Recombinant Marek’s Disease Virus as a Vector-Based Vaccine against Avian Leukosis Virus Subgroup J in Chicken
Abstract
:1. Introduction
2. Materials and Methods
2.1. Viruses and Cells
2.2. Construction of Fosmids
2.3. Recombinant MDV Rescue
2.4. Confirmation of Env and Gag Expression
2.5. Stability and Growth Properties of rMDVs
2.6. Protective Efficacy of rMDV Vaccination
2.7. Serologic Tests and Viremia Detection
2.8. Statistical Analysis
3. Results
3.1. Rescue of rMDVs
3.2. Expression of Env and Gag
3.3. In Vitro Replication and Stability of rMDVs
3.4. Antibody Responses against ALV-J Induced by rMDVs in Chickens
3.5. Vaccine Efficacy against ALV-J Challenge in Chickens
4. Discussion
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Nakamura, K.; Ogiso, M.; Tsukamoto, K.; Hamazaki, N.; Hihara, H.; Yuasa, N. Lesions of bone and bone marrow in myeloid leukosis occurring naturally in adult broiler breeders. Avian Dis. 2000, 44, 215–221. [Google Scholar] [CrossRef] [PubMed]
- Xu, B.; Dong, W.; Yu, C.; He, Z.; Lv, Y.; Sun, Y.; Feng, X.; Li, N.; Lee, L.F.; Li, M. Occurrence of avian leukosis virus subgroup J in commercial layer flocks in China. Avian Pathol. 2004, 33, 13–17. [Google Scholar] [CrossRef] [PubMed]
- Payne, L.N.; Brown, S.R.; Bumstead, N.; Howes, K.; Frazier, J.A.; Thouless, M.E. A novel subgroup of exogenous avian leukosis virus in chickens. J. Gen. Virol. 1991, 72 Pt 4, 801–807. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Yun, B.; Qin, L.; Pan, W.; Qu, Y.; Liu, Z.; Wang, Y.; Qi, X.; Gao, H.; Wang, X. Molecular epidemiology of avian leukosis virus subgroup J in layer flocks in China. J. Clin. Microbiol. 2012, 50, 953–960. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.; Cui, Z. Epidemiological and pathological studies of subgroup J avian leukosis virus infections in Chinese local “yellow” chickens. Avian Pathol. 2007, 36, 221–226. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Z.Q.; Zhang, L.; Liu, S.D.; Zhang, L.J.; Cui, Z.Z. Emerging of avian leukosis virus subgroup J in a flock of Chinese local breed. Wei Sheng Wu Xue Bao 2005, 45, 584–587. [Google Scholar] [PubMed]
- Zhang, L.; Cai, D.; Zhao, X.; Cheng, Z.; Guo, H.; Qi, C.; Liu, J.; Xu, R.; Zhao, P.; Cui, Z. Liposomes containing recombinant gp85 protein vaccine against ALV-J in chickens. Vaccine 2014, 32, 2452–2456. [Google Scholar] [CrossRef] [PubMed]
- Dou, W.; Li, H.; Cheng, Z.; Zhao, P.; Liu, J.; Cui, Z.; Liu, H.; Jing, W.; Guo, H. Maternal antibody induced by recombinant gp85 protein vaccine adjuvanted with CpG-ODN protects against ALV-J early infection in chickens. Vaccine 2013, 31, 6144–6149. [Google Scholar] [CrossRef] [PubMed]
- Xu, Q.; Ma, X.; Wang, F.; Li, H.; Xiao, Y.; Zhao, X. Design and construction of a chimeric multi-epitope gene as an epitope-vaccine strategy against ALV-J. Protein Expr. Purif. 2015, 106, 18–24. [Google Scholar] [CrossRef] [PubMed]
