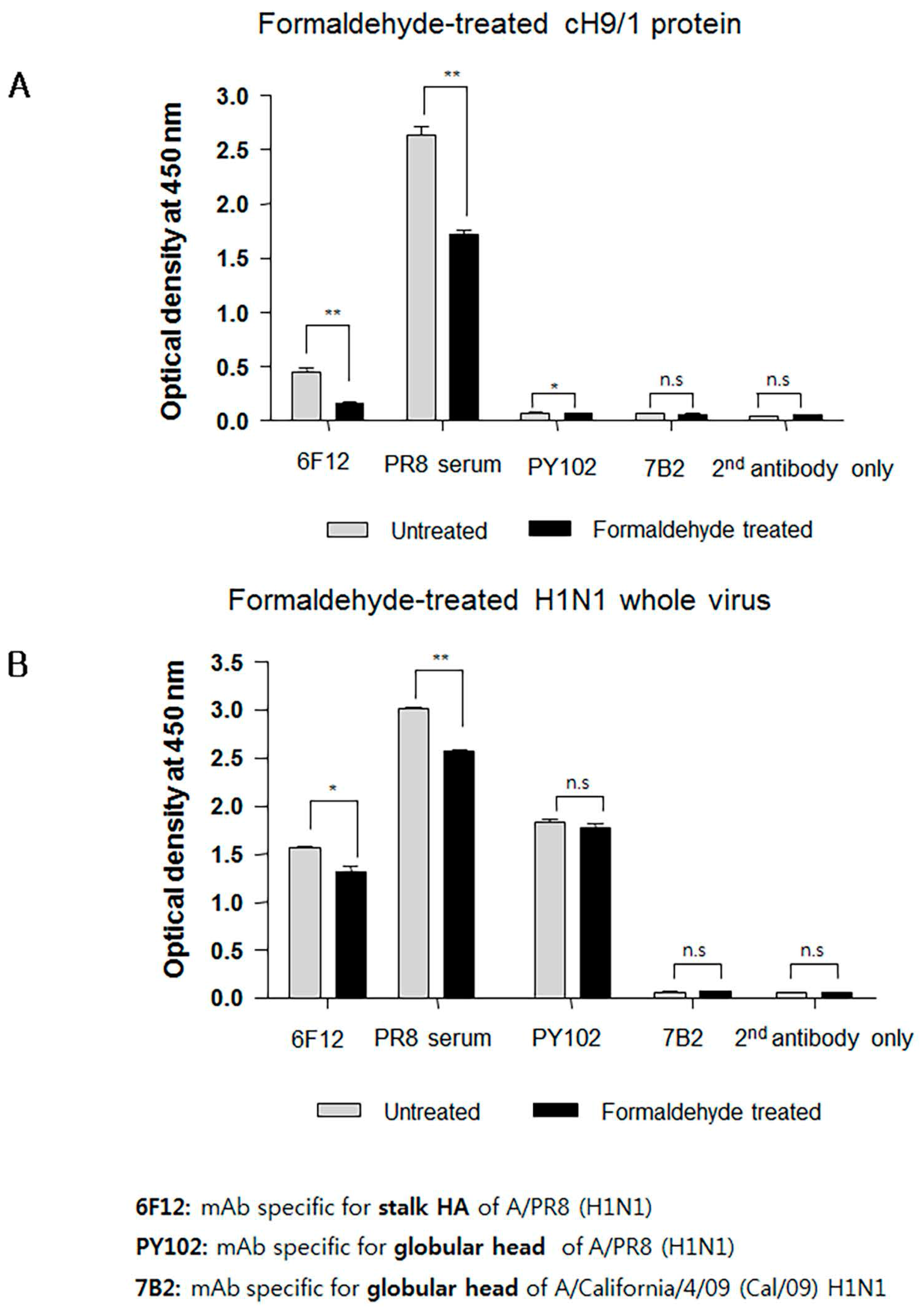
3.6. Formaldehyde Treatment Reduced Binding of HA Stalk-Specific Antibodies
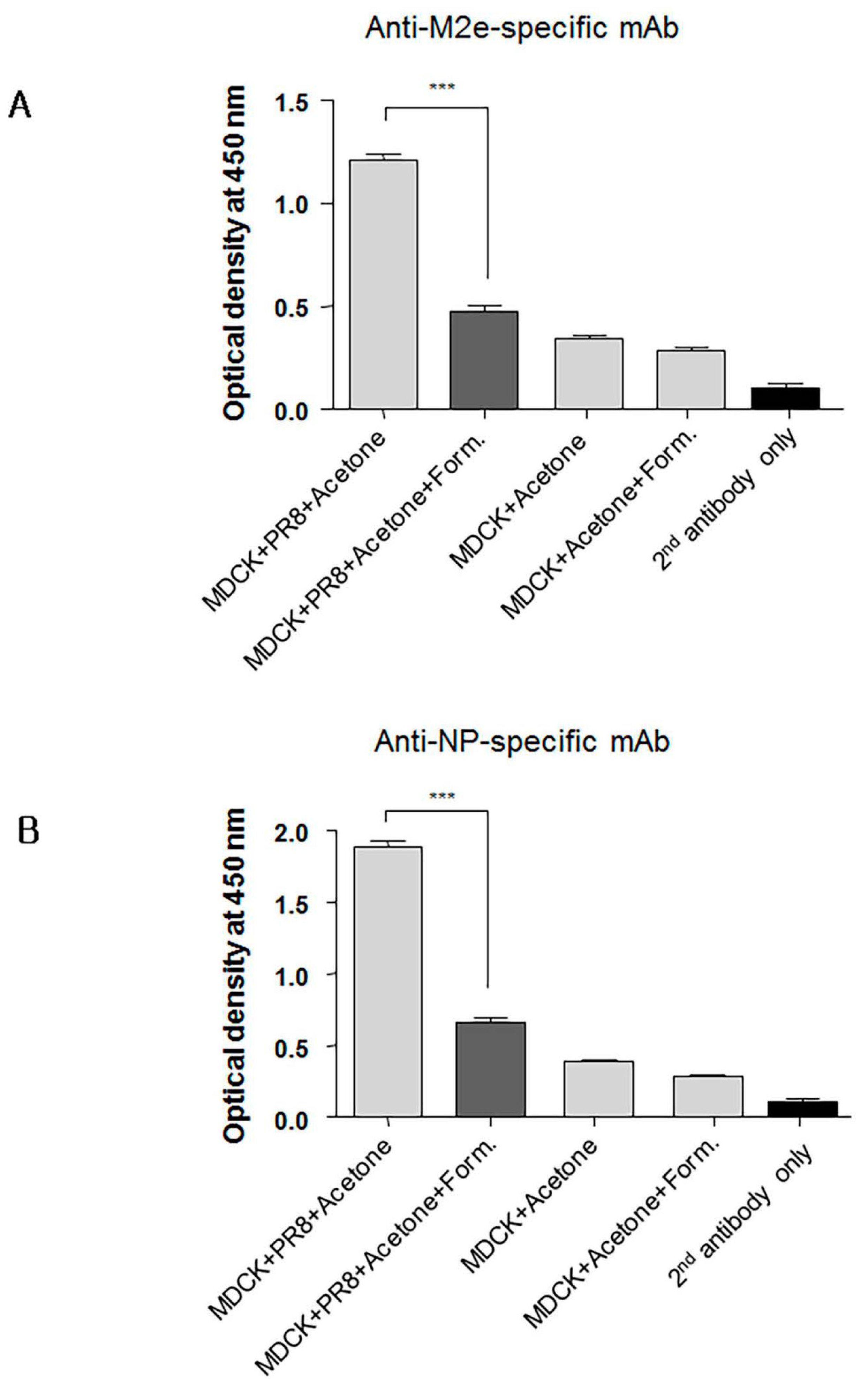
3.7. Formaldehyde Treatment Reduced Monoclonal Antibody Binding to the Conserved Proteins NP and M2
4. Discussion
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Clover, R.D.; Crawford, S.; Glezen, W.P.; Taber, L.H.; Matson, C.C.; Couch, R.B. Comparison of heterotypic protection against influenza A/Taiwan/86 (H1N1) by attenuated and inactivated vaccines to A/chile/83-like viruses. J. Infect. Dis. 1991, 163, 300–304. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.L.; Min, J.; Lamirande, E.W.; Santos, C.; Jin, H.; Kemble, G.; Subbarao, K. Comparison of a live attenuated 2009 H1N1 vaccine with seasonal influenza vaccines against 2009 pandemic H1N1 virus infection in mice and ferrets. J. Infect. Dis. 2011, 1, 930–936. [Google Scholar] [CrossRef] [PubMed]
- Cheng, X.; Zengel, J.R.; Suguitan, A.L., Jr.; Xu, Q.; Wang, W.; Lin, J.; Jin, H. Evaluation of the humoral and cellular immune responses elicited by the live attenuated and inactivated influenza vaccines and their roles in heterologous protection in ferrets. J. Infect. Dis. 2013, 208, 594–602. [Google Scholar] [CrossRef] [PubMed]
- Krammer, F.; Pica, N.; Hai, R.; Margine, I.; Palese, P. Chimeric hemagglutinin influenza virus vaccine constructs elicit broadly protective stalk-specific antibodies. J. Virol. 2013, 87, 6542–6550. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Santos, C.; Aspelund, A.; Gillim-Ross, L.; Jin, H.; Kemble, G.; Subbarao, K. Evaluation of live attenuated influenza a virus H6 vaccines in mice and ferrets. J. Virol. 2009, 83, 65–72. [Google Scholar] [CrossRef] [PubMed]
- Rao, S.S.; Kong, W.P.; Wei, C.J.; Van Hoeven, N.; Gorres, J.P.; Nason, M.; Andersen, H.; Tumpey, T.M.; Nabel, G.J. Comparative efficacy of hemagglutinin, nucleoprotein, and matrix 2 protein gene-based vaccination against H5N1 influenza in mouse and ferret. PLoS ONE 2010, 5, e9812. [Google Scholar] [CrossRef] [PubMed]
- Steel, J.; Lowen, A.C.; Wang, T.T.; Yondola, M.; Gao, Q.; Haye, K.; Garcia-Sastre, A.; Palese, P. Influenza virus vaccine based on the conserved hemagglutinin stalk domain. mBio 2010, 1. [Google Scholar] [CrossRef] [PubMed]
