The VP1u Receptor Restricts Parvovirus B19 Uptake to Permissive Erythroid Cells
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cells
2.2. Parvovirus B19-Infected Plasma
2.3. VP1u Expression
2.4. MS2 Capsid Expression
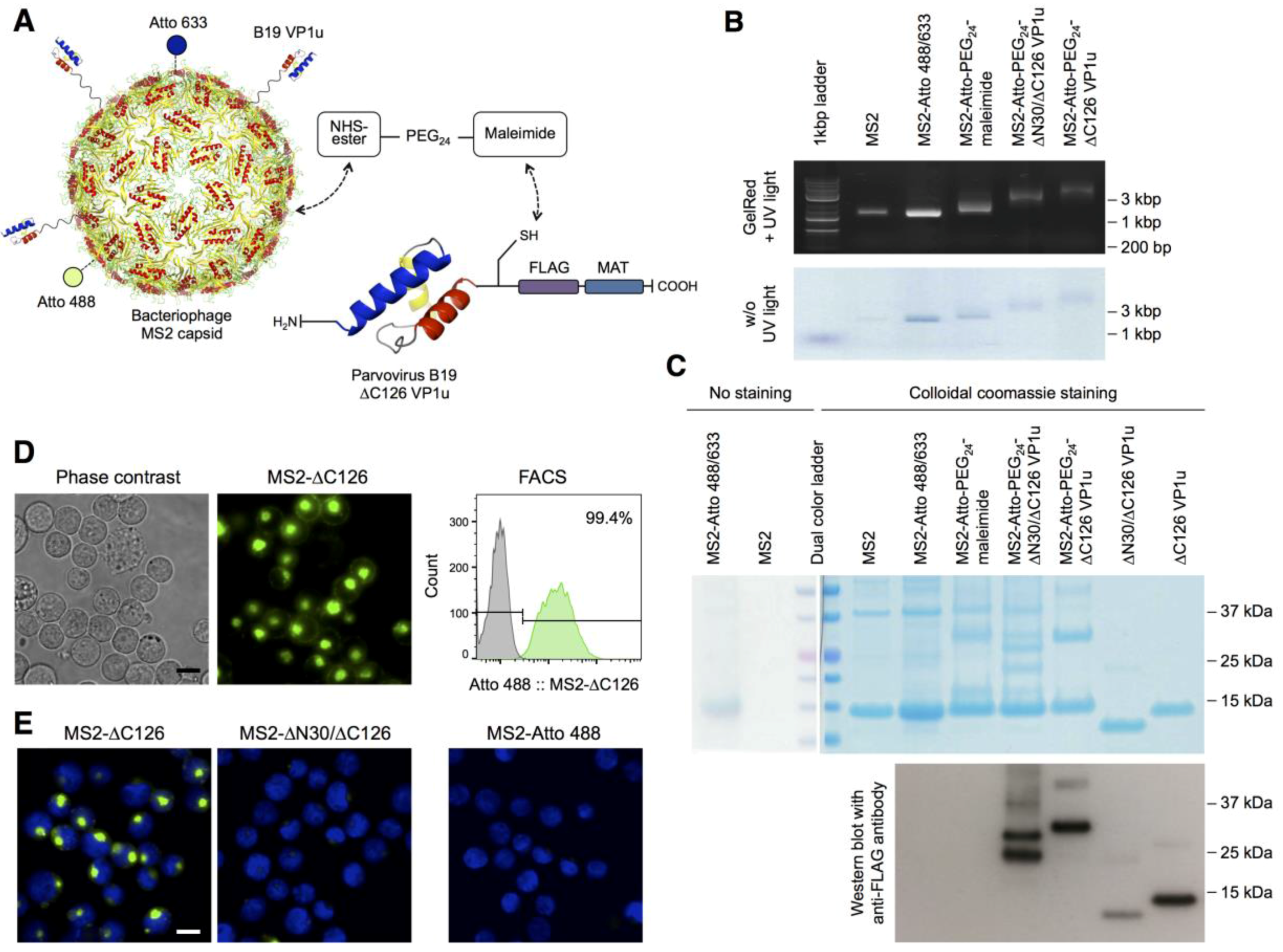
2.5. MS2-VP1u Bioconjugation
2.6. MS2 Internalization
2.7. B19V Uptake and Competition
2.8. B19V Infectivity Assays
2.9. Statistical Analysis
3. Results
3.1. Bioconjugation of the MS2-VP1u Capsid
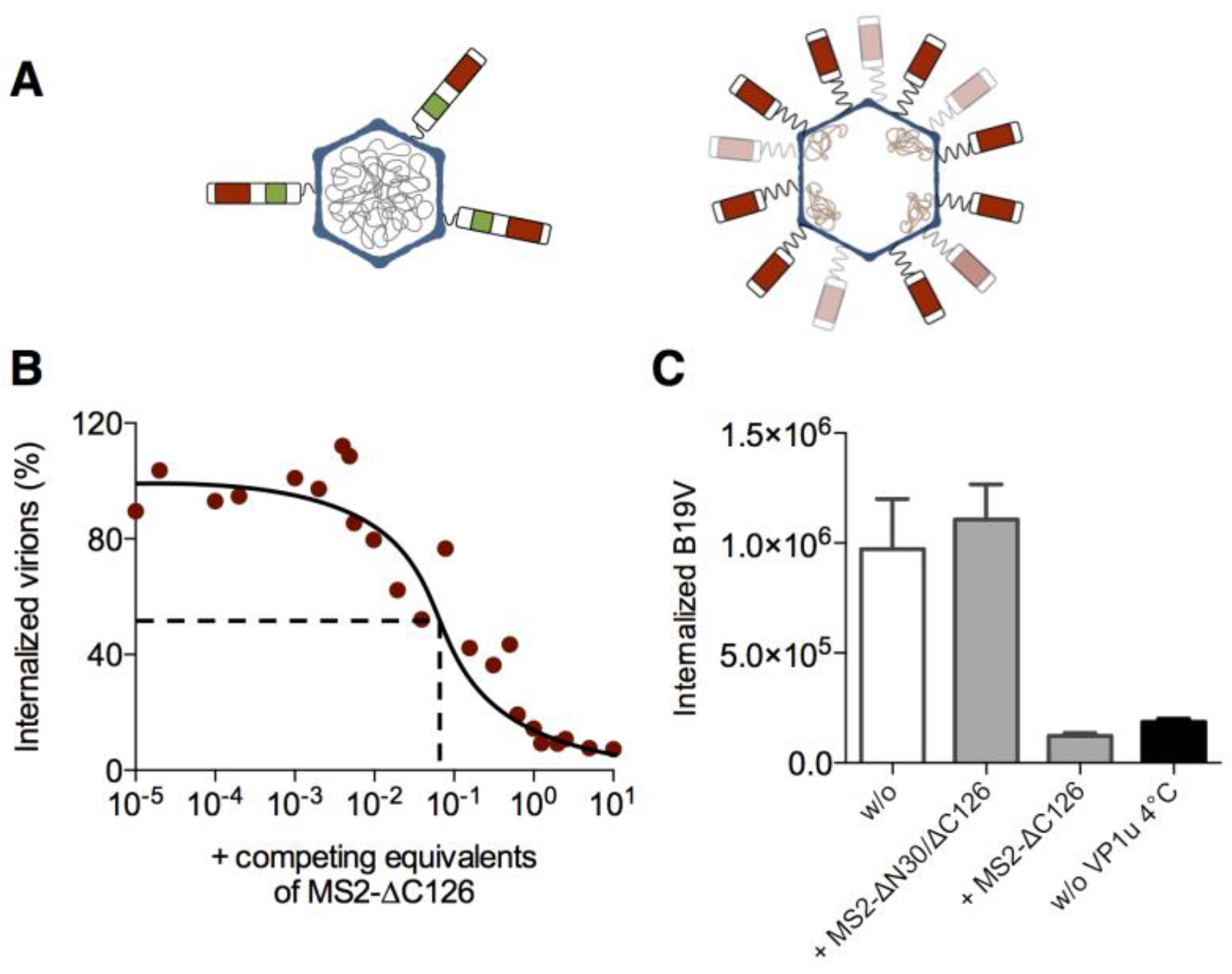
3.2. The VP1u Region Is Sufficient for Virus Internalization
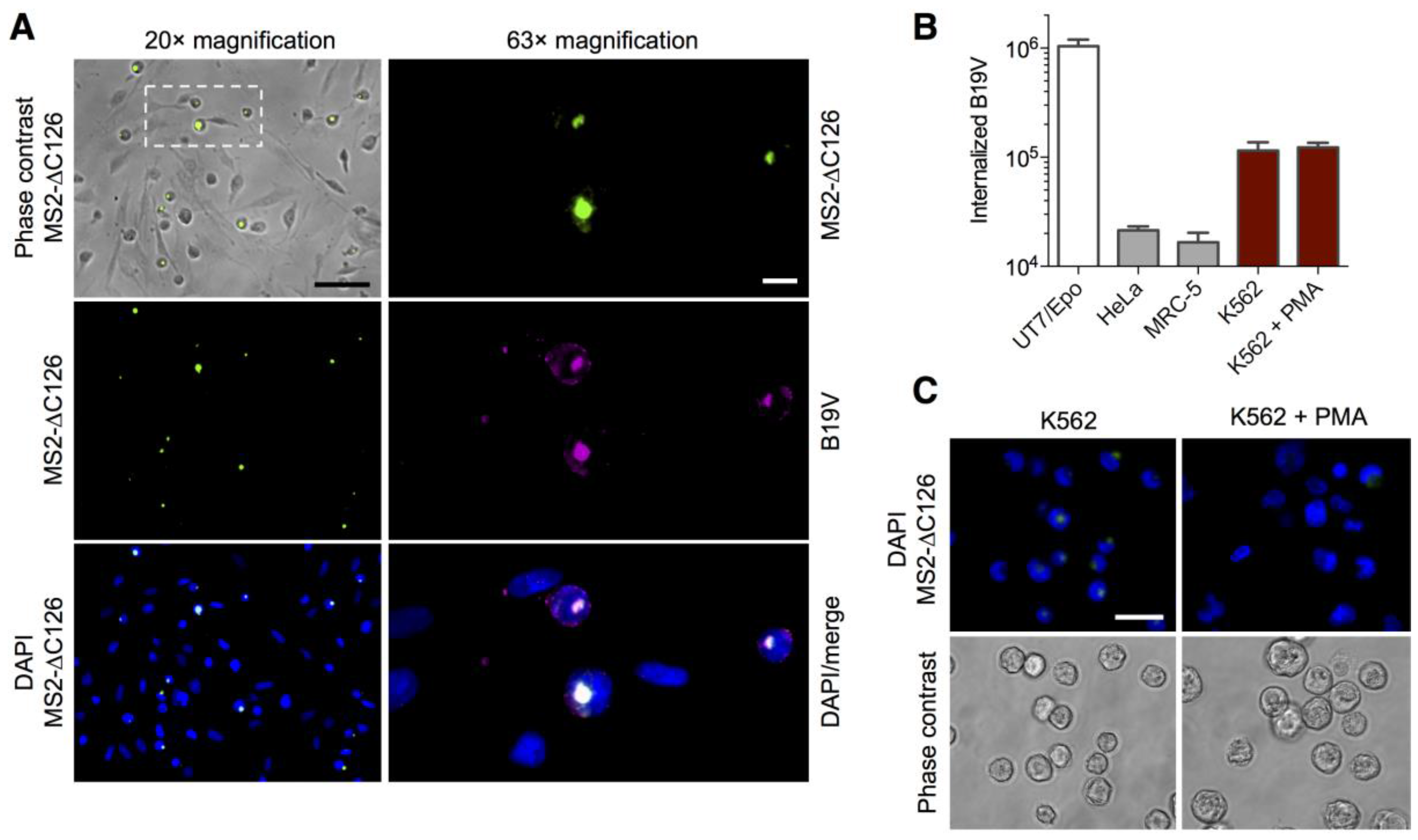
3.3. MS2-VP1u Capsids Mimic the Erythroid Specificity of Native B19V
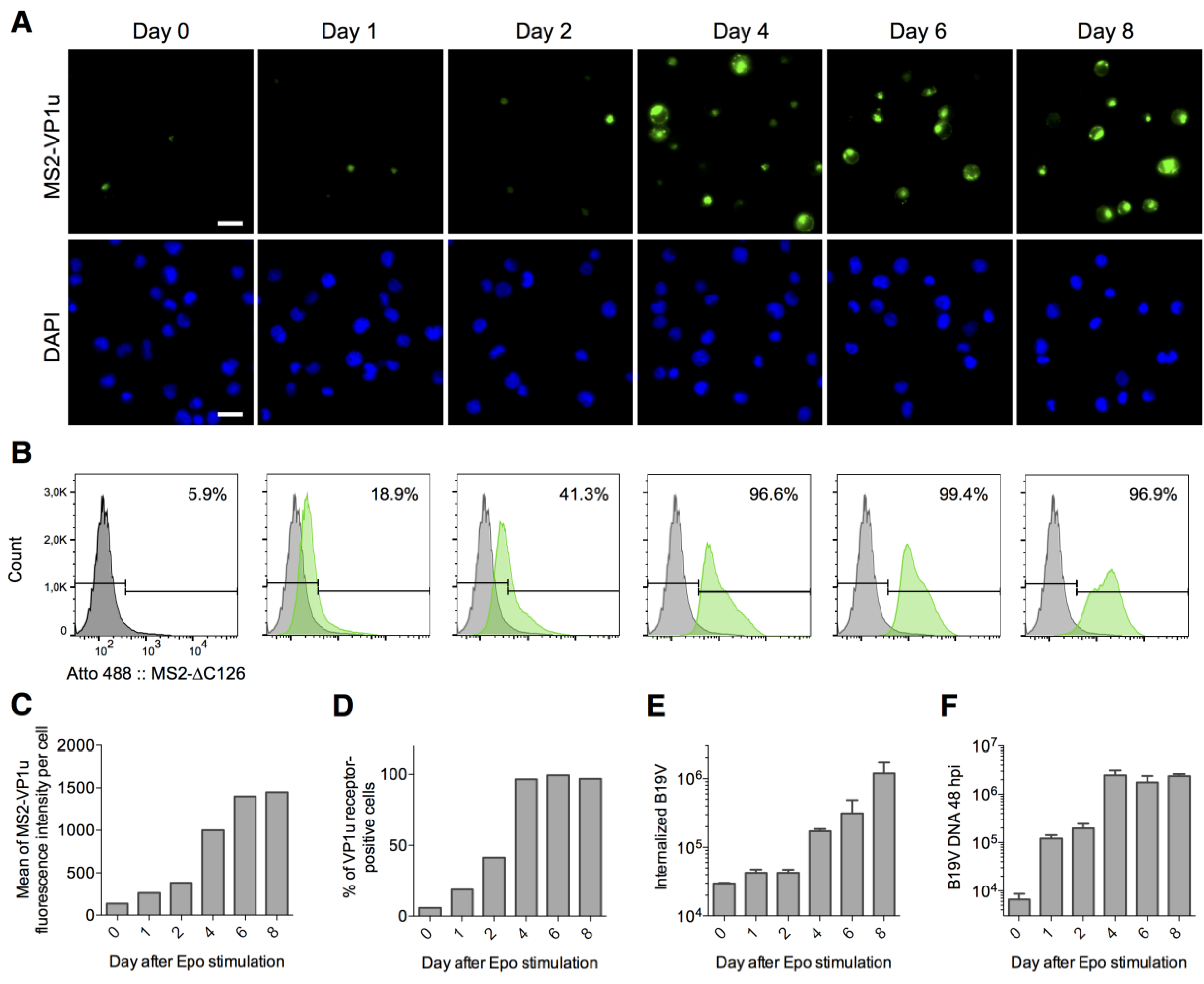
3.4. Expression of the VP1u Receptor Correlates with B19V Uptake and Cellular Permissiveness
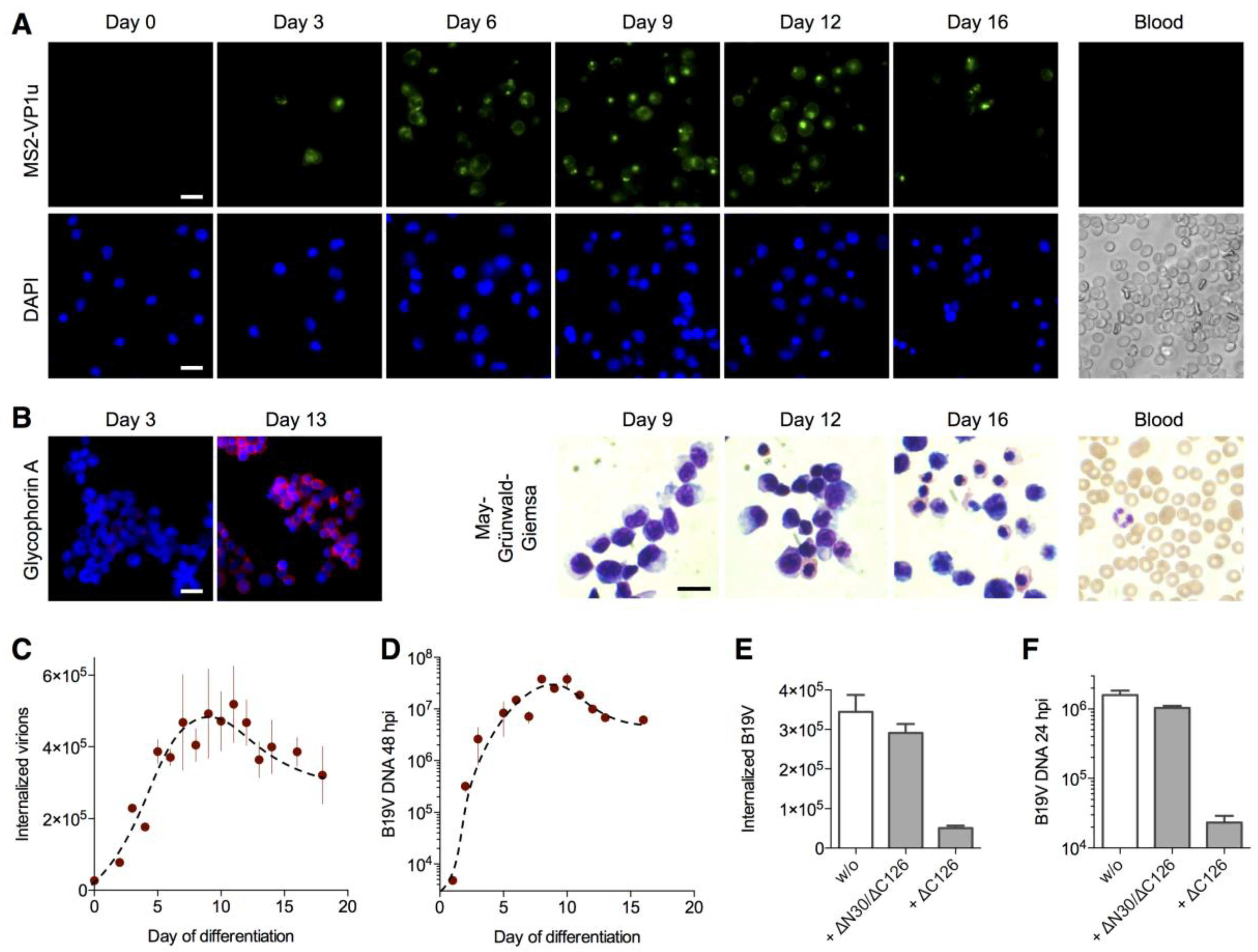
3.5. The VP1u Receptor Becomes Expressed at the Transition of Burst Forming Unit-Erythroid to the Erythropoietin-Dependent Erythroid Differentiation Stages
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Heegaard, E.D.; Brown, K.E. Human parvovirus B19. Clin. Microbiol. Rev. 2002, 15, 485–505. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, T.; Ozawa, K.; Takahashi, K.; Asano, S.; Takaku, F. Susceptibility of human erythropoietic cells to B19 parvovirus in vitro increases with differentiation. Blood 1990, 75, 603–610. [Google Scholar] [PubMed]
