IFITMs from Mycobacteria Confer Resistance to Influenza Virus When Expressed in Human Cells
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plasmids, Cloning, and Bioinformatics
MAV IFITM
GAATTCCCATGACCCAACCACCTCCTCCCCCGCCACCACCAGGATATCCCCCACAACAGCCAGCCGCTCAGGCTCCTAATAACTATCTGGTGTGGTCTATCCTTGTCACTCTCTTCTGCTGCCTGCCGTTTGGCATTGTCGCTATCGTAAAGAGCTCTCAAGTGAACGGACTTTGGGCACAGGGTAGATATGCTGAGGCACAGGCCTCCGCAGACAGTGCCAAGAAATGGGTGATATGGAGCGCAGTTATAGGCGTCGTGGTGGGAATAATCTATGGAATCCTTATGGCCGTAGGCGCCCTCAACACAAATACAAACGCGGCCCTCGCCGCGATGTTTTAGTAAGTCGAC
MAB IFITM GAATTCCCATGAGTGATGAAACCAAAAGCGACGAGCCTACAGGCGCTATCACCACACCGACCCCTCCTCCCCCACCGGCTCCTGCCTCTGTGACTGGCCCACCCAAACCCCCACCCACTAACGTGGGTTGGGCCGTCGCTAGCGTGATTTTTTTCTGGCCTCTGGCATTTAGCGCATTCACCAATGCACTGAATGTGACTCAGTTTTGGCTGACGGGGCAGTATGATCGGGCCCAGGAGTCTAGCGATCGGGCCAAGCTCCTGGGAAAGATTGCCCTCCTGACCGGGTTGGTACTGCTGTTCCTGTTCATCACCCTCCGCATTGCCTGCGCCATCTGGTGGCACTCACATGGTGGGGGATGGGGTCATCATGGCGGATGGCATAGGAGTTGGGACGACGGCGGGTGGGATGGCCCTGGCCCCATCGGGCCTATGGGTAGGCCGGGTCGCGACAACTAGTAAGTCGAC
2.2. Cells, Transfections, Palmitoylation Assay, and Virus Infections
2.3. Western Blotting, Immunoprecipitations, Immunofluorescence, and Flow Cytometry
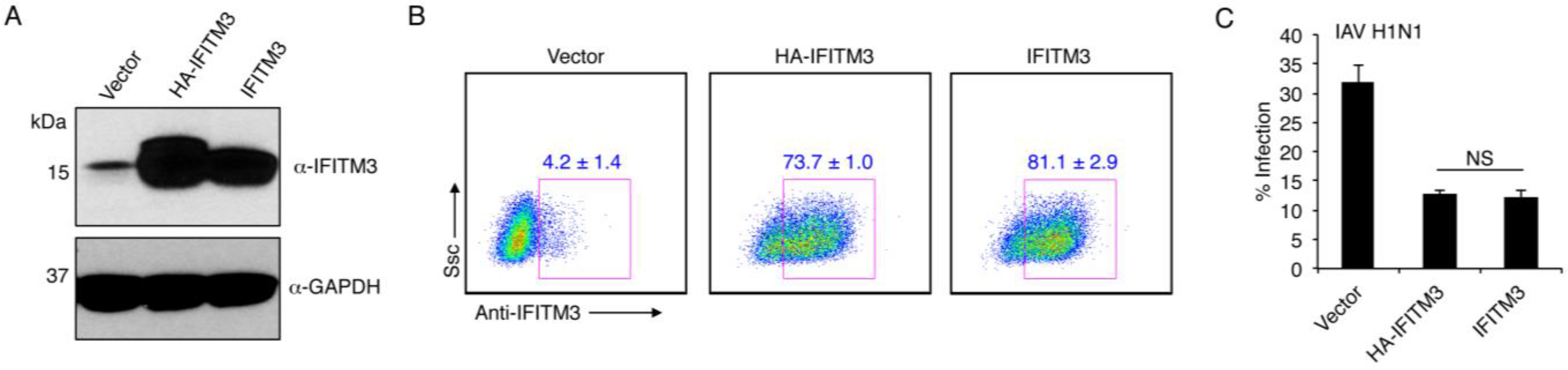
3. Results
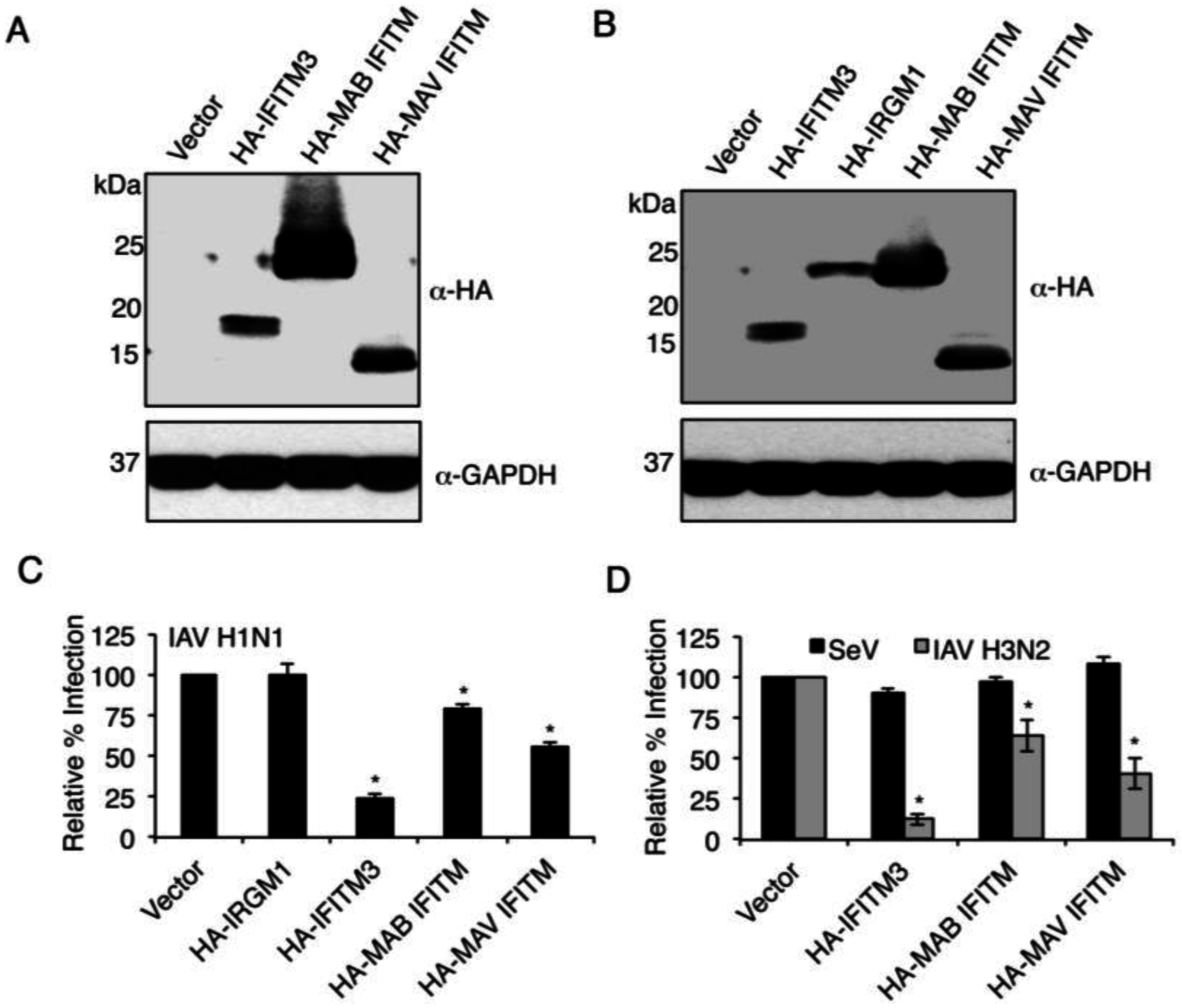
3.1. Commonalities and Differences between IFITM3 and Mycobacterial IFITMs
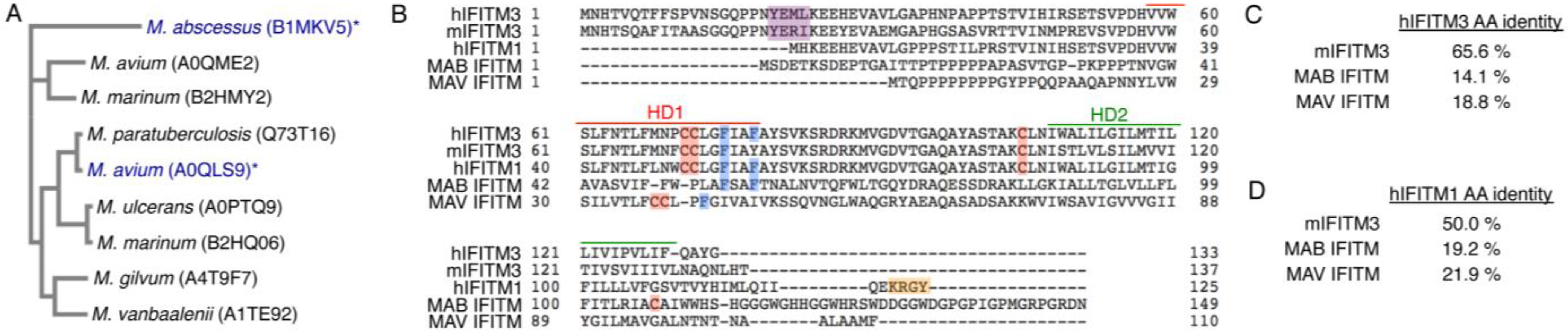
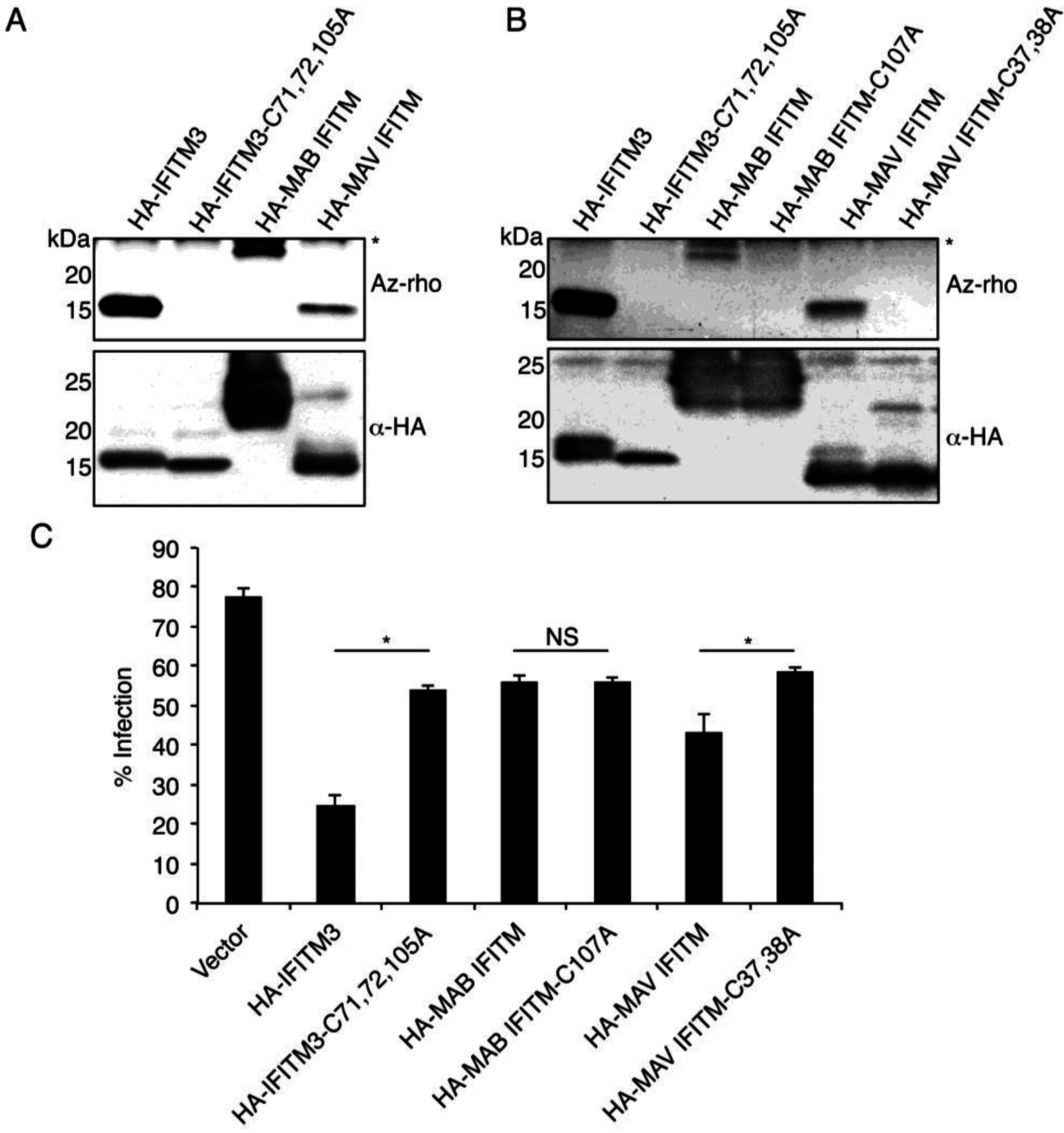
3.3. Mycobacterial IFITMs Are S-Palmitoylated in Human Cells
3.4. MAV IFITM Co-Immunoprecipitates and Co-Localizes with IFITM3

3.5. Mycobacterial IFITMs Partially Localize to Cathepsin B-Positive Cellular Compartments

4. Discussion
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Smith, S.E.; Gibson, M.S.; Wash, R.S.; Ferrara, F.; Wright, E.; Temperton, N.; Kellam, P.; Fife, M. Chicken interferon-inducible transmembrane protein 3 restricts influenza viruses and lyssaviruses in vitro. J. Virol. 2013, 87, 12957–12966. [Google Scholar] [CrossRef] [PubMed]
