Honokiol, a Lignan Biphenol Derived from the Magnolia Tree, Inhibits Dengue Virus Type 2 Infection
Abstract
:1. Introduction
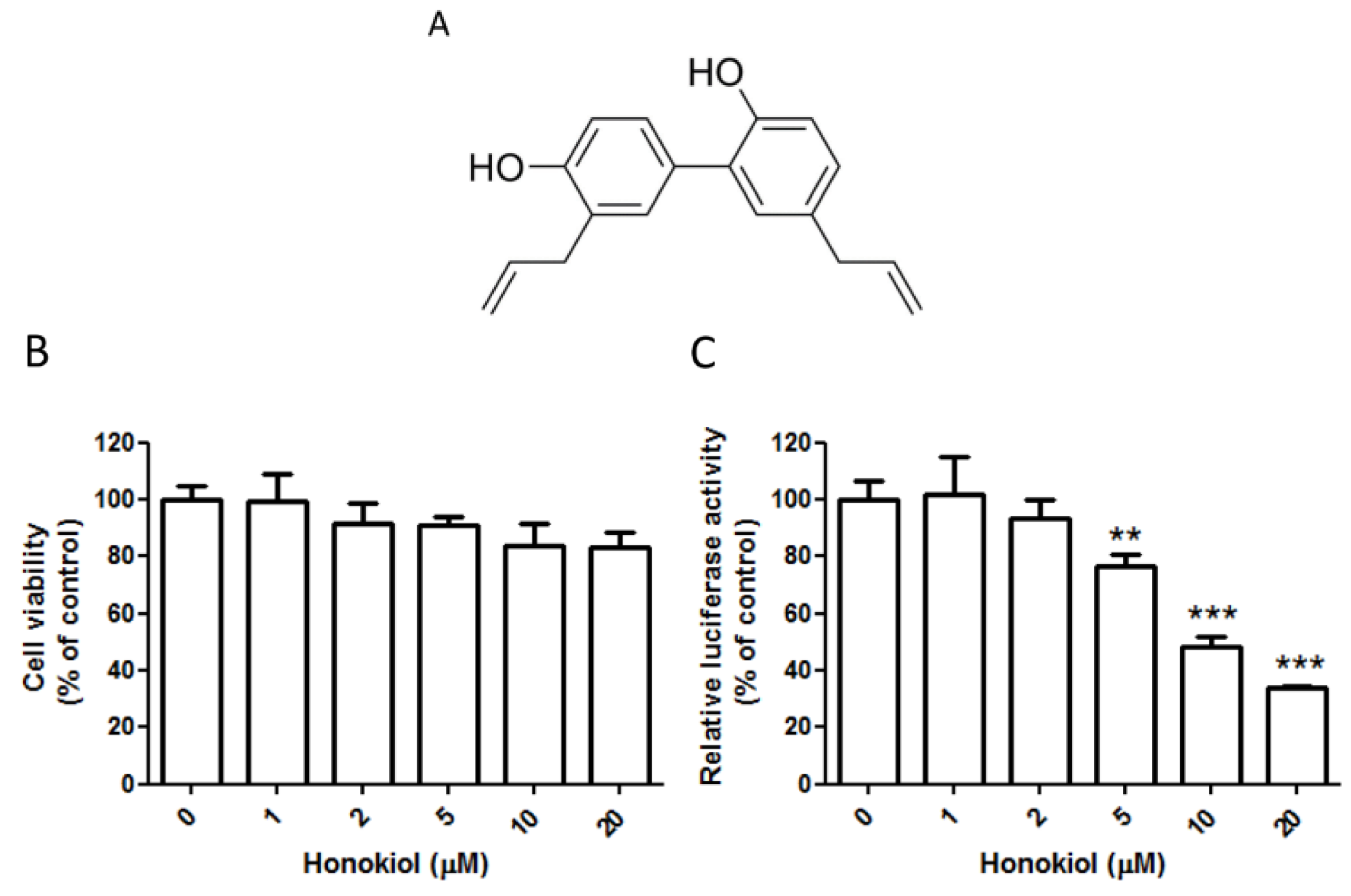
2. Results
2.1. Honokiol Effectively Inhibits the DENV Replicon
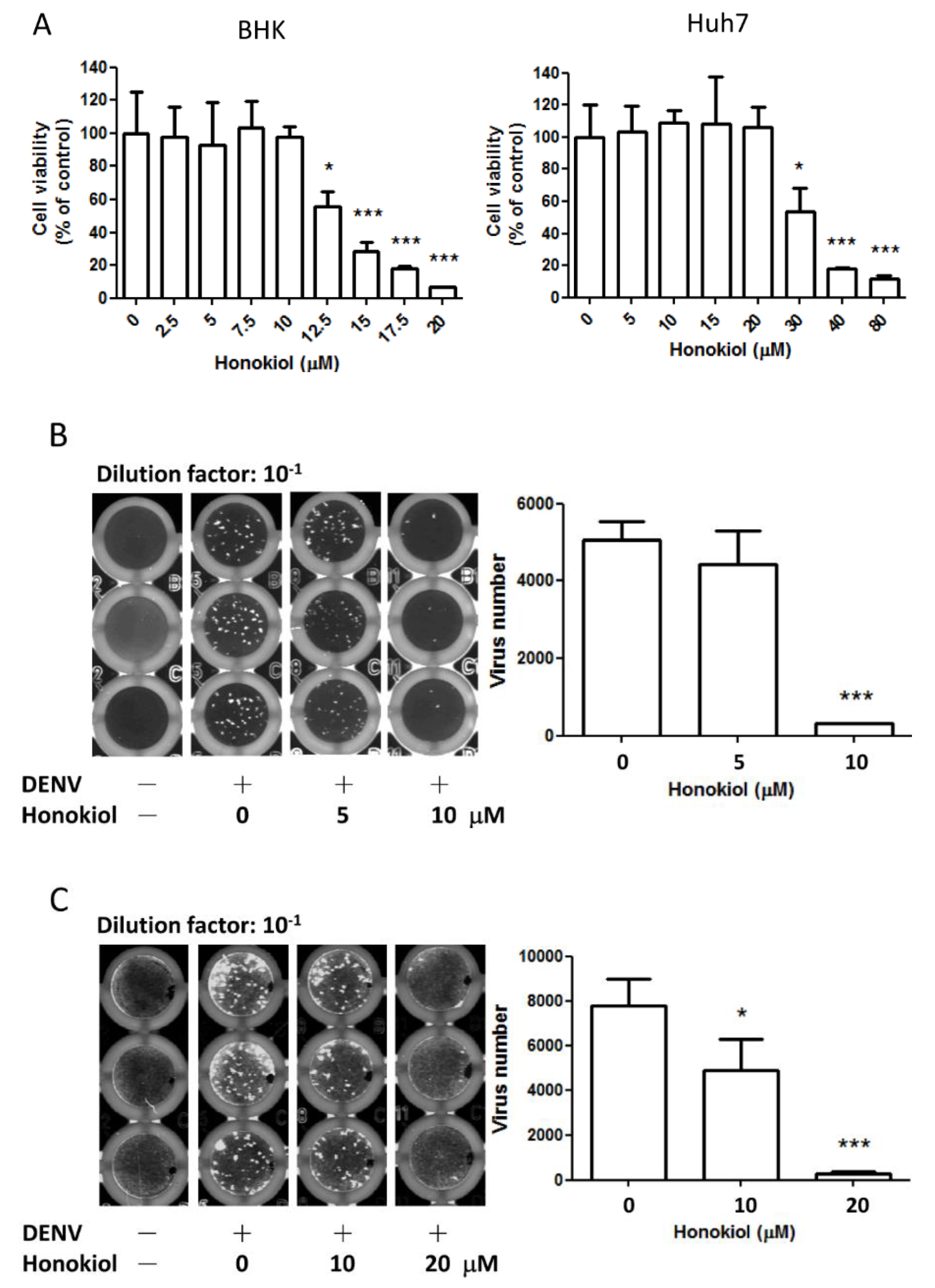
2.2. Honokiol Inhibits the DENV Infection in Vitro
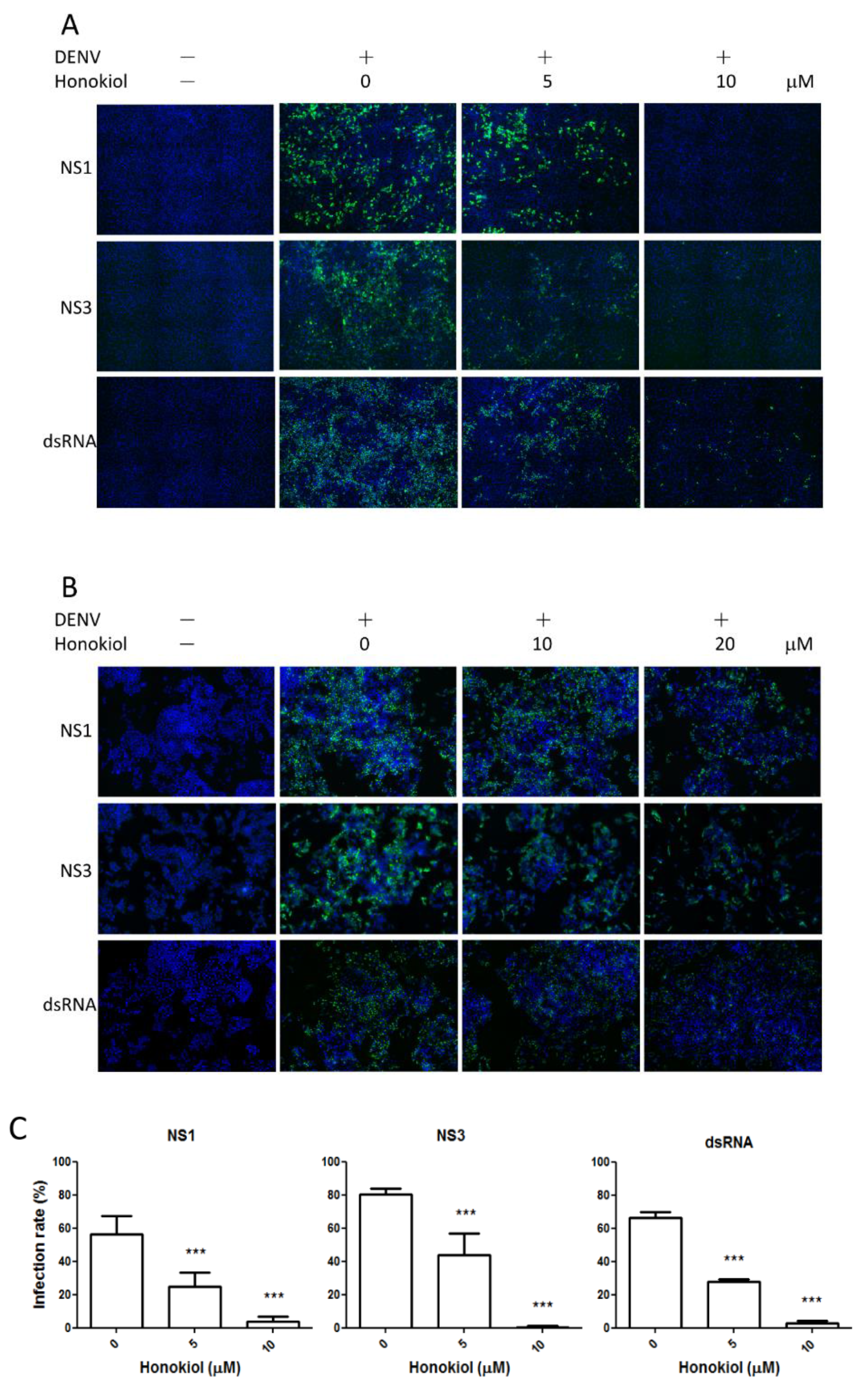
2.3. Honokiol Inhibits DENV Protein Expression and Viral RNA Replication

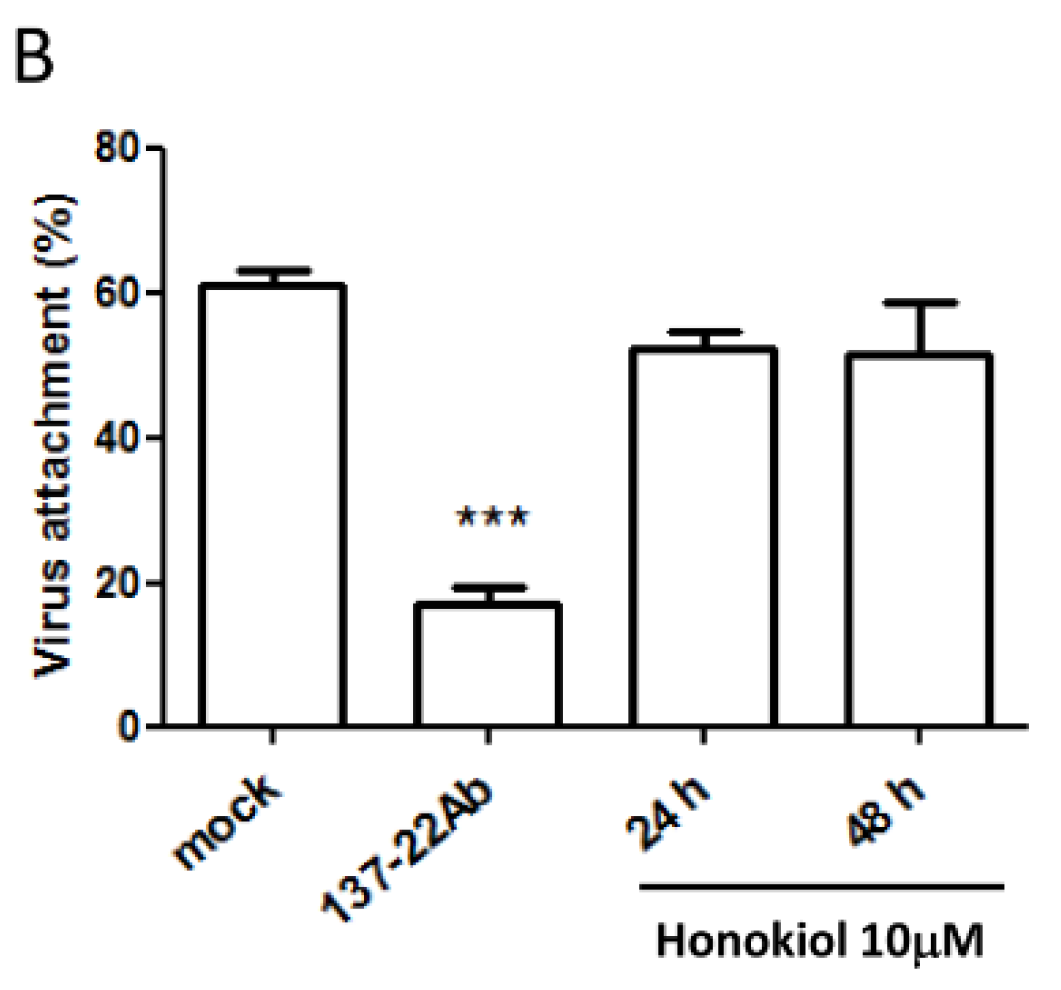
2.4. Pretreatment with Honokiol Does Not Affect the Attachment of DENV on Host Cells
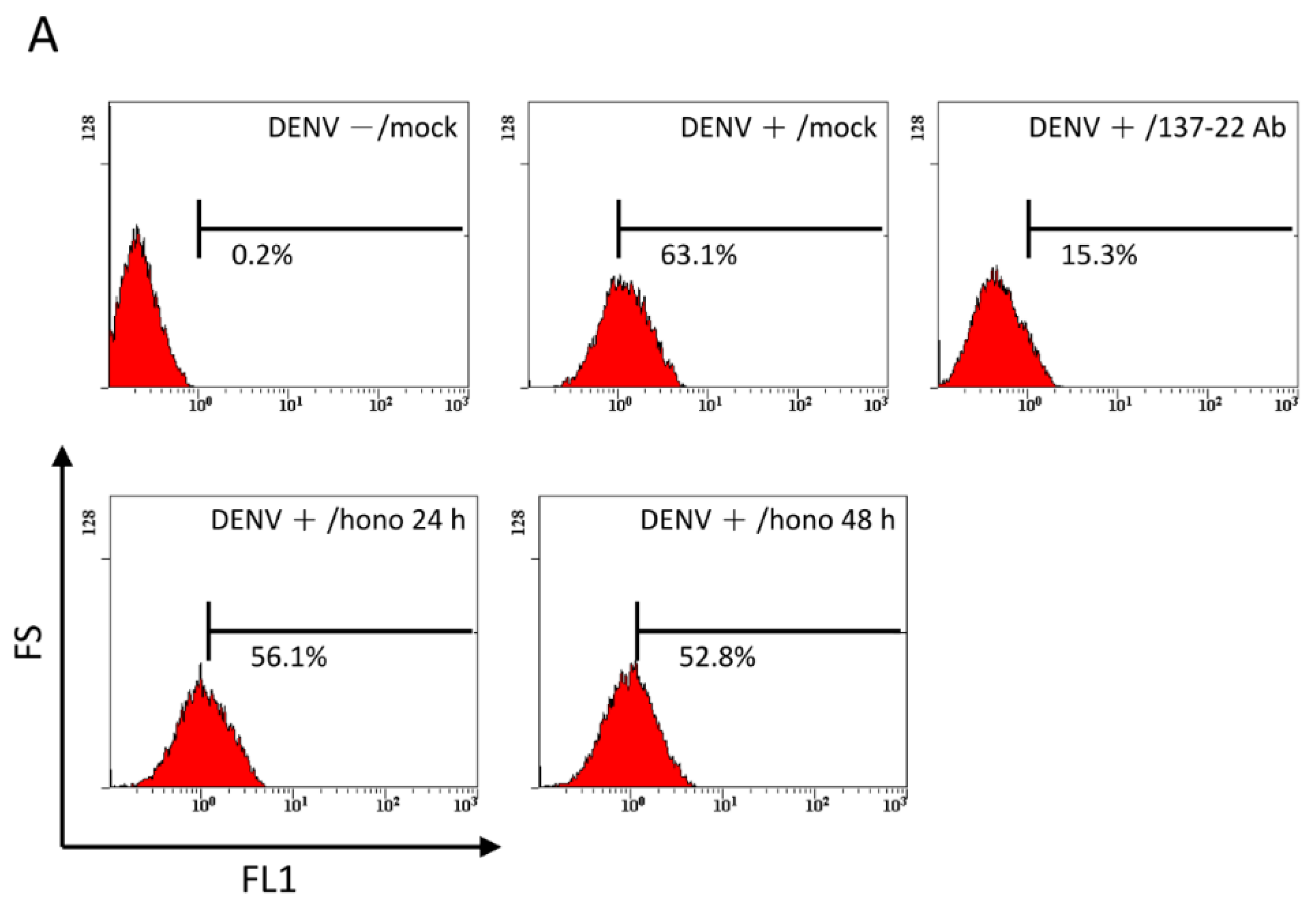
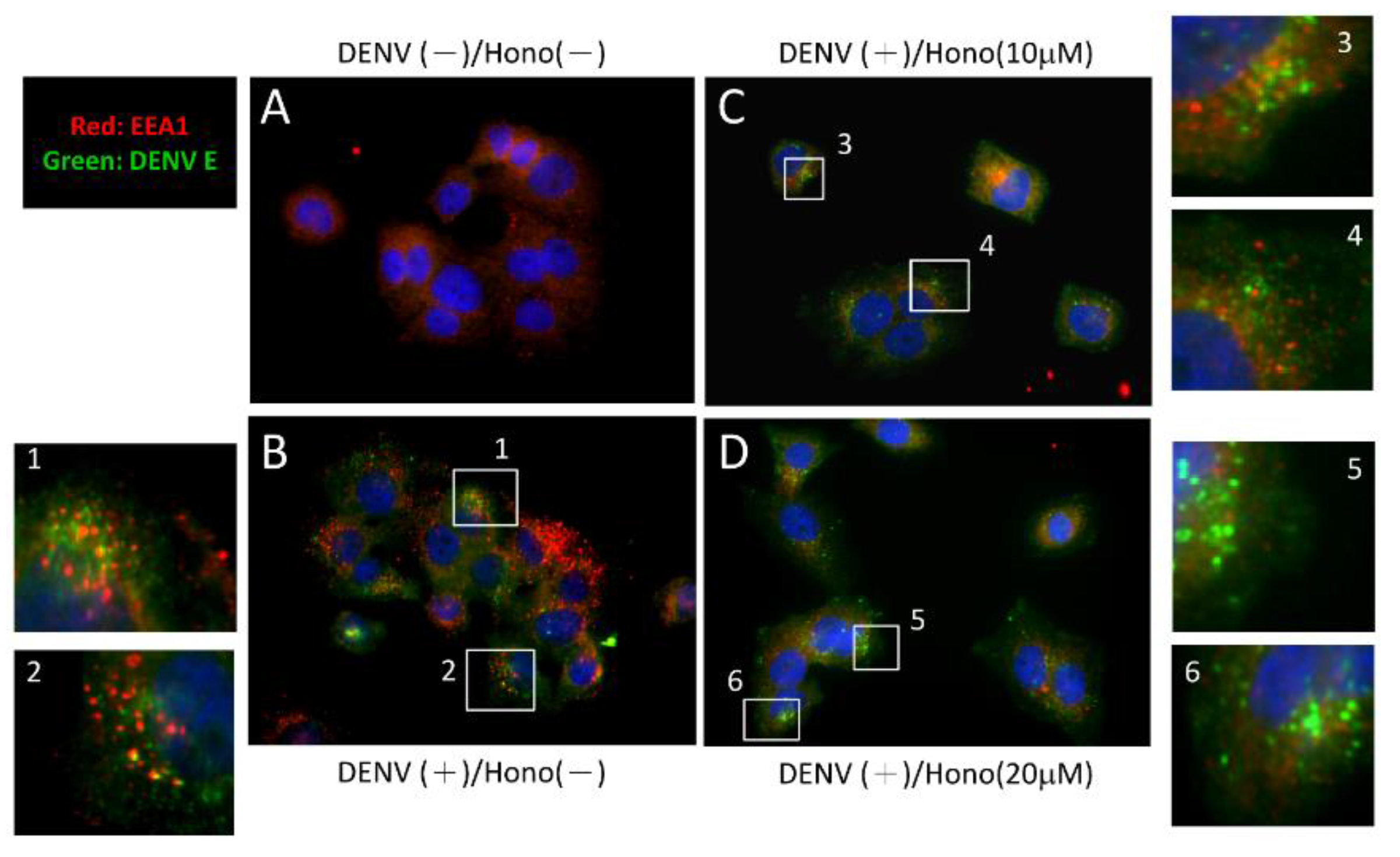
2.5. Honokiol May Interfere with the Endocytic Pathway during DENV Entry
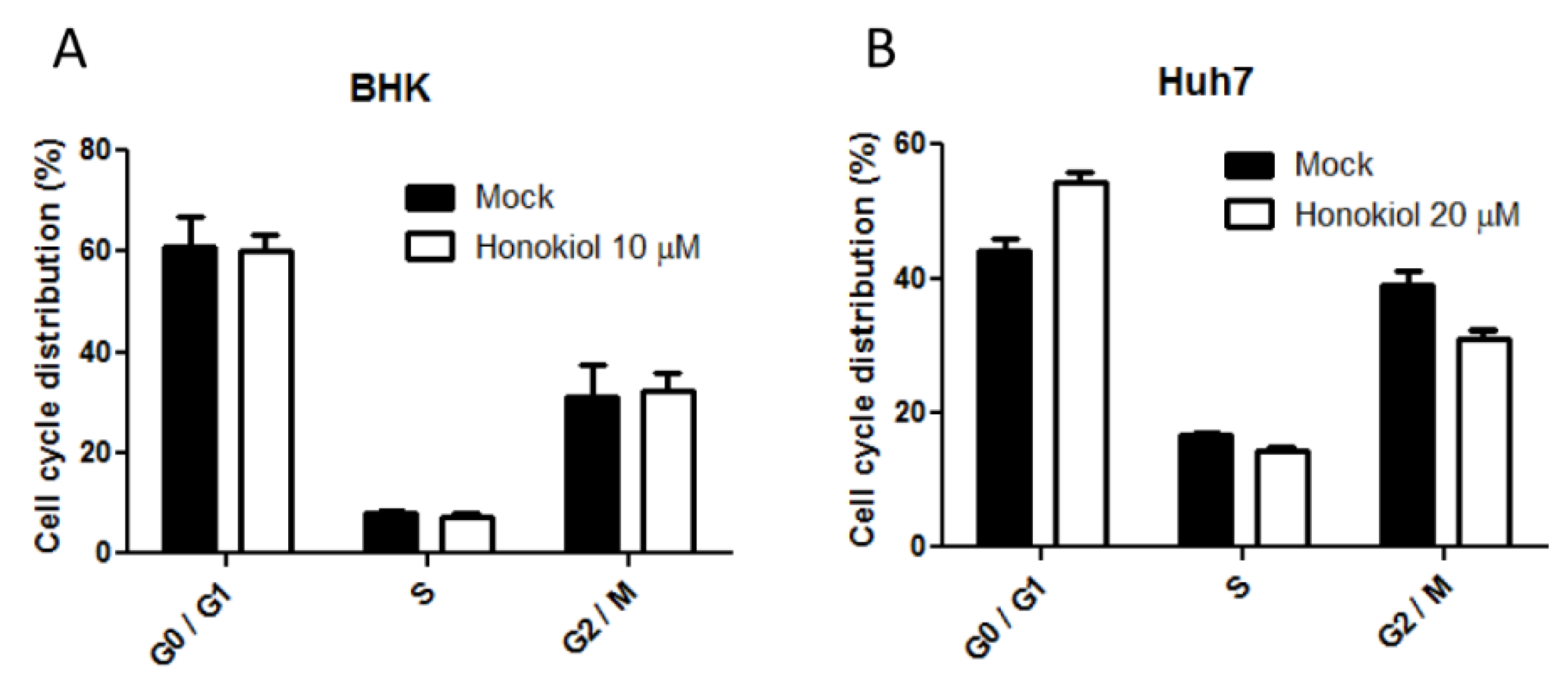
2.6. Honokiol Mediated DENV Inhibition is Not Attributed to the Influence on Cell Cycle Progression
2.7. Neither NF-κB Activation Nor IFN-β Expression was Essential in Honokiol Mediated DENV Inhibition

3. Discussion
4. Materials and Methods
4.1. Cell Lines, Virus, and Chemicals
4.2. Cell Cytotoxicity Assay
4.3. DENV Replicon Assay
4.4. Viral Yield Reduction and Fluorescence Focus Formation Assay
4.5. Immunofluorescence Staining
4.6. DENV Attachment Assay
4.7. Assay of DENV Endocytosis
4.8. Cell Cycle Analysis
4.9. Promoter Activity Assay
4.10. Statistical Analysis
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Normile, D. Tropical medicine. Surprising new dengue virus throws a spanner in disease control efforts. Science 2013, 342. [Google Scholar] [CrossRef] [PubMed]
- Halstead, S.B. Pathogenesis of dengue: Challenges to molecular biology. Science 1988, 239, 476–481. [Google Scholar] [CrossRef] [PubMed]
- Henchal, E.A.; Putnak, J.R. The dengue viruses. Clin. Microbiol. Rev. 1990, 3, 376–396. [Google Scholar] [PubMed]
- Gubler, D.J. The economic burden of dengue. Am. J. Trop. Med. Hyg. 2012, 86, 743–744. [Google Scholar] [CrossRef] [PubMed]
- Beecher, G.R. Overview of dietary flavonoids: Nomenclature, occurrence and intake. J. Nutr. 2003, 133, 3248S–3254S. [Google Scholar] [PubMed]