- Morey, A.L.; Fleming, K.A. Immunophenotyping of fetal haemopoietic cells permissive for human parvovirus B19 replication in vitro. Br. J. Haematol. 1992, 82, 302–309. [Google Scholar] [CrossRef] [PubMed]
- Wong, S.; Zhi, N.; Filippone, C.; Keyvanfar, K.; Kajigaya, S.; Brown, K.E.; Young, N.S. Ex vivo-generated CD36+ erythroid progenitors are highly permissive to human parvovirus B19 replication. J. Virol. 2008, 82, 2470–2476. [Google Scholar] [CrossRef] [PubMed]
- Chen, A.Y.; Guan, W.; Lou, S.; Liu, Z.; Kleiboeker, S.; Qiu, J. Role of erythropoietin receptor signaling in parvovirus B19 replication in human erythroid progenitor cells. J. Virol. 2010, 84, 12385–12396. [Google Scholar] [CrossRef] [PubMed]
- Bua, G.; Manaresi, E.; Bonvicini, F.; Gallinella, G. Parvovirus B19 Replication and Expression in Differentiating Erythroid Progenitor Cells. PLoS ONE 2016, 11, e0148547. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Qiu, J. Human parvovirus B19: A mechanistic overview of infection and DNA replication. Future Virol. 2015, 10, 155–167. [Google Scholar] [CrossRef] [PubMed]
- Brown, K.E.; Anderson, S.M.; Young, N.S. Erythrocyte P antigen: Cellular receptor for B19 parvovirus. Science 1993, 262, 114–117. [Google Scholar] [CrossRef] [PubMed]
- Brown, K.E.; Hibbs, J.R.; Gallinella, G.; Anderson, S.M.; Lehman, E.D.; McCarthy, P.; Young, N.S. Resistance to parvovirus B19 infection due to lack of virus receptor (erythrocyte P antigen). N. Engl. J. Med. 1994, 330, 1192–1196. [Google Scholar] [CrossRef] [PubMed]