- Zhu, R.; Wang, J.; Lei, X.Y.; Gui, J.F.; Zhang, Q.Y. Evidence for paralichthys olivaceus IFITM1 antiviral effect by impeding viral entry into target cells. Fish Shellfish Immun. 2013, 35, 918–926. [Google Scholar] [CrossRef] [PubMed]
- Benfield, C.; Smith, S.E.; Wright, E.; Wash, R.S.; Ferrara, F.; Temperton, N.J.; Kellam, P. Bat and pig interferon-induced transmembrane protein 3 restrict cell entry by influenza virus and lyssaviruses. J. Gen. Virol. 2015, 96, 991–1005. [Google Scholar] [CrossRef] [PubMed]
- Yount, J.S.; Moltedo, B.; Yang, Y.Y.; Charron, G.; Moran, T.M.; Lopez, C.B.; Hang, H.C. Palmitoylome profiling reveals S-palmitoylation-dependent antiviral activity of IFITM3. Nat. Chem. Biol. 2010, 6, 610–614. [Google Scholar] [CrossRef] [PubMed]
- Brass, A.L.; Huang, I.C.; Benita, Y.; John, S.P.; Krishnan, M.N.; Feeley, E.M.; Ryan, B.J.; Weyer, J.L.; van der Weyden, L.; Fikrig, E.; et al. The IFITM proteins mediate cellular resistance to influenza a H1N1 virus, west nile virus, and dengue virus. Cell 2009, 139, 1243–1254. [Google Scholar] [CrossRef] [PubMed]
- Huang, I.C.; Bailey, C.C.; Weyer, J.L.; Radoshitzky, S.R.; Becker, M.M.; Chiang, J.J.; Brass, A.L.; Ahmed, A.A.; Chi, X.; Dong, L.; et al. Distinct patterns of IFITM-mediated restriction of filoviruses, SARS coronavirus, and influenza a virus. PLoS Pathog. 2011, 7, e1001258. [Google Scholar] [CrossRef] [PubMed]
- Smith, S.; Weston, S.; Kellam, P.; Marsh, M. IFITM proteins-cellular inhibitors of viral entry. Curr. Opin. Virol. 2014, 4C, 71–77. [Google Scholar] [CrossRef] [PubMed]