- Clifford, M.N. Diet-derived phenols in plasma and tissues and their implications for health. Planta Med. 2004, 70, 1103–1114. [Google Scholar] [CrossRef] [PubMed]
- Naithani, R.; Huma, L.C.; Holland, L.E.; Shukla, D.; McCormick, D.L.; Mehta, R.G.; Moriarty, R.M. Antiviral activity of phytochemicals: A comprehensive review. Mini Rev. Med. Chem. 2008, 8, 1106–1133. [Google Scholar] [CrossRef] [PubMed]
- Friedman, M. Overview of antibacterial, antitoxin, antiviral, and antifungal activities of tea flavonoids and teas. Mol. Nutr. Food Res. 2007, 51, 116–134. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.J.; Lee, Y.M.; Lee, C.K.; Jung, J.K.; Han, S.B.; Hong, J.T. Therapeutic applications of compounds in the Magnolia family. Pharmacol. Ther. 2011, 130, 157–176. [Google Scholar] [CrossRef] [PubMed]
- Fried, L.E.; Arbiser, J.L. Honokiol, a multifunctional antiangiogenic and antitumor agent. Antioxid. Redox Signal. 2009, 11, 1139–1148. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Liu, X.; Zhu, Y.; Chen, S.; Zhou, D.; Wang, Y. Honokiol inhibits the inflammatory reaction during cerebral ischemia reperfusion by suppressing NF-κB activation and cytokine production of glial cells. Neurosci. Lett. 2013, 534, 123–127. [Google Scholar] [CrossRef] [PubMed]
- Hu, H.; Zhang, X.X.; Wang, Y.Y.; Chen, S.Z. Honokiol inhibits arterial thrombosis through endothelial cell protection and stimulation of prostacyclin. Acta Pharmacol. Sin. 2005, 26, 1063–1068. [Google Scholar] [CrossRef] [PubMed]
- Liou, K.T.; Shen, Y.C.; Chen, C.F.; Tsao, C.M.; Tsai, S.K. Honokiol protects rat brain from focal cerebral ischemia-reperfusion injury by inhibiting neutrophil infiltration and reactive oxygen species production. Brain Res. 2003, 992, 159–166. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.R.; Chen, H.H.; Ko, C.H.; Chan, M.H. Neuroprotective activity of honokiol and magnolol in cerebellar granule cell damage. Eur. J. Pharmacol. 