- Delavenne, X.; Zufferey, P.; Baylot, D.; Nguyen, P.; Borg, J.Y.; Fontenay, M.; Deygas, B.; Mismetti, P.; Laporte, S. Population pharmacokinetics of fondaparinux administered at prophylactic doses after major orthopaedic surgery in everyday practice. Thromb. Haemost. 2010, 104, 252–260. [Google Scholar] [CrossRef] [PubMed]
- Belshe, R.B.; Edwards, K.M.; Vesikari, T.; Black, S.V.; Walker, R.E.; Hultquist, M.; Kemble, G.; Connor, E.M.; CAIV-T Comparative Efficacy Study Group. Live attenuated versus inactivated influenza vaccine in infants and young children. N. Engl. J. Med. 2007, 356, 685–696. [Google Scholar] [CrossRef] [PubMed]
- Lee, I.S.; Liu, Y.; Narazaki, M.; Hibi, M.; Kishimoto, T.; Taga, T. Vav is associated with signal transducing molecules gp130, Grb2 and Erk2, and is tyrosine phosphorylated in response to interleukin-6. FEBS Lett. 1997, 401, 133–137. [Google Scholar] [CrossRef]
- Kingsley, C.I.; Karim, M.; Bushell, A.R.; Wood, K.J. CD25+CD4+ regulatory T cells prevent graft rejection: CTLA-4- and IL-10-dependent immunoregulation of alloresponses. J. Immunol. 2002, 168, 1080–1086. [Google Scholar] [CrossRef] [PubMed]
- Sui, J.; Hwang, W.C.; Perez, S.; Wei, G.; Aird, D.; Chen, L.M.; Santelli, E.; Stec, B.; Cadwell, G.; Ali, M.; et al. Structural and functional bases for broad-spectrum neutralization of avian and human influenza a viruses. Nat. Struct. Mol. Biol. 2009, 16, 265–273. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Zhang, X.; Zhao, B.; Wang, J.; Zhu, Z.; Teng, Z.; Shao, J.; Shen, J.; Gao, Y.; Yuan, Z.; et al. Intensive cytokine induction in pandemic H1N1 influenza virus infection accompanied by robust production of IL-10 and IL-6. PLoS ONE 2011, 6, e28680. [Google Scholar] [CrossRef] [PubMed]
- Stillé, C.J. Major-General Anthony Wayne and the Pennsylvania Line in the Continental Army; Scholar’s Bookshelf: Cranbury, NJ, USA, 2005; p. 441. [Google Scholar]
- Antel, J.P. Clinical Neuroimmunology, 2nd ed.; Oxford University Press: Oxford, UK, 2005; p. 452. [Google Scholar]
- Holgate, S.T. Are long-acting β2-agonists safe in the treatment of asthma? Pol. Arch. Med. Wewn. 2008, 118, 460–461. [Google Scholar] [PubMed]