- Perreira, J.M.; Chin, C.R.; Feeley, E.M.; Brass, A.L. IFITMs restrict the replication of multiple pathogenic viruses. J. Mol. Biol. 2013, 425, 4937–4955. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Markosyan, R.M.; Zheng, Y.M.; Golfetto, O.; Bungart, B.; Li, M.; Ding, S.; He, Y.; Liang, C.; Lee, J.C.; et al. IFITM proteins restrict viral membrane hemifusion. PLoS Pathog. 2013, 9, e1003124. [Google Scholar] [CrossRef] [PubMed]
- Desai, T.M.; Marin, M.; Chin, C.R.; Savidis, G.; Brass, A.L.; Melikyan, G.B. IFITM3 restricts influenza a virus entry by blocking the formation of fusion pores following virus-endosome hemifusion. PLoS Pathog. 2014, 10, e1004048. [Google Scholar] [CrossRef] [PubMed]
- Feeley, E.M.; Sims, J.S.; John, S.P.; Chin, C.R.; Pertel, T.; Chen, L.M.; Gaiha, G.D.; Ryan, B.J.; Donis, R.O.; Elledge, S.J.; et al. IFITM3 inhibits influenza a virus infection by preventing cytosolic entry. PLoS Pathog. 2011, 7, e1002337. [Google Scholar] [CrossRef] [PubMed]
- Chesarino, N.M.; McMichael, T.M.; Yount, J.S. Regulation of the trafficking and antiviral activity of IFITM3 by post-translational modifications. Future Microbiol. 2014, 9, 1151–1163. [Google Scholar] [CrossRef] [PubMed]
- Bailey, C.C.; Zhong, G.; Huang, I.C.; Farzan, M. IFITM-family proteins: The cell’s first line of antiviral defense. Annu. Rev. Virol. 2014, 1, 261–283. [Google Scholar] [CrossRef] [PubMed]
- Everitt, A.R.; Clare, S.; Pertel, T.; John, S.P.; Wash, R.S.; Smith, S.E.; Chin, C.R.; Feeley, E.M.; Sims, J.S.; Adams, D.J.; et al. IFITM3 restricts the morbidity and mortality associated with influenza. Nature 2012, 484, 519–523. [Google Scholar] [CrossRef] [PubMed]
- Bailey, C.C.; Huang, I.C.; Kam, C.; Farzan, M. IFITM3 limits the severity of acute influenza in mice. PLoS Pathog. 2012, 8, e1002909. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Zhang, A.; Wan, Y.; Liu, X.; Qiu, C.; Xi, X.; Ren, Y.; Wang, J.; Dong, Y.; Bao, M.; et al. Early hypercytokinemia is associated with interferon-induced transmembrane protein-3 dysfunction and predictive of fatal H7N9 infection. Proc. Natl. Acad. Sci. USA 2013, 111, 769–774. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.H.; Zhao, Y.; Li, N.; Peng, Y.C.; Giannoulatou, E.; Jin, R.H.; Yan, H.P.; Wu, H.; Liu, J.H.; Liu, N.; et al. Interferon-induced transmembrane protein-3 genetic variant rs12252-C is associated with severe influenza in chinese individuals. Nat. Commun. 2013, 4. [Google Scholar] [CrossRef] [PubMed]
- Xuan, Y.; Wang, L.N.; Li, W.; Zi, H.R.; Guo, Y.; Yan, W.J.; Chen, X.B.; Wei, P.M. IFITM3 rs12252 T>C polymorphism is associated with the risk of severe influenza: A meta-analysis. Epidemiol. Infect. 2015, 17, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Sallman Almen, M.; Bringeland, N.; Fredriksson, R.; Schioth, H.B. The dispanins: A novel gene family of ancient origin that contains 14 human members. PLoS ONE 2012, 7, e31961. [Google Scholar] [CrossRef] [PubMed]
- Chesarino, N.M.; McMichael, T.M.; Hach, J.C.; Yount, J.S. Phosphorylation of the antiviral protein IFITM3 dually regulates its endocytosis and ubiquitination. J. Biol. Chem. 2014, 289, 11986–11992. [Google Scholar] [CrossRef] [PubMed]
- Chesarino, N.M.; Hach, J.C.; Chen, J.L.; Zaro, B.W.; Rajaram, M.V.; Turner, J.; Schlesinger, L.S.; Pratt, M.R.; Hang, H.C.; Yount, J.S. Chemoproteomics reveals toll-like receptor fatty acylation. BMC Biol. 2014, 12, 91. [Google Scholar] [CrossRef] [PubMed]
- Yount, J.S.; Zhang, M.M.; Hang, H.C. Visualization and identification of fatty acylated proteins using chemical reporters. Curr. Protoc. Chem. Biol. 2011, 3, 65–79. [Google Scholar] [PubMed]
- Charron, G.; Zhang, M.M.; Yount, J.S.; Wilson, J.; Raghavan, A.S.; Shamir, E.; Hang, H.C. Robust fluorescent detection of protein fatty-acylation with chemical reporters. J. Am. Chem. Soc. 2009, 131, 4967–4975. [Google Scholar] [CrossRef] [PubMed]
