Monitoring Physiological Changes in Haloarchaeal Cell during Virus Release
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cells, Viruses and Growth of Viruses
2.2. Adsorption Assay
2.3. On-Line Electrochemical Measurements during Virus Infection
2.4. ATP Measurements during Virus Infection
3. Results
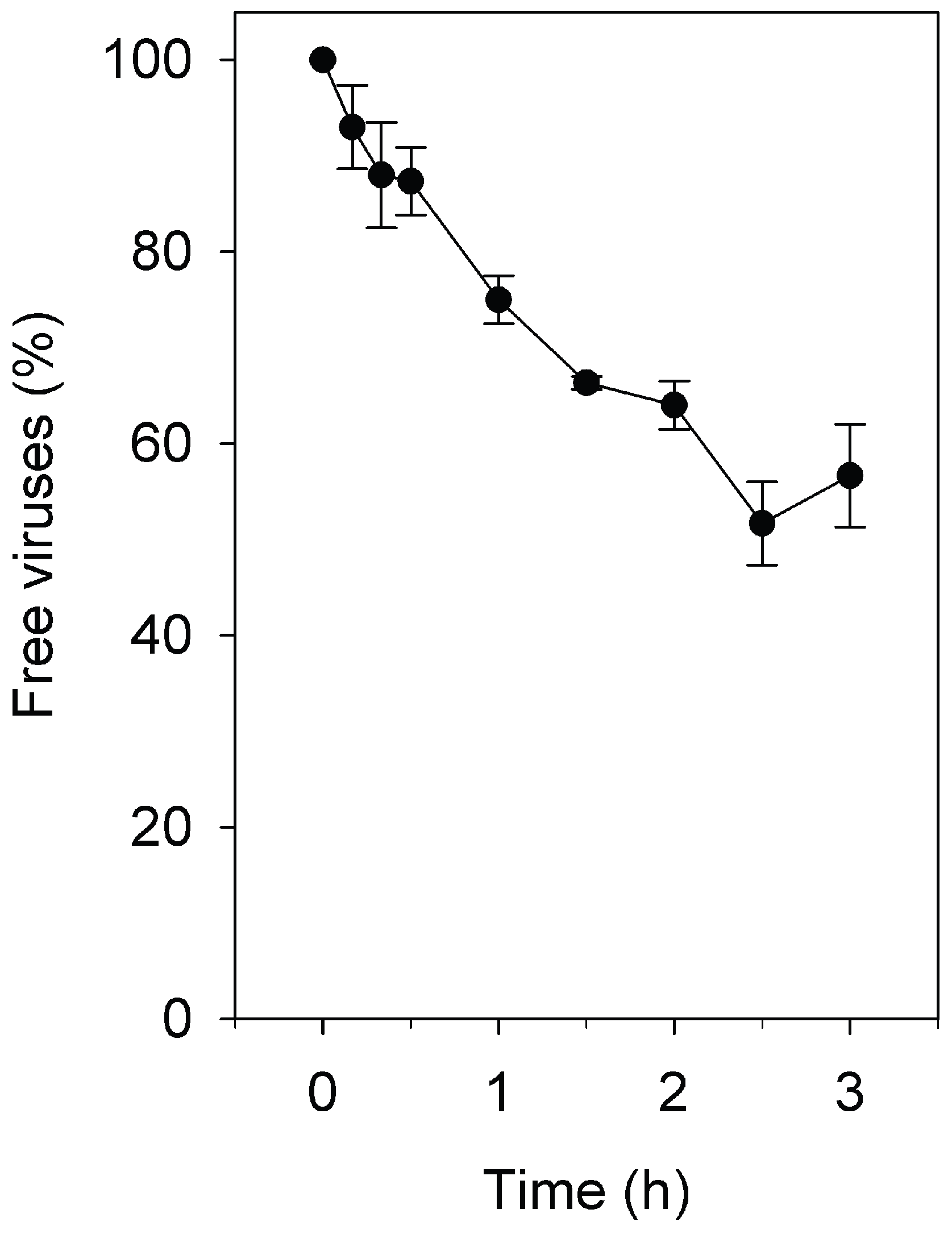
3.1. Adsorption Rate Constant of Pleomorphic Virus His2 to Har. hispanica
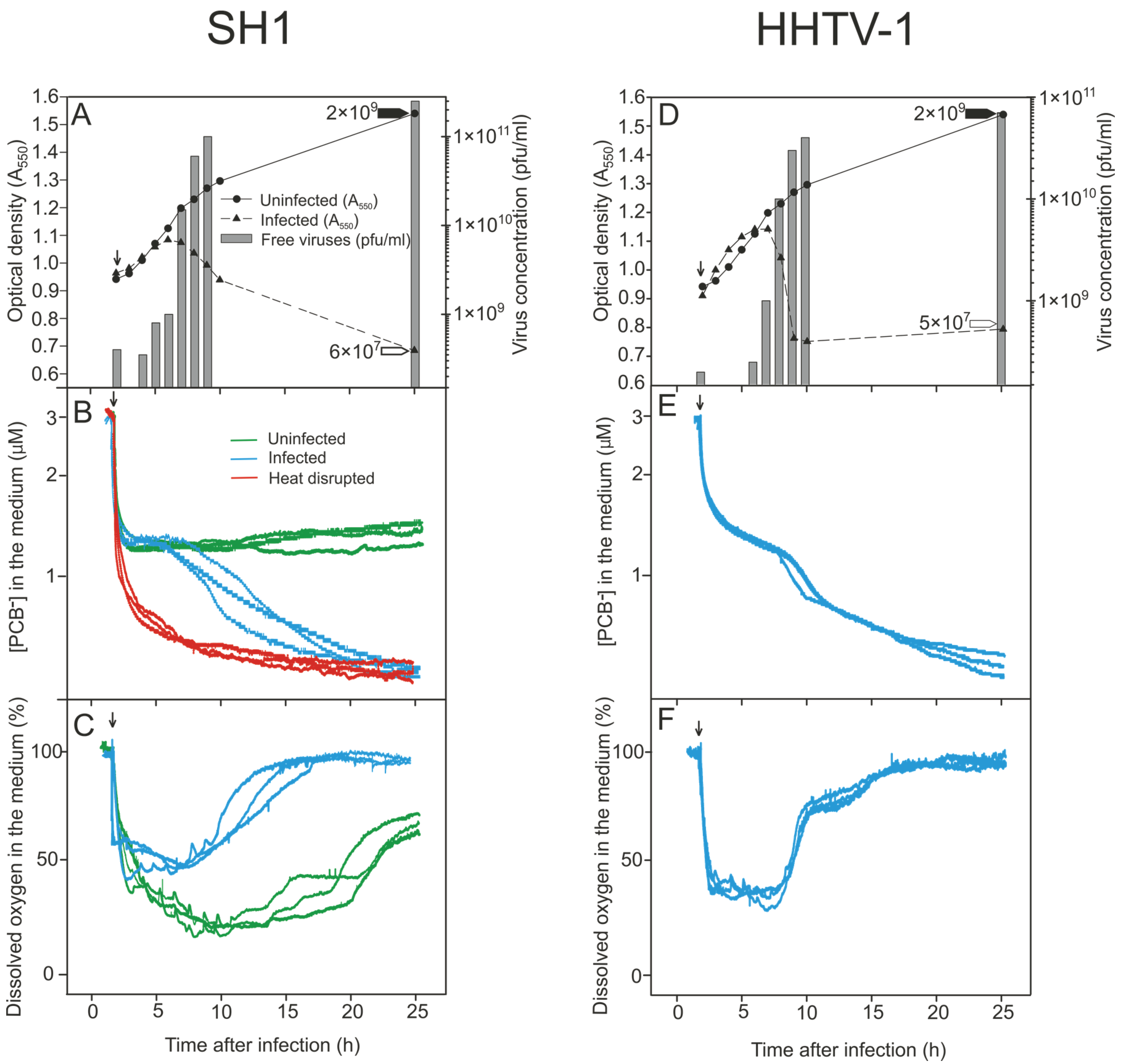
3.2. Monitoring the Turbidity and Counts of Viable Cells and Progeny Viruses during Virus Production
3.3. Binding of PCB− to Har. hispanica Cells as an Indicator of the Start of Lytic Virus Release
3.4. Changes in the Levels of Oxygen Consumption during Virus Infection
3.5. Changes in the Concentration of Intracellular and Extracellular ATP during Virus Infection
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- DeLong, E.F. Everything in moderation: Archaea as “non-extremophiles”. Curr. Opin. Genet. Dev. 1998, 8, 649–654. [Google Scholar] [CrossRef]
- Sprott, G.D. Structures of archaebacterial membrane lipids. J. Bioenerg. Biomembr. 1992, 24, 555–566. [Google Scholar] [CrossRef] [PubMed]
- Albers, S.V.; Meyer, B.H. The archaeal cell envelope. Nat. Rev. Microbiol. 2011, 9, 414–426. [Google Scholar] [CrossRef] [PubMed]
- Torsvik, T.; Dundas, I.D. Bacteriophage of Halobacterium salinarium. Nature 1974, 248, 680–681. [Google Scholar] [CrossRef] [PubMed]
- Atanasova, N.S.; Bamford, D.H.; Oksanen, H.M. Haloarchaeal virus morphotypes. Biochimie 2015, 118, 333–343. [Google Scholar] [CrossRef] [PubMed]
- Pina, M.; Bize, A.; Forterre, P.; Prangishvili, D. The archeoviruses. FEMS Microbiol. Rev. 2011, 35, 1035–1054. [Google Scholar] [CrossRef] [PubMed]
- Prangishvili, D. The wonderful world of archaeal viruses. Annu. Rev. Microbiol. 2013, 67, 565–585. [Google Scholar] [CrossRef] [PubMed]
- Quemin, E.R.; Quax, T.E. Archaeal viruses at the cell envelope: Entry and egress. Front. Microbiol. 2015, 6. [Google Scholar] [CrossRef] [PubMed]
- Bize, A.; Karlsson, E.A.; Ekefjard, K.; Quax, T.E.; Pina, M.; Prevost, M.C.; Forterre, P.; Tenaillon, O.; Bernander, R.; Prangishvili, D. A unique virus release mechanism in the Archaea. Proc. Natl. Acad. Sci. USA 2009, 106, 11306–11311. [Google Scholar] [CrossRef] [PubMed]