- Holgate, S.T.; Polosa, R. Treatment strategies for allergy and asthma. Nat. Rev. Immunol. 2008, 8, 218–230. [Google Scholar] [CrossRef] [PubMed]
- Jian, Y.R.; Chang, S.Y.; Lin, P.Y.; Yang, Y.H.; Chuang, Y.H. Inactivated influenza virus vaccine is efficient and reduces IL-4 and IL-6 in allergic asthma mice. Influenza Other Respir. Viruses 2013, 7, 1210–1217. [Google Scholar] [CrossRef] [PubMed]
- Van Kampen, K.R.; Shi, Z.; Gao, P.; Zhang, J.; Foster, K.W.; Chen, D.T.; Marks, D.; Elmets, C.A.; Tang, D.C. Safety and immunogenicity of adenovirus-vectored nasal and epicutaneous influenza vaccines in humans. Vaccine 2005, 23, 1029–1036. [Google Scholar] [CrossRef] [PubMed]
- Fiers, W.; De Filette, M.; El Bakkouri, K.; Schepens, B.; Roose, K.; Schotsaert, M.; Birkett, A.; Saelens, X. M2e-based universal influenza a vaccine. Vaccine 2009, 27, 6280–6283. [Google Scholar] [CrossRef] [PubMed]
- Schotsaert, M.; De Filette, M.; Fiers, W.; Saelens, X. Universal M2 ectodomain-based influenza a vaccines: Preclinical and clinical developments. Expert Rev. Vaccines 2009, 8, 499–508. [Google Scholar] [CrossRef] [PubMed]
- Ellebedy, A.H.; Krammer, F.; Li, G.M.; Miller, M.S.; Chiu, C.; Wrammert, J.; Chang, C.Y.; Davis, C.W.; McCausland, M.; Elbein, R.; et al. Induction of broadly cross-reactive antibody responses to the influenza HA stem region following H5N1 vaccination in humans. Proc. Natl. Acad. Sci. USA 2014, 111, 13133–13138. [Google Scholar] [CrossRef] [PubMed]
- Epstein, S.L.; Kong, W.; Misplon, J.A.; Lo, C.Y.; Tumpey, T.M.; Xu, L.; Nabel, G.J. Protection against multiple influenza a subtype by vaccination. Vaccine 2005, 16, 46–47. [Google Scholar]
- Munder, M.; Eichmann, K.; Moran, J.M.; Centeno, F.; Soler, G.; Modolell, M. Th1/Th2-regulated expression of arginase isoforms in murine macrophages and dendritic cells. J. Immunol. 1999, 163, 3771–3777. [Google Scholar] [PubMed]
- Tomy Joseph, J.M.; Lu, B.; Vogel, L.; Swayne, D.; Jin, H.; Kemble, G.; Subbarao, K. A live attenuated cold-adapted influenza a H7N3 virus vaccine provides protection against homologous and heterologous H7 viruses in mice and ferrets. Virology 2008, 378, 123–132. [Google Scholar] [CrossRef] [PubMed]
