Valganciclovir Inhibits Human Adenovirus Replication and Pathology in Permissive Immunosuppressed Female and Male Syrian Hamsters
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Syrian Hamster Experiments
2.3. Determining the EC50 of VGCV
2.4. Immunofluorescent Staining and Western Blot
2.5. Quantitative PCR (qPCR) Assay for Quantification of Ad5 Genomic DNA
3. Results
3.1. VGCV Protects Immunosuppressed Hamsters Challenged Intravenously with Ad5, Even When Administered Starting from Four Days after Ad5 Challenge
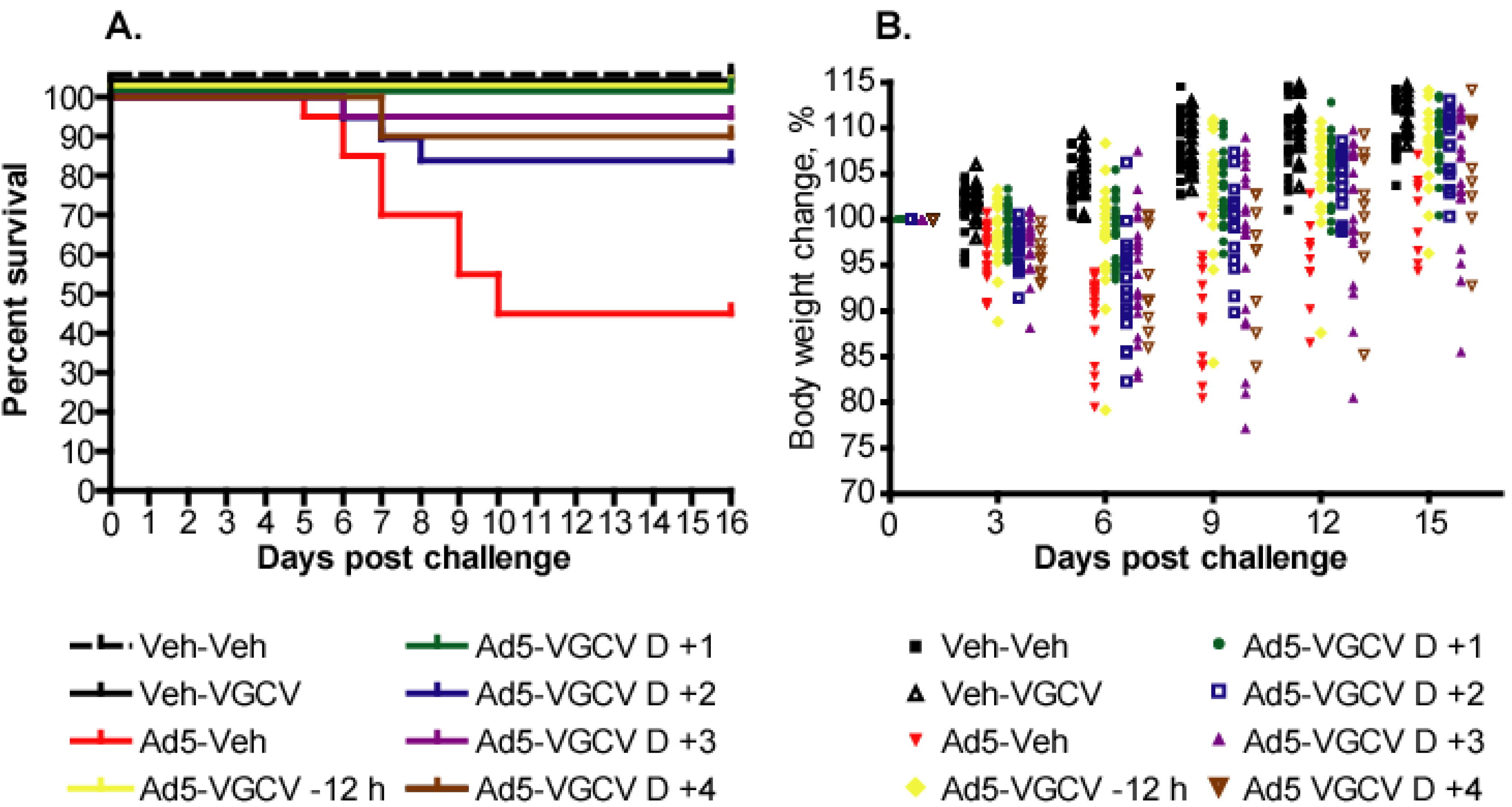
| Group | Animals affected | Severity (average histopathology scores for hepatocellular necrosis (a representative lesion) are shown) | Distribution |
|---|---|---|---|
| Vehicle-Vehicle | 0/5 | N/A | N/A |
| Vehicle-VGCV | 0/5 | N/A | N/A |
| Ad5-Vehicle | 5/5 | 3.4 | Multifocal |
| Ad5-VGCV −12 | 4/5 | 1.5 | Multifocal |
| Ad5-VGCV +1 | 5/5 | 1.8 | Multifocal |
| Ad5-VGCV +2 | 5/5 | 2.4 | Multifocal |
| Ad5-VGCV+3 | 4/5 | 1.75 | Multifocal |

3.2. VGCV Treatment Is also Effective in Male Syrian Hamsters

| Group | Animals Affected | Severity (average histopathology scores for hepatocellular necrosis (a representative lesion) are shown) | Distribution |
|---|---|---|---|
| Vehicle-Vehicle | 0/5 | N/A | N/A |
| Vehicle-VGCV | 0/5 | N/A | N/A |
| Ad5-Vehicle | 15/15 | 3.7 | Multifocal |
| Ad5-VGCV −12 | 3/5 | 0.6 | Focal/Multifocal |
| Ad5-VGCV +1 | 4/7 | 0.6 | Multifocal |
| Ad5-VGCV +2 | 10/11 | 2.1 | Multifocal |
| Ad5-VGCV+3 | 13/14 | 2.9 | Multifocal |