- Brumfield, S.K.; Ortmann, A.C.; Ruigrok, V.; Suci, P.; Douglas, T.; Young, M.J. Particle assembly and ultrastructural features associated with replication of the lytic archaeal virus Sulfolobus turreted icosahedral virus. J. Virol. 2009, 83, 5964–5970. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.N.; Pfister, P.; Leisinger, T.; Wasserfallen, A. The genome of archaeal prophage ΨM100 encodes the lytic enzyme responsible for autolysis of Methanothermobacter wolfeii. J. Bacteriol. 2001, 183, 5788–5792. [Google Scholar] [CrossRef] [PubMed]
- Pfister, P.; Wesserfallen, A.; Stettler, R.; Leisinger, T. Molecular analysis of Methanobacterium phage ψM2. Mol. Microbiol. 1998, 30, 233–244. [Google Scholar] [CrossRef] [PubMed]
- Saier, M.H., Jr.; Reddy, B.L. Holins in bacteria, eukaryotes, and archaea: Multifunctional xenologues with potential biotechnological and biomedical applications. J. Bacteriol. 2015, 197, 7–17. [Google Scholar] [CrossRef] [PubMed]
- Young, R.; Wang, I.N.; Roof, W.D. Phages will out: Strategies of host cell lysis. Trends Microbiol. 2000, 8, 120–128. [Google Scholar] [CrossRef]
- Witte, A.; Wanner, G.; Blasi, U.; Halfmann, G.; Szostak, M.; Lubitz, W. Endogenous transmembrane tunnel formation mediated by φX174 lysis protein-E. J. Bacteriol. 1990, 172, 4109–4114. [Google Scholar] [PubMed]
- Rakonjac, J.; Feng, J.N.; Model, P. Filamentous phage are released from the bacterial membrane by a two-step mechanism involving a short C-terminal fragment of pIII. J. Mol. Biol. 1999, 289, 1253–1265. [Google Scholar] [CrossRef] [PubMed]
- Prangishvili, D. Archaeal viruses: Living fossils of the ancient virosphere? Ann. N. Y. Acad. Sci. 2015, 1341, 35–40. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Liu, Y.; Wang, S.; Yang, D.; Cheng, Y.; Hu, J.; Chen, J.; Mei, Y.; Shen, P.; Bamford, D.H.; et al. Temperate membrane-containing halophilic archaeal virus SNJ1 has a circular dsDNA genome identical to that of plasmid pHH205. Virology 2012, 434, 233–241. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Wang, J.; Liu, Y.; Wang, Y.; Zhang, Z.; Oksanen, H.M.; Bamford, D.H.; Chen, X. Identification and characterization of SNJ2, the first temperate pleolipovirus integrating into the genome of the SNJ1-lysogenic archaeal strain. Mol. Microbiol. 2015. [Google Scholar] [CrossRef] [PubMed]