- Osterrieder, N.; Kamil, J.P.; Schumacher, D.; Tischer, B.K.; Trapp, S. Marek’s disease virus: From miasma to model. Nat. Rev. Microbiol. 2006, 4, 283–294. [Google Scholar] [CrossRef] [PubMed]
- Calnek, B.W. Pathogenesis of Marek’s disease virus infection. Curr. Top. Microbiol. Immunol. 2001, 255, 25–55. [Google Scholar] [PubMed]
- De Boer, G.F.; Groenendal, J.E.; Boerrigter, H.M.; Kok, G.L.; Pol, J.M. Protective efficacy of Marek’s disease virus (MDV) CVI-988 CEF65 clone C against challenge infection with three very virulent MDV strains. Avian Dis. 1986, 30, 276–283. [Google Scholar] [CrossRef] [PubMed]
- Rispens, B.H.; van Vloten, H.; Mastenbroek, N.; Maas, H.J.; Schat, K.A. Control of Marek’s disease in the Netherlands. I. Isolation of an avirulent Marek’s disease virus (strain CVI 988) and its use in laboratory vaccination trials. Avian Dis. 1972, 16, 108–125. [Google Scholar] [CrossRef] [PubMed]
- Luschow, D.; Werner, O.; Mettenleiter, T.C.; Fuchs, W. Protection of chickens from lethal avian influenza A virus infection by live-virus vaccination with infectious laryngotracheitis virus recombinants expressing the hemagglutinin (H5) gene. Vaccine 2001, 19, 4249–4259. [Google Scholar] [CrossRef]
- Sakaguchi, M.; Nakamura, H.; Sonoda, K.; Okamura, H.; Yokogawa, K.; Matsuo, K.; Hira, K. Protection of chickens with or without maternal antibodies against both Marek’s and Newcastle diseases by one-time vaccination with recombinant vaccine of Marek’s disease virus type 1. Vaccine 1998, 16, 472–479. [Google Scholar] [CrossRef]
- Sonoda, K.; Sakaguchi, M.H.; Yokogawa, K.; Tokunaga, E.; Tokiyoshi, S.; Kawaguchi, Y.; Hirai, K. Development of an Effective Polyvalent Vaccine against both Marek’s and Newcastle Diseases Based on Recombinant Marek’s Disease Virus Type 1 in Commercial Chickens with Maternal Antibodies. J. Virol. 2000, 74, 3217–3226. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Liu, C.J.; Zhang, Y.P.; Li, Z.J.; Liu, A.L.; Yan, F.H.; Cong, F.; Cheng, Y. Comparative full-length sequence analysis of Marek’s disease virus vaccine strain 814. Arch. Virol. 2012, 157, 177–183. [Google Scholar] [CrossRef] [PubMed]
- Mizuguchi, H.; Xu, Z.; Ishii-Watabe, A.; Uchida, E.; Hayakawa, T. IRES-dependent second gene expression is significantly lower than cap-dependent first gene expression in a bicistronic vector. Mol. Ther. J. Am. Soc. Gene Ther. 2000, 1, 376–382. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Li, G.; Gao, H.; Qi, X.; Gao, Y.; Qin, L.; Wang, Y.; Wang, X. Protection of chickens against reticuloendotheliosis virus infection by DNA vaccination. Vet. Microbiol. 2013, 166, 59–67. [Google Scholar] [CrossRef] [PubMed]
- Morgan, R.W.; Cantello, J.L.; McDermott, C.H. Transfection of chicken embryo fibroblasts with Marek’s disease virus DNA. Avian Dis. 1990, 34, 345–351. [Google Scholar] [CrossRef] [PubMed]
- Delong, L.I.; Liu, Z.; Yun, B.; Guan, W.U.; Gao, Y.; Qin, L.; Xiaole, Q.I.; Wang, Y.; Gao, H.; Liu, S. Expression of Capsid Protein p27 Gene of Avian Leucosis Virus and Preparation of Multiclonal Antibodies against Recombinant p27 Protein. China Poult. 2012, 34, 13–16. [Google Scholar]