- Weigel-Kelley, K.A.; Yoder, M.C.; Srivastava, A. Recombinant human parvovirus B19 vectors: Erythrocyte P antigen is necessary but not sufficient for successful transduction of human hematopoietic cells. J. Virol. 2001, 75, 4110–4116. [Google Scholar] [CrossRef] [PubMed]
- Bonsch, C.; Kempf, C.; Ros, C. Interaction of parvovirus B19 with human erythrocytes alters virus structure and cell membrane integrity. J. Virol. 2008, 82, 11784–11791. [Google Scholar] [CrossRef] [PubMed]
- Bonsch, C.; Zuercher, C.; Lieby, P.; Kempf, C.; Ros, C. The globoside receptor triggers structural changes in the B19 virus capsid that facilitate virus internalization. J. Virol. 2010, 84, 11737–11746. [Google Scholar] [CrossRef] [PubMed]
- Weigel-Kelley, K.A.; Yoder, M.C.; Srivastava, A. Alpha5beta1 integrin as a cellular coreceptor for human parvovirus B19: Requirement of functional activation of beta1 integrin for viral entry. Blood 2003, 102, 3927–3933. [Google Scholar] [CrossRef] [PubMed]
- Munakata, Y.; Saito-Ito, T.; Kumura-Ishii, K.; Huang, J.; Kodera, T.; Ishii, T.; Hirabayashi, Y.; Koyanagi, Y.; Sasaki, T. Ku80 autoantigen as a cellular coreceptor for human parvovirus B19 infection. Blood 2005, 106, 3449–3456. [Google Scholar] [CrossRef] [PubMed]
- Leisi, R.; Ruprecht, N.; Kempf, C.; Ros, C. Parvovirus B19 uptake is a highly selective process controlled by VP1u: A novel determinant of viral tropism. J. Virol. 2013, 87, 13161–13167. [Google Scholar] [CrossRef] [PubMed]