- Quan, F.S.; Compans, R.W.; Nguyen, H.H.; Kang, S.M. Induction of heterosubtypic immunity to influenza virus by intranasal immunization. J. Virol. 2008, 82, 1350–1359. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.H.; Park, H.J.; Han, G.Y.; Song, M.K.; Pereboev, A.; Hong, J.S.; Chang, J.; Byun, Y.H.; Seong, B.L.; Nguyen, H.H. Intranasal adenovirus-vectored vaccine for induction of long-lasting humoral immunity-mediated broad protection against influenza in mice. J. Virol. 2014, 88, 9693–9703. [Google Scholar] [CrossRef] [PubMed]
- Kolpe, A.; Schepens, B.; Fiers, W.; Saelens, X. M2-based influenza vaccines: Recent advances and clinical potential. Expert Rev. Vaccines 2017, 16, 123–136. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.N.; Kim, M.C.; Lee, Y.T.; Kim, Y.J.; Kang, S.M. Mechanisms of cross-protection by influenza virus M2-based vaccines. Immune Netw. 2015, 15, 213–221. [Google Scholar] [CrossRef] [PubMed]
- Eliasson, D.G.; Omokanye, A.; Schon, K.; Wenzel, U.A.; Bernasconi, V.; Bemark, M.; Kolpe, A.; El Bakkouri, K.; Ysenbaert, T.; Deng, L.; et al. M2e-tetramer-specific memory CD4 T cells are broadly protective against influenza infection. Mucosal Immunol. 2017. [Google Scholar] [CrossRef] [PubMed]
- Kreijtz, J.H.; Suezer, Y.; de Mutsert, G.; van den Brand, J.M.; van Amerongen, G.; Schnierle, B.S.; Kuiken, T.; Fouchier, R.A.; Lower, J.; Osterhaus, A.D.; et al. Recombinant modified vaccinia virus ankara expressing the hemagglutinin gene confers protection against homologous and heterologous H5N1 influenza virus infections in macaques. J. Infect. Dis. 2009, 199, 405–413. [Google Scholar] [CrossRef] [PubMed]
- Bommakanti, G.; Citron, M.P.; Hepler, R.W.; Callahan, C.; Heidecker, G.J.; Najar, T.A.; Lu, X.; Joyce, J.G.; Shiver, J.W.; Casimiro, D.R.; et al. Design of an HA2-based escherichia coli expressed influenza immunogen that protects mice from pathogenic challenge. Proc. Natl. Acad. Sci. USA 2010, 107, 13701–13706. [Google Scholar] [CrossRef] [PubMed]
- Krammer, F. The quest for a universal flu vaccine: Headless Ha 2.0. Cell Host Microbe 2015, 18, 395–397. [Google Scholar] [CrossRef] [PubMed]
- Stevens, T.L.; Bossie, A.; Sanders, V.M.; Fernandez-Botran, R.; Coffman, R.L.; Mosmann, T.R.; Vitetta, E.S. Regulation of antibody isotype secretion by subsets of antigen-specific helper T cells. Nature 1988, 334, 255–258. [Google Scholar] [CrossRef] [PubMed]