- Schumacher, D.; Tischer, B.K.; Fuchs, W.; Osterrieder, N. Reconstitution of Marek’s disease virus serotype 1 (MDV-1) from DNA cloned as a bacterial artificial chromosome and characterization of a glycoprotein B-negative MDV-1 mutant. J. Virol. 2000, 74, 11088–11098. [Google Scholar] [CrossRef] [PubMed]
- Cui, H.; Gao, H.; Cui, X.; Zhao, Y.; Shi, X.; Li, Q.; Yan, S.; Gao, M.; Wang, M.; Liu, C.; et al. Avirulent Marek’s disease virus type 1 strain 814 vectored vaccine expressing avian influenza (AI) virus H5 haemagglutinin induced better protection than turkey herpesvirus vectored AI vaccine. PLoS ONE 2013, 8, e53340. [Google Scholar] [CrossRef] [PubMed]
- Jarosinski, K.W.; Margulis, N.G.; Kamil, J.P.; Spatz, S.J.; Nair, V.K.; Osterrieder, N. Horizontal transmission of Marek’s disease virus requires US2, the UL13 protein kinase, and gC. J. Virol. 2007, 81, 10575–10587. [Google Scholar] [CrossRef] [PubMed]
- Jang, H.K.; Ono, M.; Kim, T.J.; Izumiya, Y.; Damiani, A.M.; Matsumura, T.; Niikura, M.; Kai, C.; Mikami, T. The genetic organization and transcriptional analysis of the short unique region in the genome of nononcogenic Marek’s disease virus serotype 2. Virus Res. 1998, 58, 137–147. [Google Scholar] [CrossRef]
- Cantello, J.L.; Anderson, A.S.; Francesconi, A.; Morgan, R.W. Isolation of a Marek’s disease virus (MDV) recombinant containing the lacZ gene of Escherichia coli stably inserted within the MDV US2 gene. J. Virol. 1991, 65, 1584–1588. [Google Scholar] [PubMed]
- Hicar, M.D.; Chen, X.; Briney, B.; Hammonds, J.; Wang, J.J.; Kalams, S.; Spearman, P.W.; Crowe, J.E., Jr. Pseudovirion particles bearing native HIV envelope trimers facilitate a novel method for generating human neutralizing monoclonal antibodies against HIV. J. Acquir. Immune Defic. Syndr. 2010, 54, 223–235. [Google Scholar] [CrossRef] [PubMed]
- Witter, R.L. Avian tumor viruses: Persistent and evolving pathogens. Acta Vet. Hung. 1997, 45, 251–266. [Google Scholar] [PubMed]
- Baigent, S.J.; Smith, L.P.; Nair, V.K.; Currie, R.J. Vaccinal control of Marek’s disease: Current challenges, and future strategies to maximize protection. Vet. Immunol. Immunopathol. 2006, 112, 78–86. [Google Scholar] [CrossRef] [PubMed]
- Cunningham, C.; Davison, A.J. A cosmid-based system for constructing mutants of herpes simplex virus type 1. Virology 1993, 197, 116–124. [Google Scholar] [CrossRef] [PubMed]
- Brune, W.; Messerle, M.; Koszinowski, U.H. Forward with BACs: New tools for herpesvirus genomics. Trends Genet. 2000, 16, 254–259. [Google Scholar] [CrossRef]
- Zhao, Y.; Petherbridge, L.; Smith, L.P.; Baigent, S.; Nair, V. Self-excision of the BAC sequences from the recombinant Marek’s disease virus genome increases replication and pathogenicity. Virol. J. 2008, 5, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Wagner, M. Systematic excision of vector sequences from the BAC-cloned herpesvirus genome during virus reconstitution. J. Virol. 1999, 73, 7056–7060. [Google Scholar] [PubMed]