2006, 537, 64–69. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Duan, X.; Yang, G.; Zhang, X.; Deng, L.; Zheng, H.; Deng, C.; Wen, J.; Wang, N.; Peng, C.; et al. Honokiol crosses BBB and BCSFB, and inhibits brain tumor growth in rat 9L intracerebral gliosarcoma model and human U251 xenograft glioma model. PLoS ONE 2011, 6, e18490. [Google Scholar] [CrossRef] [PubMed]
- Lan, K.H.; Wang, Y.W.; Lee, W.P.; Lan, K.L.; Tseng, S.H.; Hung, L.R.; Yen, S.H.; Lin, H.C.; Lee, S.D. Multiple effects of Honokiol on the life cycle of hepatitis C virus. Liver Int. 2012, 32, 989–997. [Google Scholar] [CrossRef] [PubMed]
- Thisyakorn, U.; Thisyakorn, C. Latest developments and future directions in dengue vaccines. Ther. Adv. Vaccines 2014, 2, 3–9. [Google Scholar] [CrossRef] [PubMed]
- Guzman, M.G.; Kouri, G. Dengue: An update. Lancet Infect. Dis. 2002, 2, 33–42. [Google Scholar] [CrossRef]
- Malavige, G.N.; Fernando, S.; Fernando, D.J.; Seneviratne, S.L. Dengue viral infections. Postgrad. Med. J. 2004, 80, 588–601. [Google Scholar] [CrossRef] [PubMed]
- Kouri, G.P.; Guzman, M.G.; Bravo, J.R.; Triana, C. Dengue haemorrhagic fever/dengue shock syndrome: Lessons from the Cuban epidemic, 1981. Bull. World Health Organ. 1989, 67, 375–380. [Google Scholar] [PubMed]
- Hsu, Y.C.; Chen, N.C.; Chen, P.C.; Wang, C.C.; Cheng, W.C.; Wu, H.N. Identification of a small-molecule inhibitor of dengue virus using a replicon system. Arch. Virol. 2012, 157, 681–688. [Google Scholar] [CrossRef] [PubMed]
- Grove, J.; Marsh, M. The cell biology of receptor-mediated virus entry. J. Cell Biol. 2011, 195, 1071–1082. [Google Scholar] [CrossRef] [PubMed]
- Perera-Lecoin, M.; Meertens, L.; Carnec, X.; Amara, A. Flavivirus entry receptors: An update. Viruses 2014, 6, 69–88. [Google Scholar] [CrossRef] [PubMed]
- Van der Schaar, H.M.; Rust, M.J.; Chen, C.; van der Ende-Metselaar, H.; Wilschut, J.; Zhuang, X.; Smit, J.M. Dissecting the cell entry pathway of dengue virus by single-particle tracking in living cells. PLoS Pathog. 2008, 4, e1000244. [Google Scholar] [CrossRef] [PubMed]