- Leisi, R.; von Nordheim, M.; Kempf, C.; Ros, C. Specific targeting of proerythroblasts and erythroleukemic cells by the VP1u region of parvovirus B19. Bioconjug. Chem. 2015, 26, 1923–1930. [Google Scholar] [CrossRef] [PubMed]
- Ozawa, K.; Young, N. Characterization of capsid and noncapsid proteins of B19 parvovirus propagated in human erythroid bone marrow cell cultures. J. Virol. 1987, 61, 2627–2630. [Google Scholar] [PubMed]
- Cotmore, S.F.; McKie, V.C.; Anderson, L.J.; Astell, C.R.; Tattersall, P. Identification of the major structural and nonstructural proteins encoded by human parvovirus B19 and mapping of their genes by procaryotic expression of isolated genomic fragments. J. Virol. 1986, 60, 548–557. [Google Scholar] [PubMed]
- Kurtzman, G.J.; Cohen, B.J.; Field, A.M.; Oseas, R.; Blaese, R.M.; Young, N.S. Immune response to B19 parvovirus and an antibody defect in persistent viral infection. J. Clin. Investig. 1989, 84, 1114–1123. [Google Scholar] [CrossRef] [PubMed]
- Chandramouli, S.; Medina-Selby, A.; Coit, D.; Schaefer, M.; Spencer, T.; Brito, L.A.; Zhang, P.; Otten, G.; Mandl, C.W.; Mason, P.W.; et al. Generation of a parvovirus B19 vaccine candidate. Vaccine 2013, 31, 3872–3878. [Google Scholar] [CrossRef] [PubMed]
- Saikawa, T.; Anderson, S.; Momoeda, M.; Kajigaya, S.; Young, N.S. Neutralizing linear epitopes of B19 parvovirus cluster in the VP1 unique and VP1-VP2 junction regions. J. Virol. 1993, 67, 3004–3009. [Google Scholar] [PubMed]