| Ad5-VGCV+4 | 9/10 | 3.1 | Multifocal |
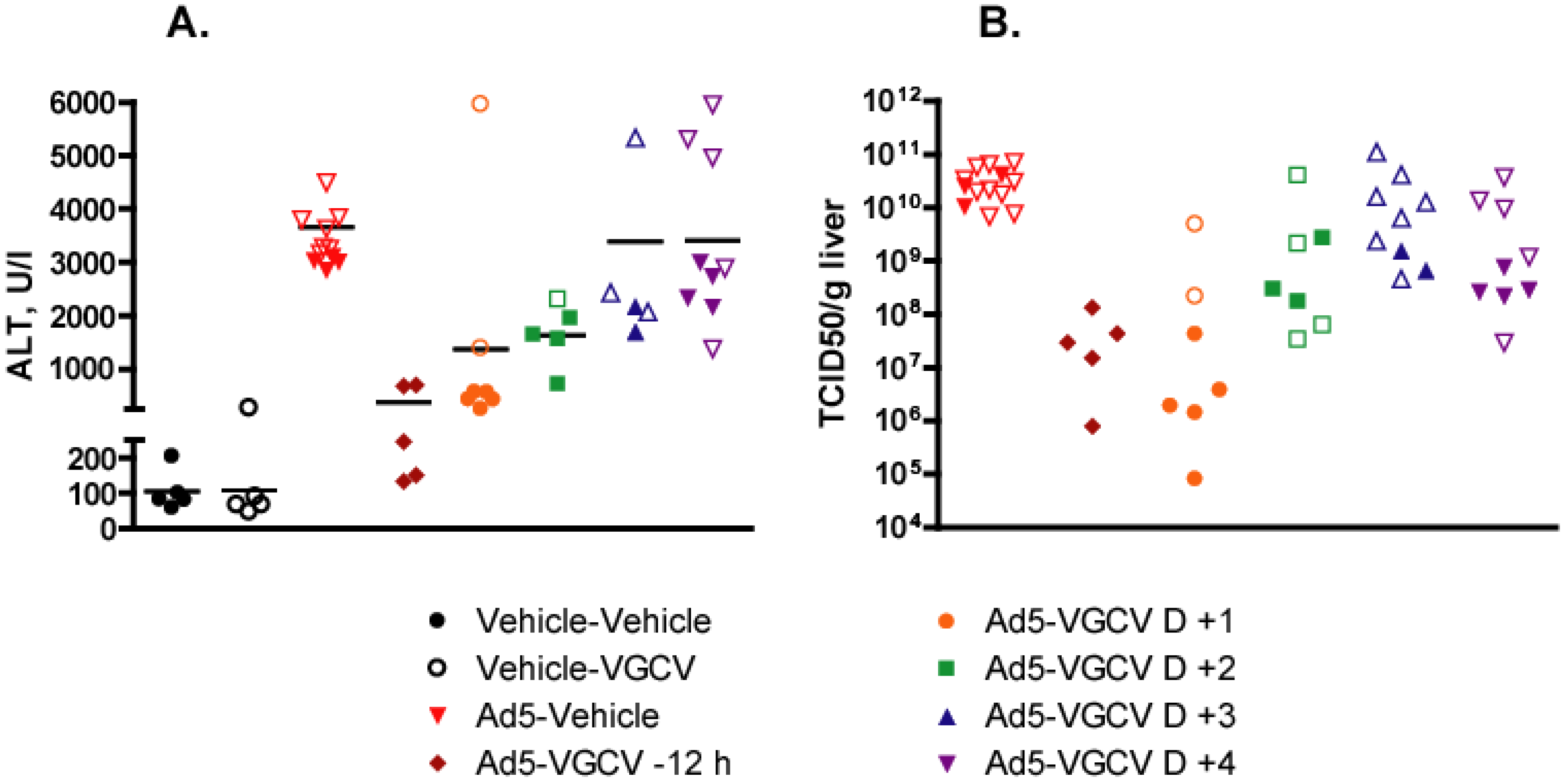
3.3. VGCV Inhibits the Growth of Human Ads in Vitro

3.4. Treatment with VGCV Prevents Progression of Adenovirus Infection into the Late Stage

3.5. VGCV Inhibits Ad5 DNA Replication

3.6. VGCV Inhibits Ad5 Replication by Mechanisms other than that Described for Herpesviruses
4. Discussion
Supplementary Files
Supplementary File 1Acknowledgments
Author Contributions
Conflicts of Interest
References
- Wold, W.S.M.; Ison, M.G. Adenoviruses. In Fields Virology; Knipe, D.M., Howley, P.M., Eds.; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2013; p. 1732. [Google Scholar]
- Lion, T. Adenovirus infections in immunocompetent and immunocompromised patients. Clin. Microbiol. Rev. 2014, 27, 441–62. [Google Scholar]
- Matthes-Martin, S.; Boztug, H.; Lion, T. Diagnosis and treatment of adenovirus infection in immunocompromised patients. Expert review of anti-infective therapy 2013, 11, 1017–28. [Google Scholar]
- Matthes-Martin, S.; Feuchtinger, T.; Shaw, P.J.; Engelhard, D.; Hirsch, H.H.; Cordonnier, C.; Fourth European Conference on Infections in Leukemia. European guidelines for diagnosis and treatment of adenovirus infection in leukemia and stem cell transplantation: Summary of ECIL-4 (2011). Transplant infectious disease: An official journal of the Transplantation Society 2012, 14, 555–63. [Google Scholar]
- Sandkovsky, U.; Vargas, L.; Florescu, D.F. Adenovirus: Current epidemiology and emerging approaches to prevention and treatment. Current infectious disease reports 2014, 16, 416. [Google Scholar]