- Zaitseva, E.; Yang, S.T.; Melikov, K.; Pourmal, S.; Chernomordik, L.V. Dengue virus ensures its fusion in late endosomes using compartment-specific lipids. PLoS Pathog. 2010, 6, e1001131. [Google Scholar] [CrossRef] [PubMed]
- Smit, J.M.; Moesker, B.; Rodenhuis-Zybert, I.; Wilschut, J. Flavivirus cell entry and membrane fusion. Viruses 2011, 3, 160–171. [Google Scholar] [CrossRef] [PubMed]
- Hahm, E.R.; Singh, S.V. Honokiol causes G0-G1 phase cell cycle arrest in human prostate cancer cells in association with suppression of retinoblastoma protein level/phosphorylation and inhibition of E2F1 transcriptional activity. Mol. Cancer Ther. 2007, 6, 2686–2695. [Google Scholar] [CrossRef] [PubMed]
- Phoolcharoen, W.; Smith, D.R. Internalization of the dengue virus is cell cycle modulated in HepG2, but not Vero cells. J. Med. Virol. 2004, 74, 434–441. [Google Scholar] [CrossRef] [PubMed]
- Kawai, T.; Akira, S. The roles of TLRs, RLRs and NLRs in pathogen recognition. Int. Immunol. 2009, 21, 317–337. [Google Scholar] [CrossRef] [PubMed]
- Lenardo, M.J.; Fan, C.M.; Maniatis, T.; Baltimore, D. The involvement of NF-κB in beta-interferon gene regulation reveals its role as widely inducible mediator of signal transduction. Cell 1989, 57, 287–294. [Google Scholar] [CrossRef]
- Chang, T.H.; Chen, S.R.; Yu, C.Y.; Lin, Y.S.; Chen, Y.S.; Kubota, T.; Matsuoka, M.; Lin, Y.L. Dengue virus serotype 2 blocks extracellular signal-regulated kinase and nuclear factor-κB activation to downregulate cytokine production. PLoS ONE 2012, 7, e41635. [Google Scholar] [CrossRef] [PubMed]
- Wati, S.; Li, P.; Burrell, C.J.; Carr, J.M. Dengue virus (DV) replication in monocyte-derived macrophages is not affected by tumor necrosis factor alpha (TNF-α), and DV infection induces altered responsiveness to TNF-alpha stimulation. J. Virol. 2007, 81, 10161–10171. [Google Scholar] [CrossRef] [PubMed]