- Anderson, S.; Momoeda, M.; Kawase, M.; Kajigaya, S.; Young, N.S. Peptides derived from the unique region of B19 parvovirus minor capsid protein elicit neutralizing antibodies in rabbits. Virology 1995, 206, 626–632. [Google Scholar] [CrossRef]
- Leisi, R.; Di Tommaso, C.; Kempf, C.; Ros, C. The Receptor-Binding Domain in the VP1u Region of Parvovirus B19. Viruses 2016, 8, 61. [Google Scholar] [CrossRef] [PubMed]
- Mastico, R.A.; Talbot, S.J.; Stockley, P.G. Multiple presentation of foreign peptides on the surface of an RNA-free spherical bacteriophage capsid. J. Gen. Virol. 1993, 74 Pt 4, 541–548. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.; Brown, W.L.; Stockley, P.G. Cell-specific delivery of bacteriophage-encapsidated ricin A chain. Bioconjug. Chem. 1995, 6, 587–595. [Google Scholar] [CrossRef] [PubMed]
- Galaway, F.A.; Stockley, P.G. MS2 viruslike particles: A robust, semisynthetic targeted drug delivery platform. Mol. Pharm. 2013, 10, 59–68. [Google Scholar] [CrossRef] [PubMed]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef] [PubMed]
- Stonehouse, N.J.; Valegard, K.; Golmohammadi, R.; van den Worm, S.; Walton, C.; Stockley, P.G.; Liljas, L. Crystal structures of MS2 capsids with mutations in the subunit FG loop. J. Mol. Biol. 1996, 256, 330–339. [Google Scholar] [CrossRef] [PubMed]
- Valegard, K.; Liljas, L.; Fridborg, K.; Unge, T. The three-dimensional structure of the bacterial virus MS2. Nature 1990, 345, 36–41. [Google Scholar] [CrossRef] [PubMed]
- Peabody, D.S.; Al-Bitar, L. Isolation of viral coat protein mutants with altered assembly and aggregation properties. Nucleic Acids Res. 2001, 29, E113. [Google Scholar] [CrossRef] [PubMed]