- Moran, T.M.; Park, H.; Fernandez-Sesma, A.; Schulman, J.L. Th2 responses to inactivated influenza virus can be converted to Th1 responses and facilitate recovery from heterosubtypic virus infection. J. Infect. Dis. 1999, 180, 579–585. [Google Scholar] [CrossRef] [PubMed]
- Mosmann, T.R.; Coffman, R.L. Heterogeneity of cytokine secretion patterns and functions of helper T cells. Adv. Immunol. 1989, 46, 111–147. [Google Scholar] [PubMed]
- Coffman, R.L.; Lebman, D.A.; Rothman, P. Mechanism and regulation of immunoglobulin isotype switching. Adv. Immunol. 1993, 54, 229–270. [Google Scholar] [PubMed]
- Spellberg, B.; Edwards, J.E., Jr. Type 1/type 2 immunity in infectious diseases. Clin. Infect. Dis. 2001, 32, 76–102. [Google Scholar] [CrossRef] [PubMed]
- Diehl, S.; Chow, C.W.; Weiss, L.; Palmetshofer, A.; Twardzik, T.; Rounds, L.; Serfling, E.; Davis, R.J.; Anguita, J.; Rincon, M. Induction of NFATc2 expression by interleukin 6 promotes T helper type 2 differentiation. J. Exp. Med. 2002, 196, 39–49. [Google Scholar] [CrossRef] [PubMed]
- Mogensen, T.H.; Paludan, S.R. Molecular pathways in virus-induced cytokine production. Microbiol. Mol. Biol. Rev. 2001, 65, 131–150. [Google Scholar] [CrossRef] [PubMed]
- Wrammert, J.; Koutsonanos, D.; Li, G.M.; Edupuganti, S.; Sui, J.; Morrissey, M.; McCausland, M.; Skountzou, I.; Hornig, M.; Lipkin, W.I.; et al. Broadly cross-reactive antibodies dominate the human B cell response against 2009 pandemic H1N1 influenza virus infection. J. Exp. Med. 2011, 208, 181–193. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, H.H.; Tumpey, T.M.; Park, H.J.; Byun, Y.H.; Tran, L.D.; Nguyen, V.D.; Kilgore, P.E.; Czerkinsky, C.; Katz, J.M.; Seong, B.L.; et al. Prophylactic and therapeutic efficacy of avian antibodies against influenza virus H5N1 and H1N1 in mice. PLoS ONE 2010, 5, e10152. [Google Scholar] [CrossRef] [PubMed]
- Shim, B.S.; Choi, Y.K.; Yun, C.H.; Lee, E.G.; Jeon, Y.S.; Park, S.M.; Cheon, I.S.; Joo, D.H.; Cho, C.H.; Song, M.S.; et al. Sublingual immunization with M2-based vaccine induces broad protective immunity against influenza. PLoS ONE 2011, 6, e27953. [Google Scholar] [CrossRef] [PubMed]