- Moltedo, B.; Li, W.; Yount, J.S.; Moran, T.M. Unique type I interferon responses determine the functional fate of migratory lung dendritic cells during influenza virus infection. PLoS Pathog. 2011, 7, e1002345. [Google Scholar] [CrossRef] [PubMed]
- Yount, J.S.; Kraus, T.A.; Horvath, C.M.; Moran, T.M.; Lopez, C.B. A novel role for viral-defective interfering particles in enhancing dendritic cell maturation. J. Immunol. 2006, 177, 4503–4513. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Liu, J.; Li, M.; Yang, H.; Zhang, C.Y. Evolutionary dynamics of the interferon-induced transmembrane gene family in vertebrates. PLoS ONE 2012, 7, e49265. [Google Scholar] [CrossRef] [PubMed]
- Hickford, D.; Frankenberg, S.; Shaw, G.; Renfree, M.B. Evolution of vertebrate interferon inducible transmembrane proteins. BMC Genomics 2012, 13. [Google Scholar] [CrossRef] [PubMed]
- Jia, R.; Pan, Q.; Ding, S.; Rong, L.; Liu, S.L.; Geng, Y.; Qiao, W.; Liang, C. The N-terminal region of IFITM3 modulates its antiviral activity by regulating ifitm3 cellular localization. J. Virol. 2012, 86, 13697–13707. [Google Scholar] [CrossRef] [PubMed]
- Jia, R.; Xu, F.; Qian, J.; Yao, Y.; Miao, C.; Zheng, Y.M.; Liu, S.L.; Guo, F.; Geng, Y.; Qiao, W.; et al. Identification of an endocytic signal essential for the antiviral action of ifitm3. Cell. Microbiol. 2014, 16, 1080–1093. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Jia, R.; Li, M.; Zheng, Y.; Miao, C.; Yao, Y.; Geng, Y.; Qiao, W.; Albritton, L.M.; Liang, C.; et al. A sorting signal suppresses ifitm1 restriction of viral entry. J. Biol. Chem. 2015, 290, 4248–4259. [Google Scholar] [CrossRef] [PubMed]
- Diamond, M.S.; Farzan, M. The broad-spectrum antiviral functions of ifit and ifitm proteins. Nat. Rev. Immunol. 2013, 13, 46–57. [Google Scholar] [CrossRef] [PubMed]