- Zhou, F.; Li, Q.; Wong, S.W.; Gao, S.J. Autoexcision of bacterial artificial chromosome facilitated by terminal repeat-mediated homologous recombination: A novel approach for generating traceless genetic mutants of herpesviruses. J. Virol. 2010, 84, 2871–2880. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Taaffe, J.; Parker, C.; Solorzano, A.; Cao, H.; Garcia-Sastre, A.; Lu, S. Hemagglutinin (HA) proteins from H1 and H3 serotypes of influenza A viruses require different antigen designs for the induction of optimal protective antibody responses as studied by codon-optimized HA DNA vaccines. J. Virol. 2006, 80, 11628–11637. [Google Scholar] [CrossRef] [PubMed]
- Lee, L.F.; Lupiani, B.; Silva, R.F.; Kung, H.J.; Reddy, S.M. Recombinant Marek’s disease virus (MDV) lacking the Meq oncogene confers protection against challenge with a very virulent plus strain of MDV. Vaccine 2008, 26, 1887–1892. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Yu, K.; Zhang, H.; Zhang, P.; Li, C.; Tian, G.; Li, Y.; Wang, X.; Ge, J.; Bu, Z.; et al. Enhanced protective efficacy of H5 subtype avian influenza DNA vaccine with codon optimized HA gene in a pCAGGS plasmid vector. Antivir. Res. 2007, 75, 234–241. [Google Scholar] [CrossRef] [PubMed]
- Han, J.J.; Mhatre, A.N.; Wareing, M.; Pettis, R.; Gao, W.Q.; Zufferey, R.N.; Trono, D.; Lalwani, A.K. Transgene expression in the guinea pig cochlea mediated by a lentivirus-derived gene transfer vector. Hum. Gene Ther. 1999, 10, 1867–1873. [Google Scholar] [CrossRef] [PubMed]
- Baigent, S.J.; Petherbridge, L.J.; Smith, L.P.; Zhao, Y.; Chesters, P.M.; Nair, V.K. Herpesvirus of turkey reconstituted from bacterial artificial chromosome clones induces protection against Marek’s disease. J. Gen. Virol. 2006, 87 Pt 4, 769–776. [Google Scholar] [CrossRef] [PubMed]
- Tsukamoto, K.; Kojima, C.; Komori, Y.; Tanimura, N.; Mase, M.; Yamaguchi, S. Protection of Chickens against Very Virulent Infectious Bursal Disease Virus (IBDV) and Marek’s Disease Virus (MDV) with a Recombinant MDV Expressing IBDV VP2. Virology 1999, 257, 352–362. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Q.; Li, S.; Yu, H.; Xia, N.; Modis, Y. Virus-like particle-based human vaccines: Quality assessment based on structural and functional properties. Trends Biotechnol. 2013, 31, 654–663. [Google Scholar] [CrossRef] [PubMed]
- Crooks, E.T.; Moore, P.L.; Franti, M.; Cayanan, C.S.; Zhu, P.; Jiang, P.; Vries, R.P.D.; Wiley, C.; Zharkikh, I.; Schülke, N. A comparative immunogenicity study of HIV-1 virus-like particles bearing various forms of envelope proteins, particles bearing no envelope and soluble monomeric gp120. Virology 2007, 366, 245–262. [Google Scholar] [CrossRef] [PubMed]