- Witte, A.; Baranyi, U.; Klein, R.; Sulzner, M.; Luo, C.; Wanner, G.; Kruger, D.H.; Lubitz, W. Characterization of Natronobacterium magadii phage ΦCh1, a unique archaeal phage containing DNA and RNA. Mol. Microbiol. 1997, 23, 603–616. [Google Scholar] [CrossRef] [PubMed]
- Schnabel, H. An immune strain of Halobacterium halobium carries the invertible L segment of phage φH as a plasmid. Proc. Natl. Acad. Sci. USA 1984, 81, 1017–1020. [Google Scholar] [CrossRef] [PubMed]
- Schnabel, H.; Zillig, W.; Pfaffle, M.; Schnabel, R.; Michel, H.; Delius, H. Halobacterium halobium phage φH. EMBO J. 1982, 1, 87–92. [Google Scholar] [PubMed]
- Pietilä, M.K.; Atanasova, N.S.; Manole, V.; Liljeroos, L.; Butcher, S.J.; Oksanen, H.M.; Bamford, D.H. Virion architecture unifies globally distributed pleolipoviruses infecting halophilic archaea. J. Virol. 2012, 86, 5067–5079. [Google Scholar] [CrossRef] [PubMed]
- Pietilä, M.K.; Atanasova, N.S.; Oksanen, H.M.; Bamford, D.H. Modified coat protein forms the flexible spindle-shaped virion of haloarchaeal virus His1. Environ. Microbiol. 2013, 15, 1674–1686. [Google Scholar] [CrossRef] [PubMed]
- Juez, G.; Rodriguez-Valera, F.; Ventosa, A.; Kushner, D.J. Haloarcula hispanica spec. nov. and Haloferax gibbonsii spec, nov., two new species of extremely halophilic archaebacteria. Syst. Appl. Microbiol. 1986, 8, 75–79. [Google Scholar] [CrossRef]
- Bath, C.; Dyall-Smith, M.L. His1, an archaeal virus of the Fuselloviridae family that infects Haloarcula hispanica. J. Virol. 1998, 72, 9392–9395. [Google Scholar] [PubMed]
- Bamford, D.H.; Ravantti, J.J.; Rönnholm, G.; Laurinavičius, S.; Kukkaro, P.; Dyall-Smith, M.; Somerharju, P.; Kalkkinen, N.; Bamford, J.K.H. Constituents of SH1, a novel lipid-containing virus infecting the halophilic euryarchaeon Haloarcula hispanica. J. Virol. 2005, 79, 9097–9107. [Google Scholar] [CrossRef] [PubMed]
- Bath, C.; Cukalac, T.; Porter, K.; Dyall-Smith, M.L. His1 and His2 are distantly related, spindle-shaped haloviruses belonging to the novel virus group, Salterprovirus. Virology 2006, 350, 228–239. [Google Scholar] [CrossRef] [PubMed]