- Yount, J.S.; Zhang, M.M.; Hang, H.C. Emerging roles for protein S-palmitoylation in immunity from chemical proteomics. Curr. Opin. Chem. Biol. 2013, 17, 27–33. [Google Scholar] [CrossRef] [PubMed]
- Hach, J.C.; McMichael, T.; Chesarino, N.M.; Yount, J.S. Palmitoylation on conserved and non-conserved cysteines of murine IFITM1 regulates its stability and anti-influenza a virus activity. J. Virol. 2013, 87, 9923–9927. [Google Scholar] [CrossRef] [PubMed]
- John, S.P.; Chin, C.R.; Perreira, J.M.; Feeley, E.M.; Aker, A.M.; Savidis, G.; Smith, S.E.; Elia, A.E.; Everitt, A.R.; Vora, M.; et al. The CD225 domain of IFITM3 is required for both IFITM protein association and inhibition of influenza A virus and dengue virus replication. J. Virol. 2013, 87, 7837–7852. [Google Scholar] [CrossRef] [PubMed]
- Weidner, J.M.; Jiang, D.; Pan, X.B.; Chang, J.; Block, T.M.; Guo, J.T. Interferon-induced cell membrane proteins, ifitm3 and tetherin, inhibit vesicular stomatitis virus infection via distinct mechanisms. J. Virol. 2010, 84, 12646–12657. [Google Scholar] [CrossRef] [PubMed]
- Yount, J.S.; Karssemeijer, R.A.; Hang, H.C. S-palmitoylation and ubiquitination differentially regulate interferon-induced transmembrane protein 3 (IFITM3)-mediated resistance to influenza virus. J. Biol. Chem. 2012, 287, 19631–19641. [Google Scholar] [CrossRef] [PubMed]
- Amini-Bavil-Olyaee, S.; Choi, Y.J.; Lee, J.H.; Shi, M.; Huang, I.C.; Farzan, M.; Jung, J.U. The antiviral effector IFITM3 disrupts intracellular cholesterol homeostasis to block viral entry. Cell Host Microbe 2013, 13, 452–464. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Pan, Q.; Rong, L.; He, W.; Liu, S.L.; Liang, C. The IFITM proteins inhibit HIV-1 infection. J. Virol. 2011, 85, 2126–2137. [Google Scholar] [CrossRef] [PubMed]
- Chutiwitoonchai, N.; Hiyoshi, M.; Hiyoshi-Yoshidomi, Y.; Hashimoto, M.; Tokunaga, K.; Suzu, S. Characteristics of IFITM, the newly identified ifn-inducible anti-HIV-1 family proteins. Microbes Infect. 2013, 15, 280–290. [Google Scholar] [CrossRef] [PubMed]