- Coen, D.M.; Richman, D.D. Antiviral Agents. In Fields Virology, 6th ed.; Knipe, D.M., Howley, P.M., Eds.; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2013; Vol. 1, pp. 338–373. [Google Scholar]
- Florescu, D.F.; Pergam, S.A.; Neely, M.N.; Qiu, F.; Johnston, C.; Way, S.; Sande, J.; Lewinsohn, D.A.; Guzman-Cottrill, J.A.; Graham, M.L.; Papanicolaou, G.; Kurtzberg, J.; Rigdon, J.; Painter, W.; Mommeja-Marin, H.; Lanier, R.; Anderson, M.; van der Horst, C. Safety and efficacy of CMX001 as salvage therapy for severe adenovirus infections in immunocompromised patients. Biol. Blood Marrow Transplant. 2012, 18, 731–8. [Google Scholar]
- Paolino, K.; Sande, J.; Perez, E.; Loechelt, B.; Jantausch, B.; Painter, W.; Anderson, M.; Tippin, T.; Lanier, E.R.; Fry, T.; DeBiasi, R.L. Eradication of disseminated adenovirus infection in a pediatric hematopoietic stem cell transplantation recipient using the novel antiviral agent CMX001. J. Clin. Virol. 2011, 50, 167–70. [Google Scholar]
- Tollefson, A.E.; Spencer, J.F.; Ying, B.; Buller, R.M.; Wold, W.S.; Toth, K. Cidofovir and brincidofovir reduce the pathology caused by systemic infection with human type 5 adenovirus in immunosuppressed Syrian hamsters, while ribavirin is largely ineffective in this model. Antiviral Res. 2014, 112, 38–46. [Google Scholar]