- Kukkaro, P.; Bamford, D.H. Virus-host interactions in environments with a wide range of ionic strengths. Environ. Microbiol. Rep. 2009, 1, 71–77. [Google Scholar] [CrossRef] [PubMed]
- Jaakkola, S.T.; Penttinen, R.K.; Vilen, S.T.; Jalasvuori, M.; Rönnholm, G.; Bamford, J.K.; Bamford, D.H.; Oksanen, H.M. Closely related archaeal Haloarcula hispanica icosahedral viruses HHIV-2 and SH1 have nonhomologous genes encoding host recognition functions. J. Virol. 2012, 86, 4734–4742. [Google Scholar] [CrossRef] [PubMed]
- Porter, K.; Kukkaro, P.; Bamford, J.K.; Bath, C.; Kivelä, H.M.; Dyall-Smith, M.L.; Bamford, D.H. SH1: A novel, spherical halovirus isolated from an Australian hypersaline lake. Virology 2005, 335, 22–33. [Google Scholar] [CrossRef] [PubMed]
- Porter, K.; Tang, S.L.; Chen, C.P.; Chiang, P.W.; Hong, M.J.; Dyall-Smith, M. PH1: An archaeovirus of Haloarcula hispanica related to SH1 and HHIV-2. Archaea 2013. [Google Scholar] [CrossRef] [PubMed]
- Atanasova, N.S.; Demina, T.A.; Buivydas, A.; Bamford, D.H.; Oksanen, H.M. Archaeal viruses multiply: Temporal screening in a solar saltern. Viruses 2015, 7, 1902–1926. [Google Scholar] [CrossRef] [PubMed]
- Mei, Y.; He, C.; Huang, Y.; Liu, Y.; Zhang, Z.; Chen, X.; Shen, P. Salinity regulation of the interaction of halovirus SNJ1 with its host and alteration of the halovirus replication strategy to adapt to the variable ecosystem. PLoS ONE 2015, 10, e0123874. [Google Scholar] [CrossRef] [PubMed]
- Jäälinoja, H.T.; Roine, E.; Laurinmäki, P.; Kivelä, H.M.; Bamford, D.H.; Butcher, S.J. Structure and host-cell interaction of SH1, a membrane-containing, halophilic euryarchaeal virus. Proc. Natl. Acad. Sci. USA 2008, 105, 8008–8013. [Google Scholar] [CrossRef] [PubMed]
- Kivelä, H.M.; Roine, E.; Kukkaro, P.; Laurinavičius, S.; Somerharju, P.; Bamford, D.H. Quantitative dissociation of archaeal virus SH1 reveals distinct capsid proteins and a lipid core. Virology 2006, 356, 4–11. [Google Scholar] [CrossRef] [PubMed]