- Tartour, K.; Appourchaux, R.; Gaillard, J.; Nguyen, X.N.; Durand, S.; Turpin, J.; Beaumont, E.; Roch, E.; Berger, G.; Mahieux, R.; et al. IFITM proteins are incorporated onto HIV-1 virion particles and negatively imprint their infectivity. Retrovirology 2014, 11, 103. [Google Scholar] [CrossRef] [PubMed]
- Compton, A.A.; Bruel, T.; Porrot, F.; Mallet, A.; Sachse, M.; Euvrard, M.; Liang, C.; Casartelli, N.; Schwartz, O. IFITM proteins incorporated into HIV-1 virions impair viral fusion and spread. Cell Host Microbe 2014, 16, 736–747. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, T.; Kawakami, E.; Shoemaker, J.E.; Lopes, T.J.; Matsuoka, Y.; Tomita, Y.; Kozuka-Hata, H.; Gorai, T.; Kuwahara, T.; Takeda, E.; et al. Influenza virus-host interactome screen as a platform for antiviral drug development. Cell Host Microbe 2014, 16, 795–805. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.B.; Davis, A.S.; Taylor, G.A.; Deretic, V. Human irgm induces autophagy to eliminate intracellular mycobacteria. Science 2006, 313, 1438–1441. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, S.; Choi, H.P.; Matsuzawa, T.; Pypaert, M.; MacMicking, J.D. Targeting of the gtpase irgm1 to the phagosomal membrane via ptdins(3,4)p-2 and ptdins(3,4,5)p-3 promotes immunity to mycobacteria. Nat. Immunol. 2009, 10, 907–1017. [Google Scholar] [CrossRef] [PubMed]
- Yount, J.S.; Charron, G.; Hang, H.C. Bioorthogonal proteomics of 15-hexadecynyloxyacetic acid chemical reporter reveals preferential targeting of fatty acid modified proteins and biosynthetic enzymes. Bioorg. Med. Chem. 2012, 20, 650–654. [Google Scholar] [CrossRef] [PubMed]
- Charron, G.; Tsou, L.K.; Maguire, W.; Yount, J.S.; Hang, H.C. Alkynyl-farnesol reporters for detection of protein S-prenylation in cells. Mol. Biosyst. 2011, 7, 67–73. [Google Scholar] [CrossRef] [PubMed]