- Hai, R.; Krammer, F.; Tan, G.S.; Pica, N.; Eggink, D.; Maamary, J.; Margine, I.; Albrecht, R.A.; Palese, P. Influenza viruses expressing chimeric hemagglutinins: Globular head and stalk domains derived from different subtypes. J. Virol. 2012, 86, 5774–5781. [Google Scholar] [CrossRef] [PubMed]
- Tan, G.S.; Krammer, F.; Eggink, D.; Kongchanagul, A.; Moran, T.M.; Palese, P. A pan-H1 anti-hemagglutinin monoclonal antibody with potent broad-spectrum efficacy in vivo. J. Virol. 2012, 86, 6179–6188. [Google Scholar] [CrossRef] [PubMed]
- Xu-Amano, J.; Jackson, R.J.; Fujihashi, K.; Kiyono, H.; Staats, H.F.; McGhee, J.R. Helper Th1 and Th2 cell responses following mucosal or systemic immunization with cholera toxin. Vaccine 1994, 12, 903–911. [Google Scholar] [CrossRef]
- Taga, T.; Kishimoto, T. Gp130 and the interleukin-6 family of cytokines. Annu. Rev. Immunol. 1997, 15, 797–819. [Google Scholar] [CrossRef] [PubMed]
- Amanna, I.J.; Slifka, M.K. Wanted, dead or alive: New viral vaccines. Antivir. Res. 2009, 84, 119–130. [Google Scholar] [CrossRef] [PubMed]
- Arulanandam, B.P.; Raeder, R.H.; Nedrud, J.G.; Bucher, D.J.; Le, J.; Metzger, D.W. Iga immunodeficiency leads to inadequate Th cell priming and increased susceptibility to influenza virus infection. J. Immunol. 2001, 166, 226–231. [Google Scholar] [CrossRef] [PubMed]
- Van Riet, E.; Ainai, A.; Suzuki, T.; Hasegawa, H. Mucosal IgA responses in influenza virus infections; thoughts for vaccine design. Vaccine 2012, 30, 5893–5900. [Google Scholar] [CrossRef] [PubMed]
- Tamura, S.; Kurata, T. Defense mechanisms against influenza virus infection in the respiratory tract mucosa. Jpn. J. Infect. Dis. 2004, 57, 236–247. [Google Scholar] [PubMed]
- Gorse, G.J.; Otto, E.E.; Powers, D.C.; Chambers, G.W.; Eickhoff, C.S.; Newman, F.K. Induction of mucosal antibodies by live attenuated and inactivated influenza virus vaccines in the chronically ill elderly. J. Infect. Dis. 1996, 173, 285–290. [Google Scholar] [CrossRef] [PubMed]
- Brandtzaeg, P. Role of mucosal immunity in influenza. Dev. Biol. 2003, 115, 39–48. [Google Scholar]
- Haan, L.; Verweij, W.R.; Holtrop, M.; Brands, R.; van Scharrenburg, G.J.; Palache, A.M.; Agsteribbe, E.; Wilschut, J. Nasal or intramuscular immunization of mice with influenza subunit antigen and the B subunit of escherichia coli heat-labile toxin induces IgA- or IgG-mediated protective mucosal immunity. Vaccine 2001, 19, 2898–2907. [Google Scholar] [CrossRef]