- Gil-Carton, D.; Jaakkola, S.T.; Charro, D.; Peralta, B.; Castano-Diez, D.; Oksanen, H.M.; Bamford, D.H.; Abrescia, N.G. Insight into the assembly of viruses with vertical single beta-barrel major capsid proteins. Structure 2015, 23, 1866–1877. [Google Scholar] [CrossRef] [PubMed]
- Senčilo, A.; Jacobs-Sera, D.; Russell, D.A.; Ko, C.C.; Bowman, C.A.; Atanasova, N.S.; Österlund, E.; Oksanen, H.M.; Bamford, D.H.; Hatfull, G.F.; et al. Snapshot of haloarchaeal tailed virus genomes. RNA Biol. 2013, 10, 803–816. [Google Scholar] [CrossRef] [PubMed]
- Krupovič, M.; Prangishvili, D.; Hendrix, R.W.; Bamford, D.H. Genomics of bacterial and archaeal viruses: Dynamics within the prokaryotic virosphere. Microbiol. Mol. Biol. Rev. 2011, 75, 610–635. [Google Scholar] [CrossRef] [PubMed]
- Hendrix, R.W. Bacteriophage genomics. Curr. Opin. Microbiol. 2003, 6, 506–511. [Google Scholar] [CrossRef] [PubMed]
- Atanasova, N.S.; Roine, E.; Oren, A.; Bamford, D.H.; Oksanen, H.M. Global network of specific virus-host interactions in hypersaline environments. Environ. Microbiol. 2012, 14, 426–440. [Google Scholar] [CrossRef] [PubMed]
- Krupovič, M.; Quemin, E.R.; Bamford, D.H.; Forterre, P.; Prangishvili, D. Unification of the globally distributed spindle-shaped viruses of the Archaea. J. Virol. 2014, 88, 2354–2358. [Google Scholar] [CrossRef] [PubMed]
- Hong, C.; Pietilä, M.K.; Fu, C.J.; Schmid, M.F.; Bamford, D.H.; Chiu, W. Lemon-shaped halo archaeal virus His1 with uniform tail but variable capsid structure. Proc. Natl. Acad. Sci. USA 2015, 112, 2449–2454. [Google Scholar] [CrossRef] [PubMed]
- Hanhijärvi, K.J.; Ziedaite, G.; Pietilä, M.K.; Haeggstrom, E.; Bamford, D.H. DNA ejection from an archaeal virus—A single-molecule approach. Biophys. J. 2013, 104, 2264–2272. [Google Scholar] [CrossRef] [PubMed]
- Pietilä, M.K.; Roine, E.; Sencilo, A.; Bamford, D.H.; Oksanen, H.M. Pleolipoviridae, a newly proposed family comprising archaeal pleomorphic viruses with single-stranded or double-stranded DNA genomes. Arch. Virol. 2015. [Google Scholar] [CrossRef] [PubMed]
- Porter, K.; Russ, B.E.; Dyall-Smith, M.L. Virus-host interactions in salt lakes. Curr. Opin. Microbiol. 2007, 10, 418–424. [Google Scholar] [CrossRef] [PubMed]