- Plevka, P.; Tars, K.; Liljas, L. Structure and stability of icosahedral particles of a covalent coat protein dimer of bacteriophage MS2. Protein Sci. 2009, 18, 1653–1661. [Google Scholar] [CrossRef] [PubMed]
- Wolfisberg, R.; Ruprecht, N.; Kempf, C.; Ros, C. Impaired genome encapsidation restricts the in vitro propagation of human parvovirus B19. J. Virol. Methods 2013, 193, 215–225. [Google Scholar] [CrossRef] [PubMed]
- Gigler, A.; Dorsch, S.; Hemauer, A.; Williams, C.; Kim, S.; Young, N.S.; Zolla-Pazner, S.; Wolf, H.; Gorny, M.K.; Modrow, S. Generation of neutralizing human monoclonal antibodies against parvovirus B19 proteins. J. Virol. 1999, 73, 1974–1979. [Google Scholar] [PubMed]
- Filippone, C.; Franssila, R.; Kumar, A.; Saikko, L.; Kovanen, P.E.; Soderlund-Venermo, M.; Hedman, K. Erythroid progenitor cells expanded from peripheral blood without mobilization or preselection: Molecular characteristics and functional competence. PLoS ONE 2010, 5, e9496. [Google Scholar] [CrossRef] [PubMed]
- Hao, Q.L.; Shah, A.J.; Thiemann, F.T.; Smogorzewska, E.M.; Crooks, G.M. A functional comparison of CD34 + CD38- cells in cord blood and bone marrow. Blood 1995, 86, 3745–3753. [Google Scholar] [PubMed]
- Gothot, A.; Pyatt, R.; McMahel, J.; Rice, S.; Srour, E.F. Functional heterogeneity of human CD34(+) cells isolated in subcompartments of the G0/G1 phase of the cell cycle. Blood 1997, 90, 4384–4393. [Google Scholar] [PubMed]