- Plante, M.; Jones, T.; Allard, F.; Torossian, K.; Gauthier, J.; St-Felix, N.; White, G.L.; Lowell, G.H.; Burt, D.S. Nasal immunization with subunit proteosome influenza vaccines induces serum HAI, mucosal IgA and protection against influenza challenge. Vaccine 2001, 20, 218–225. [Google Scholar] [CrossRef]
- Verweij, W.R.; de Haan, L.; Holtrop, M.; Agsteribbe, E.; Brands, R.; van Scharrenburg, G.J.; Wilschut, J. Mucosal immunoadjuvant activity of recombinant escherichia coli heat-labile enterotoxin and its B subunit: Induction of systemic IgG and secretory IgA responses in mice by intranasal immunization with influenza virus surface antigen. Vaccine 1998, 16, 2069–2076. [Google Scholar] [CrossRef]
- Dreyfus, C.; Laursen, N.S.; Kwaks, T.; Zuijdgeest, D.; Khayat, R.; Ekiert, D.C.; Lee, J.H.; Metlagel, Z.; Bujny, M.V.; Jongeneelen, M.; et al. Highly conserved protective epitopes on influenza B viruses. Science 2012, 337, 1343–1348. [Google Scholar] [CrossRef] [PubMed]
- Ekiert, D.C.; Bhabha, G.; Elsliger, M.A.; Friesen, R.H.; Jongeneelen, M.; Throsby, M.; Goudsmit, J.; Wilson, I.A. Antibody recognition of a highly conserved influenza virus epitope. Science 2009, 324, 246–251. [Google Scholar] [CrossRef] [PubMed]
- Suguitan, A.L., Jr.; McAuliffe, J.; Mills, K.L.; Jin, H.; Duke, G.; Lu, B.; Luke, C.J.; Murphy, B.; Swayne, D.E.; Kemble, G.; et al. Live, attenuated influenza a H5N1 candidate vaccines provide broad cross-protection in mice and ferrets. PLoS Med. 2006, 3, e360. [Google Scholar] [CrossRef] [PubMed]
- Thomson, C.A.; Wang, Y.; Jackson, L.M.; Olson, M.; Wang, W.; Liavonchanka, A.; Keleta, L.; Silva, V.; Diederich, S.; Jones, R.B.; et al. Pandemic H1N1 influenza infection and vaccination in humans induces cross-protective antibodies that target the hemagglutinin stem. Front. Immunol. 2012, 3, 87. [Google Scholar] [CrossRef] [PubMed]