- Roine, E.; Oksanen, H.M. Viruses from the hypersaline environment. In Halophiles and Hypersaline Environments: Current Research and Future Trends; Ventosa, A., Oren, A., Ma, Y., Eds.; Springer-Verlag: Berlin, Germany, 2011; pp. 153–172. [Google Scholar]
- Daugelavičius, R.; Gaidelytė, A.; Cvirkaite-Krupovič, V.; Bamford, D.H. On-line monitoring of changes in host cell physiology during the one-step growth cycle of Bacillus phage Bam35. J. Microbiol. Methods. 2007, 69, 174–179. [Google Scholar] [CrossRef] [PubMed]
- Daugelavičius, R.; Bamford, J.K.H.; Bamford, D.H. Changes in host cell energetics in response to bacteriophage PRD1 DNA entry. J. Bacteriol. 1997, 179, 5203–5210. [Google Scholar] [PubMed]
- Kivelä, H.M.; Daugelavičius, R.; Hankkio, R.H.; Bamford, J.K.H.; Bamford, D.H. Penetration of membrane-containing double-stranded-DNA bacteriophage PM2 into Pseudoalteromonas hosts. J. Bacteriol. 2004, 186, 5342–5354. [Google Scholar] [CrossRef] [PubMed]
- Krupovič, M.; Daugelavičius, R.; Bamford, D.H. A novel lysis system in PM2, a lipid-containing marine double-stranded DNA bacteriophage. Mol. Microbiol. 2007, 64, 1635–1648. [Google Scholar] [CrossRef] [PubMed]
- Poranen, M.M.; Daugelavičius, R.; Ojala, P.M.; Hess, M.W.; Bamford, D.H. A novel virus-host cell membrane interaction: Membrane voltage-dependent endocytic-like entry of bacteriophage φ6 nucleocapsid. J. Cell Biol. 1999, 147, 671–681. [Google Scholar] [CrossRef] [PubMed]
- Jakutytė, L.; Baptista, C.; Sao-Jose, C.; Daugelavičius, R.; Carballido-Lopez, R.; Tavares, P. Bacteriophage SPP1 infection of Bacillus subtilis: Evidence for a preferential polar route for entry in a Gram-positive bacterium. FEBS J. 2011, 278, 461. [Google Scholar] [CrossRef] [PubMed]
- Nuttall, S.D.; Dyall-Smith, M.L. HF1 and HF2: Novel bacteriophages of halophilic archaea. Virology 1993, 197, 678–684. [Google Scholar] [CrossRef] [PubMed]
- Dyall-Smith, M. Halohandbook: Protocols for Halobacterial Genetics. Available online: http://www.microbiol.unimelb.edu.au/micro/staff/mds/ (accessed on 12 May 2009).
- Adams, M.H. Bacteriophages; Interscience Publishers: New York, NY, USA, 1959. [Google Scholar]
- Daugelavičius, R.; Bakienė, E.; Beržinskienė, J.; Bamford, D.H. Binding of lipophilic anions to microbial cells. Bioelectrochem. Bioenerg. 1997, 42, 263–274. [Google Scholar] [CrossRef]