- Ahn, K.S.; Sethi, G.; Shishodia, S.; Sung, B.; Arbiser, J.L.; Aggarwal, B.B. Honokiol potentiates apoptosis, suppresses osteoclastogenesis, and inhibits invasion through modulation of nuclear factor-kappaB activation pathway. Mol. Cancer Res. 2006, 4, 621–633. [Google Scholar] [CrossRef] [PubMed]
- Tse, A.K.; Wan, C.K.; Shen, X.L.; Yang, M.; Fong, W.F. Honokiol inhibits TNF-alpha-stimulated NF-kappaB activation and NF-κB-regulated gene expression through suppression of IKK activation. Biochem. Pharmacol. 2005, 70, 1443–1457. [Google Scholar] [CrossRef] [PubMed]
- Le Bon, A.; Tough, D.F. Links between innate and adaptive immunity via type I interferon. Curr. Opin. Immunol. 2002, 14, 432–436. [Google Scholar] [CrossRef]
- Stetson, D.B.; Medzhitov, R. Type I interferons in host defense. Immunity 2006, 25, 373–381. [Google Scholar] [CrossRef] [PubMed]
- Munoz-Jordan, J.L. Subversion of interferon by dengue virus. Curr. Top. Microbiol. Immunol. 2010, 338, 35–44. [Google Scholar] [PubMed]
- Ashour, J.; Laurent-Rolle, M.; Shi, P.Y.; Garcia-Sastre, A. NS5 of dengue virus mediates STAT2 binding and degradation. J. Virol. 2009, 83, 5408–5418. [Google Scholar] [CrossRef] [PubMed]
- Mazzon, M.; Jones, M.; Davidson, A.; Chain, B.; Jacobs, M. Dengue virus NS5 inhibits interferon-alpha signaling by blocking signal transducer and activator of transcription 2 phosphorylation. J. Infect. Dis. 2009, 200, 1261–1270. [Google Scholar] [CrossRef] [PubMed]
- Munoz-Jordan, J.L.; Laurent-Rolle, M.; Ashour, J.; Martinez-Sobrido, L.; Ashok, M.; Lipkin, W.I.; Garcia-Sastre, A. Inhibition of α/β interferon signaling by the NS4B protein of flaviviruses. J. Virol. 2005, 79, 8004–8013. [Google Scholar] [CrossRef] [PubMed]
- Morrison, J.; Aguirre, S.; Fernandez-Sesma, A. Innate immunity evasion by Dengue virus. Viruses 2012, 4, 397–413. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Chen, Z.; Kato, N.; Gale, M., Jr.; Lemon, S.M. Distinct poly(I–C) and virus-activated signaling pathways leading to interferon-beta production in hepatocytes. J. Biol. Chem. 2005, 280, 16739–16747. [Google Scholar] [CrossRef] [PubMed]