- Throsby, M.; van den Brink, E.; Jongeneelen, M.; Poon, L.L.; Alard, P.; Cornelissen, L.; Bakker, A.; Cox, F.; van Deventer, E.; Guan, Y.; et al. Heterosubtypic neutralizing monoclonal antibodies cross-protective against H5N1 and H1N1 recovered from human IgM+ memory B cells. PLoS ONE 2008, 3, e3942. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Coucke, D.; Schotsaert, M.; Libert, C.; Pringels, E.; Vervaet, C.; Foreman, P.; Saelens, X.; Remon, J.P. Spray-dried powders of starch and crosslinked poly(acrylic acid) as carriers for nasal delivery of inactivated influenza vaccine. Vaccine 2009, 27, 1279–1286. [Google Scholar] [CrossRef] [PubMed]
- Markine-Goriaynoff, D.; Coutelier, J.P. Increased efficacy of the immunoglobulin G2a subclass in antibody-mediated protection against lactate dehydrogenase-elevating virus-induced polioencephalomyelitis revealed with switch mutants. J. Virol. 2002, 76, 432–435. [Google Scholar] [CrossRef] [PubMed]
- Brown, F. Review of accidents caused by incomplete inactivation of viruses. Dev. Biol. Stand. 1993, 81, 103–107. [Google Scholar] [PubMed]
- Branch, A. Vaccination against whooping cough. Can. Med. Assoc. J. 1938, 38, 65–66. [Google Scholar] [PubMed]
- Hoft, D.F.; Babusis, E.; Worku, S.; Spencer, C.T.; Lottenbach, K.; Truscott, S.M.; Abate, G.; Sakala, I.G.; Edwards, K.M.; Creech, C.B.; et al. Live and inactivated influenza vaccines induce similar humoral responses, but only live vaccines induce diverse T-cell responses in young children. J. Infect. Dis. 2011, 204, 845–853. [Google Scholar] [CrossRef] [PubMed]
- Houser, K.V.; Katz, J.M.; Tumpey, T.M. Seasonal trivalent inactivated influenza vaccine does not protect against newly emerging variants of influenza a (H3N2v) virus in ferrets. J. Virol. 2013, 87, 1261–1263. [Google Scholar] [CrossRef] [PubMed]



© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, E.H.; Han, G.-Y.; Nguyen, H. An Adenovirus-Vectored Influenza Vaccine Induces Durable Cross-Protective Hemagglutinin Stalk Antibody Responses in Mice. Viruses 2017, 9, 234. https://doi.org/10.3390/v9080234
Kim EH, Han G-Y, Nguyen H. An Adenovirus-Vectored Influenza Vaccine Induces Durable Cross-Protective Hemagglutinin Stalk Antibody Responses in Mice. Viruses. 2017; 9(8):234. https://doi.org/10.3390/v9080234
Chicago/Turabian StyleKim, Eun Hye, Gye-Yeong Han, and Huan Nguyen. 2017. "An Adenovirus-Vectored Influenza Vaccine Induces Durable Cross-Protective Hemagglutinin Stalk Antibody Responses in Mice" Viruses 9, no. 8: 234. https://doi.org/10.3390/v9080234