- Koury, M.J.; Bondurant, M.C. Maintenance by erythropoietin of viability and maturation of murine erythroid precursor cells. J. Cell. Physiol. 1988, 137, 65–74. [Google Scholar] [CrossRef] [PubMed]
- Koury, M.J.; Bondurant, M.C. Erythropoietin retards DNA breakdown and prevents programmed death in erythroid progenitor cells. Science 1990, 248, 378–381. [Google Scholar] [CrossRef] [PubMed]
- Pop, R.; Shearstone, J.R.; Shen, Q.; Liu, Y.; Hallstrom, K.; Koulnis, M.; Gribnau, J.; Socolovsky, M. A key commitment step in erythropoiesis is synchronized with the cell cycle clock through mutual inhibition between PU.1 and S-phase progression. PLoS Biol. 2010, 8. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Liu, X.; Jaenisch, R.; Lodish, H.F. Generation of committed erythroid BFU-E and CFU-E progenitors does not require erythropoietin or the erythropoietin receptor. Cell 1995, 83, 59–67. [Google Scholar] [CrossRef]
- Kelley, L.L.; Koury, M.J.; Bondurant, M.C.; Koury, S.T.; Sawyer, S.T.; Wickrema, A. Survival or death of individual proerythroblasts results from differing erythropoietin sensitivities: A mechanism for controlled rates of erythrocyte production. Blood 1993, 82, 2340–2352. [Google Scholar] [PubMed]
- Jobin, C.; Cloutier, M.; Simard, C.; Neron, S. Heterogeneity of in vitro-cultured CD34+ cells isolated from peripheral blood. Cytotherapy 2015, 17, 1472–1484. [Google Scholar] [CrossRef] [PubMed]