- Gubler, D.J. Epidemic dengue/dengue hemorrhagic fever as a public health, social and economic problem in the 21st century. Trends Microbiol. 2002, 10, 100–103. [Google Scholar] [CrossRef]
- Guzman, M.G.; Halstead, S.B.; Artsob, H.; Buchy, P.; Farrar, J.; Gubler, D.J.; Hunsperger, E.; Kroeger, A.; Margolis, H.S.; Martinez, E.; et al. Dengue: A continuing global threat. Nat. Rev. Microbiol. 2010, 8, S7–S16. [Google Scholar] [CrossRef] [PubMed]
- Durbin, A.P.; Whitehead, S.S. Next-generation dengue vaccines: Novel strategies currently under development. Viruses 2011, 3, 1800–1814. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.; Wang, T.; Wu, Y.F.; Gu, Y.; Xu, X.L.; Zheng, S.; Hu, X. Honokiol: A potent chemotherapy candidate for human colorectal carcinoma. World J. Gastroenterol. 2004, 10, 3459–3463. [Google Scholar] [PubMed]
- Su, W.J.; Huang, X.; Qin, E.; Jiang, L.; Ren, P. Pharmacokinetics of honokiol in rat after oral administration of Cortex of Magnolia officinalis and its compound preparation Houpu Sanwu Decoction. Zhong Yao Cai 2008, 31, 255–258. [Google Scholar] [PubMed]
- Varatharaj, A. Encephalitis in the clinical spectrum of dengue infection. Neurol. India 2010, 58, 585–591. [Google Scholar] [CrossRef] [PubMed]
- Solomon, T.; Dung, N.M.; Vaughn, D.W.; Kneen, R.; Thao, L.T.; Raengsakulrach, B.; Loan, H.T.; Day, N.P.; Farrar, J.; Myint, K.S.; et al. Neurological manifestations of dengue infection. Lancet 2000, 355, 1053–1059. [Google Scholar] [CrossRef]
- Battle, T.E.; Arbiser, J.; Frank, D.A. The natural product honokiol induces caspase-dependent apoptosis in B-cell chronic lymphocytic leukemia (B-CLL) cells. Blood 2005, 106, 690–697. [Google Scholar] [CrossRef] [PubMed]
- Saeed, M.; Kuete, V.; Kadioglu, O.; Bortzler, J.; Khalid, H.; Greten, H.J.; Efferth, T. Cytotoxicity of the bisphenolic honokiol from Magnolia officinalis against multiple drug-resistant tumor cells as determined by pharmacogenomics and molecular docking. Phytomedicine 2014, 21, 1525–1533. [Google Scholar] [CrossRef] [PubMed]