- Berkower, I.; Raymond, M.; Muller, J.; Spadaccini, A.; Aberdeen, A. Assembly, structure, and antigenic properties of virus-like particles rich in HIV-1 envelope gp120. Virology 2004, 321, 75–86. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.J.; Zhang, S.; Xiao-Xue, G.U.; Yuan, L.; Wang, B.Y.; Zhai, X.Y.; Tian, K.G. Epidemiological Study of Avian Leukemia in Different Import Poultry Strains from 2009 to 2010 in Some Areas of China. China Anim. Husb. Vet. Med. 2012, 39, 200–202. [Google Scholar]
- Pavón, J.; Galvis, O.; Echeverría, F.; Castaño, J.G.; Echeverry, M.; Robledo, S.; Jiménez-Piqué, E.; Mestra, A.; Anglada, M. Development of an antigen-capture ELISA for the detection of avian leukosis virus p27 antigen. J. Virol. Methods 2013, 187, 278–283. [Google Scholar]
- Li, Y.; Reddy, K.; Reid, S.M.; Cox, W.J.; Brown, I.H.; Britton, P.; Nair, V.; Iqbal, M. Recombinant herpesvirus of turkeys as a vector-based vaccine against highly pathogenic H7N1 avian influenza and Marek’s disease. Vaccine 2011, 29, 8257–8266. [Google Scholar] [CrossRef] [PubMed]
- Fadly, A.M.; Smith, E.J. Isolation and some characteristics of a subgroup J-like avian leukosis virus associated with myeloid leukosis in meat-type chickens in the United States. Avian Dis. 1999, 43, 391–400. [Google Scholar] [CrossRef] [PubMed]
- Tong, K. Study on the Immunization of Chickens against Marek’s Disease—Report on a Naturally Avirulent Vaccine Strain of Marek’ Disease Herpesvirus. Chin. J. Anim. Vet. Sci. 1984. [Google Scholar]
- Zhang, F.; Liu, C.J.; Zhang, Y.P.; Liu, A.L.; Yan, F.H.; Hou, G.Y.; Cong, F. Sequencing analysis of repeat regions of Marek’s disease virus vaccine strain 814. Chin. J. Prev. Vet. Med. 2010, 32, 294–297. [Google Scholar]
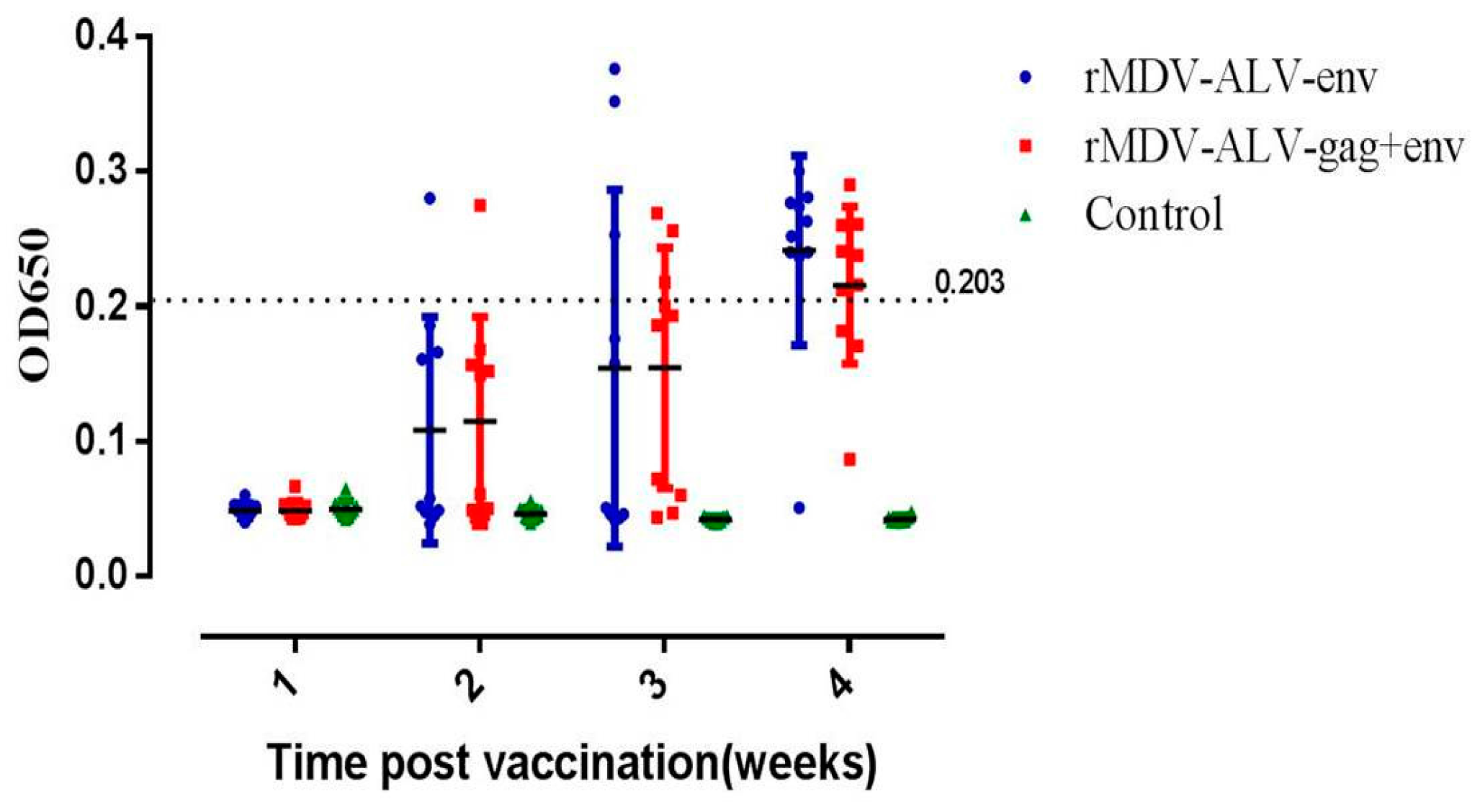
| Group | 1st Week | 2nd Week | ||
|---|---|---|---|---|
| P.V.R | P.R. | P.V.R. | P.R. | |
| rMDV/ALV-env | 26.7% (4/15) | 73.3% (11/15) | 0.0% (0/15) | 100.0% (15/15) |
| rMDV/ALV-gag+env | 33.3% (4/12) | 67.7% (8/12) | 0.0% (0/12) | 100% (12/12) |
| No immune challenge | 55.5% (5/9) | 45.5% (4/9) | 25.0% (2/8) | 75% (6/8) |
| No immune no challenge | 0.0% (0/5) | 100% (5/5) | 0.0% (0/5) | 100% (5/5) |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Y.; Li, K.; Gao, Y.; Gao, L.; Zhong, L.; Zhang, Y.; Liu, C.; Zhang, Y.; Wang, X. Recombinant Marek’s Disease Virus as a Vector-Based Vaccine against Avian Leukosis Virus Subgroup J in Chicken. Viruses 2016, 8, 301. https://doi.org/10.3390/v8110301
Liu Y, Li K, Gao Y, Gao L, Zhong L, Zhang Y, Liu C, Zhang Y, Wang X. Recombinant Marek’s Disease Virus as a Vector-Based Vaccine against Avian Leukosis Virus Subgroup J in Chicken. Viruses. 2016; 8(11):301. https://doi.org/10.3390/v8110301
Chicago/Turabian StyleLiu, Yongzhen, Kai Li, Yulong Gao, Li Gao, Li Zhong, Yao Zhang, Changjun Liu, Yanping Zhang, and Xiaomei Wang. 2016. "Recombinant Marek’s Disease Virus as a Vector-Based Vaccine against Avian Leukosis Virus Subgroup J in Chicken" Viruses 8, no. 11: 301. https://doi.org/10.3390/v8110301