- Koury, M.J. Tracking erythroid progenitor cells in times of need and times of plenty. Exp. Hematol. 2016, 44, 653–663. [Google Scholar] [CrossRef] [PubMed]
- Hara, H.; Ogawa, M. Erthropoietic precursors in mice with phenylhydrazine-induced anemia. Am. J. Hematol. 1976, 1, 453–458. [Google Scholar] [CrossRef] [PubMed]
- Chasis, J.A.; Mohandas, N. Erythroblastic islands: Niches for erythropoiesis. Blood 2008, 112, 470–478. [Google Scholar] [CrossRef] [PubMed]
- Rouger, P.; Gane, P.; Salmon, C. Tissue distribution of H, Lewis and P antigens as shown by a panel of 18 monoclonal antibodies. Rev. Fr. Transfus. Immunohematol. 1987, 30, 699–708. [Google Scholar] [CrossRef]
- Nasir, W.; Nilsson, J.; Olofsson, S.; Bally, M.; Rydell, G.E. Parvovirus B19 VLP recognizes globoside in supported lipid bilayers. Virology 2014, 456–457, 364–369. [Google Scholar] [CrossRef] [PubMed]
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Leisi, R.; Von Nordheim, M.; Ros, C.; Kempf, C. The VP1u Receptor Restricts Parvovirus B19 Uptake to Permissive Erythroid Cells. Viruses 2016, 8, 265. https://doi.org/10.3390/v8100265
Leisi R, Von Nordheim M, Ros C, Kempf C. The VP1u Receptor Restricts Parvovirus B19 Uptake to Permissive Erythroid Cells. Viruses. 2016; 8(10):265. https://doi.org/10.3390/v8100265
Chicago/Turabian StyleLeisi, Remo, Marcus Von Nordheim, Carlos Ros, and Christoph Kempf. 2016. "The VP1u Receptor Restricts Parvovirus B19 Uptake to Permissive Erythroid Cells" Viruses 8, no. 10: 265. https://doi.org/10.3390/v8100265