- Vaughn, D.W.; Green, S.; Kalayanarooj, S.; Innis, B.L.; Nimmannitya, S.; Suntayakorn, S.; Endy, T.P.; Raengsakulrach, B.; Rothman, A.L.; Ennis, F.A.; et al. Dengue viremia titer, antibody response pattern, and virus serotype correlate with disease severity. J. Infect. Dis. 2000, 181, 2–9. [Google Scholar] [CrossRef] [PubMed]
- Nayak, V.; Dessau, M.; Kucera, K.; Anthony, K.; Ledizet, M.; Modis, Y. Crystal structure of dengue virus type 1 envelope protein in the postfusion conformation and its implications for membrane fusion. J. Virol. 2009, 83, 4338–4344. [Google Scholar] [CrossRef] [PubMed]
- Harrison, S.C. Viral membrane fusion. Nat. Struct. Mol. Biol. 2008, 15, 690–698. [Google Scholar] [CrossRef] [PubMed]
- Chu, L.W.; Huang, Y.L.; Lee, J.H.; Huang, L.Y.; Chen, W.J.; Lin, Y.H.; Chen, J.Y.; Xiang, R.; Lee, C.H.; Ping, Y.H. Single-virus tracking approach to reveal the interaction of Dengue virus with autophagy during the early stage of infection. J. Biomed. Opt. 2014, 19, 011018. [Google Scholar] [CrossRef] [PubMed]
- Green, A.M.; Beatty, P.R.; Hadjilaou, A.; Harris, E. Innate immunity to dengue virus infection and subversion of antiviral responses. J. Mol. Biol. 2014, 426, 1148–1160. [Google Scholar] [CrossRef] [PubMed]
- Fensterl, V.; Sen, G.C. Interferons and viral infections. Biofactors 2009, 35, 14–20. [Google Scholar] [CrossRef] [PubMed]
- Munoz-Jordan, J.L.; Sanchez-Burgos, G.G.; Laurent-Rolle, M.; Garcia-Sastre, A. Inhibition of interferon signaling by dengue virus. Proc. Natl. Acad. Sci. USA 2003, 100, 14333–14338. [Google Scholar] [CrossRef] [PubMed]
- Huang, K.J.; Li, S.Y.; Chen, S.C.; Liu, H.S.; Lin, Y.S.; Yeh, T.M.; Liu, C.C.; Lei, H.Y. Manifestation of thrombocytopenia in dengue-2-virus-infected mice. J. Gen. Virol. 2000, 81, 2177–2182. [Google Scholar] [CrossRef] [PubMed]