- Peng, T.; Hang, H.C. Bifunctional fatty acid chemical reporter for analyzing S-palmitoylated membrane protein-protein interactions in mammalian cells. J. Am. Chem. Soc. 2015, 137, 556–559. [Google Scholar] [CrossRef] [PubMed]
- Wee, Y.S.; Roundy, K.M.; Weis, J.J.; Weis, J.H. Interferon-inducible transmembrane proteins of the innate immune response act as membrane organizers by influencing clathrin and V-atpase localization and function. Innate Immun. 2012, 18, 834–845. [Google Scholar] [CrossRef] [PubMed]
- Lin, T.Y.; Chin, C.R.; Everitt, A.R.; Clare, S.; Perreira, J.M.; Savidis, G.; Aker, A.M.; John, S.P.; Sarlah, D.; Carreira, E.M.; et al. Amphotericin B increases influenza A virus infection by preventing IFITM3-mediated restriction. Cell Rep. 2013, 5, 895–890. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Guo, F.; Liu, F.; Cuconati, A.; Chang, J.; Block, T.M.; Guo, J.T. Interferon induction of IFITM proteins promotes infection by human coronavirus oc43. Proc. Natl. Acad. Sci. USA 2014, 111, 6756–6761. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Melvin, W.J.; McMichael, T.M.; Chesarino, N.M.; Hach, J.C.; Yount, J.S. IFITMs from Mycobacteria Confer Resistance to Influenza Virus When Expressed in Human Cells. Viruses 2015, 7, 3035-3052. https://doi.org/10.3390/v7062759
Melvin WJ, McMichael TM, Chesarino NM, Hach JC, Yount JS. IFITMs from Mycobacteria Confer Resistance to Influenza Virus When Expressed in Human Cells. Viruses. 2015; 7(6):3035-3052. https://doi.org/10.3390/v7062759
Chicago/Turabian StyleMelvin, William J., Temet M. McMichael, Nicholas M. Chesarino, Jocelyn C. Hach, and Jacob S. Yount. 2015. "IFITMs from Mycobacteria Confer Resistance to Influenza Virus When Expressed in Human Cells" Viruses 7, no. 6: 3035-3052. https://doi.org/10.3390/v7062759