- Daugelavičius, R.; Bakienė, E.; Beržinskienė, J.; Bamford, D.H. Use of lipophilic anions for estimation of biomass and cell viability. Biotechnol. Bioeng. 2001, 71, 208–216. [Google Scholar] [CrossRef]
- Mihali, C.; Vaum, N. Use of plasticizers for electrochemical sensors. In Recent Advances in Plasticizers; Luqman, M., Ed.; InTech: Baia Mare, Romania, 2012; pp. 125–140. Available online: http://www.intechopen.com/books/recent-advances-in-plasticizers/use-of-plasticizers-for-electrochemicalsensors (accessed on 24 August 2015).
- Woese, C.R.; Fox, G.E. Phylogenetic structure of prokaryotic domain: The primary kingdoms. Proc. Natl. Acad. Sci. USA 1977, 74, 5088–5090. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharya, A.; Kohrer, C.; Mandal, D.; RajBhandary, U.L. Nonsense suppression in archaea. Proc. Natl. Acad. Sci. USA 2015, 112, 6015–6020. [Google Scholar] [CrossRef] [PubMed]
- Wirth, J.F.; Snyder, J.C.; Hochstein, R.A.; Ortmann, A.C.; Willits, D.A.; Douglas, T.; Young, M.J. Development of a genetic system for the archaeal virus Sulfolobus turreted icosahedral virus (STIV). Virology 2011, 415, 6–11. [Google Scholar] [CrossRef] [PubMed]
- Porter, K.; Dyall-Smith, M.L. Transfection of haloarchaea by the DNAs of spindle and round haloviruses and the use of transposon mutagenesis to identify non-essential regions. Mol. Microbiol. 2008, 70, 1236–1245. [Google Scholar] [CrossRef] [PubMed]
- Shao, Y.P.; Wang, I.N. Bacteriophage adsorption rate and optimal lysis time. Genetics 2008, 180, 471–482. [Google Scholar] [CrossRef] [PubMed]
- Aalto, A.P.; Bitto, D.; Ravantti, J.J.; Bamford, D.H.; Huiskonen, J.T.; Oksanen, H.M. Snapshot of virus evolution in hypersaline environments from the characterization of a membrane-containing Salisaeta icosahedral phage 1. Proc. Natl. Acad. Sci. USA 2012, 109, 7079–7084. [Google Scholar] [CrossRef] [PubMed]
- Pietilä, M.K.; Laurinmäki, P.; Russell, D.A.; Ko, C.C.; Jacobs-Sera, D.; Butcher, S.J.; Bamford, D.H.; Hendrix, R.W. Insights into head-tailed viruses infecting extremely halophilic archaea. J. Virol. 2013, 87, 3248–3260. [Google Scholar] [CrossRef] [PubMed]