- Huang, K.J.; Yang, Y.C.; Lin, Y.S.; Huang, J.H.; Liu, H.S.; Yeh, T.M.; Chen, S.H.; Liu, C.C.; Lei, H.Y. The dual-specific binding of dengue virus and target cells for the antibody-dependent enhancement of dengue virus infection. J. Immunol. 2006, 176, 2825–2832. [Google Scholar] [CrossRef] [PubMed]
- Hopman, A.H.N.; Ramaekers, F.C.S.; Speel, E.J.M. Rapid synthesis of biotin-, digoxigenin-, trinitrophenyl-, and fluorochrome-labeled tyramides and their application for in situ hybridization using CARD amplification. J. Histochem. Cytochem. 1998, 46, 771–777. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.H.; Lai, L.C.; Cheng, H.C.; Chen, K.R.; Syue, Y.Z.; Lu, H.C.; Lin, W.Y.; Chen, S.H.; Huang, H.S.; Shiau, A.L.; et al. TBK1-associated protein in endolysosomes (TAPE) is an innate immune regulator modulating the TLR3 and TLR4 signaling pathways. J. Biol. Chem. 2011, 286, 7043–7051. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fang, C.-Y.; Chen, S.-J.; Wu, H.-N.; Ping, Y.-H.; Lin, C.-Y.; Shiuan, D.; Chen, C.-L.; Lee, Y.-R.; Huang, K.-J. Honokiol, a Lignan Biphenol Derived from the Magnolia Tree, Inhibits Dengue Virus Type 2 Infection. Viruses 2015, 7, 4894-4910. https://doi.org/10.3390/v7092852
Fang C-Y, Chen S-J, Wu H-N, Ping Y-H, Lin C-Y, Shiuan D, Chen C-L, Lee Y-R, Huang K-J. Honokiol, a Lignan Biphenol Derived from the Magnolia Tree, Inhibits Dengue Virus Type 2 Infection. Viruses. 2015; 7(9):4894-4910. https://doi.org/10.3390/v7092852
Chicago/Turabian StyleFang, Chih-Yeu, Siang-Jyun Chen, Huey-Nan Wu, Yueh-Hsin Ping, Ching-Yen Lin, David Shiuan, Chi-Long Chen, Ying-Ray Lee, and Kao-Jean Huang. 2015. "Honokiol, a Lignan Biphenol Derived from the Magnolia Tree, Inhibits Dengue Virus Type 2 Infection" Viruses 7, no. 9: 4894-4910. https://doi.org/10.3390/v7092852
APA StyleFang, C.-Y., Chen, S.-J., Wu, H.-N., Ping, Y.-H., Lin, C.-Y., Shiuan, D., Chen, C.-L., Lee, Y.-R., & Huang, K.-J. (2015). Honokiol, a Lignan Biphenol Derived from the Magnolia Tree, Inhibits Dengue Virus Type 2 Infection. Viruses, 7(9), 4894-4910. https://doi.org/10.3390/v7092852