- Rajaure, M.; Berry, J.; Kongari, R.; Cahill, J.; Young, R. Membrane fusion during phage lysis. Proc. Natl. Acad. Sci. USA 2015, 112, 5497–5502. [Google Scholar] [CrossRef] [PubMed]
- Young, R. Phage lysis: Three steps, three choices, one outcome. J. Microbiol. 2014, 52, 243–258. [Google Scholar] [CrossRef] [PubMed]
- Casjens, S.R. The DNA-packaging nanomotor of tailed bacteriophages. Nat. Rev. Microbiol. 2011, 9, 647–657. [Google Scholar] [CrossRef] [PubMed]


| Virus | Virion Morphotype | Lipids 1 | Genome 3 (GenBank Acc. No.) | Virus Family 4 | Adsorption Rate Constant (mL/min) | Virus Release Starts (h p.i.) | Virus Exit | Progeny Viruses at 25 h p.i. (pfu/mL) | References |
|---|---|---|---|---|---|---|---|---|---|
| SH1 | Icosahedral | Internal membrane | 30,889 bp (AY950802) | Spherolipoviridae | 1.1 × 10−11 | ~6 | Lysis | 1.5 × 1011 | [27,30,31] This study |
| HHTV-1 | Icosahedral tailed | No lipids | 49,107 bp (KC292025) | Unclassified siphovirus | 2.9 × 10−13 | ~7 | Lysis | 7 × 1010 | [29,38] This study |
| His1 | Spindle-shaped | Lipid modified MCP 2 | 14,462 bp (AF191796) | Salterprovirus genus 5 | 1.9 × 10−12 | ~4 | No lysis | 3 × 1010 | [24,28] This study |
| His2 | Pleomorphic | Membrane envelope | 16,067 bp (AF191797) | “Pleolipoviridae” | 5.0 × 10−12 | ~4 | No lysis | 5 × 1011 | [23,28] This study |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Svirskaitė, J.; Oksanen, H.M.; Daugelavičius, R.; Bamford, D.H. Monitoring Physiological Changes in Haloarchaeal Cell during Virus Release. Viruses 2016, 8, 59. https://doi.org/10.3390/v8030059
Svirskaitė J, Oksanen HM, Daugelavičius R, Bamford DH. Monitoring Physiological Changes in Haloarchaeal Cell during Virus Release. Viruses. 2016; 8(3):59. https://doi.org/10.3390/v8030059
Chicago/Turabian StyleSvirskaitė, Julija, Hanna M. Oksanen, Rimantas Daugelavičius, and Dennis H. Bamford. 2016. "Monitoring Physiological Changes in Haloarchaeal Cell during Virus Release" Viruses 8, no. 3: 59. https://doi.org/10.3390/v8030059
APA StyleSvirskaitė, J., Oksanen, H. M., Daugelavičius, R., & Bamford, D. H. (2016). Monitoring Physiological Changes in Haloarchaeal Cell during Virus Release. Viruses, 8(3), 59. https://doi.org/10.3390/v8030059