- Toth, K.; Spencer, J.F.; Dhar, D.; Sagartz, J.E.; Buller, R.M.; Painter, G.R.; Wold, W.S. M. Hexadecyloxypropyl-cidofovir, CMX001, prevents adenovirus-induced mortality in a permissive, immunosuppressed animal model. Proc. Natl. Acad. Sci. U. S. A. 2008, 105, 7293–7297. [Google Scholar]
- Wold, W.S.; Toth, K. Syrian hamster as an animal model to study oncolytic adenoviruses and to evaluate the efficacy of antiviral compounds. Adv. Cancer Res. 2012, 115, 69–92. [Google Scholar]
- Dhar, D.; Spencer, J.F.; Toth, K.; Wold, W.S.M. Effect of preexisting immunity on oncolytic adenovirus vector INGN 007 antitumor efficacy in immunocompetent and immunosuppressed Syrian hamsters. J. Virol. 2009, 83, 2130–2139. [Google Scholar]
- Dhar, D.; Toth, K.; Wold, W.S. M. Cycles of transient high-dose cyclophosphamide administration and intratumoral oncolytic adenovirus vector injection for long-term tumor suppression in Syrian hamsters. Cancer Gene Ther. 2014. [Google Scholar]
- Thomas, M.A.; Spencer, J.F.; Toth, K.; Sagartz, J.E.; Phillips, N.; Wold, W.S. M. Immunosuppression enhances oncolytic adenovirus replication and anti tumor efficacy in the Syrian hamster model. Mol. Ther. 2008, 16, 1665–1673. [Google Scholar]
- Lichtenstein, D.L.; Spencer, J.F.; Doronin, K.; Patra, D.; Meyer, J.M.; Shashkova, E.V.; Kuppuswamy, M.; Dhar, D.; Thomas, M.A.; Tollefson, A.E.; Zumstein, L.A.; Wold, W.S.; Toth, K. An acute toxicology study with INGN 007, an oncolytic adenovirus vector, in mice and permissive Syrian hamsters; comparisons with wild-type Ad5 and a replication-defective adenovirus vector. Cancer Gene Ther. 2009, 16, 644–54. [Google Scholar]
- Ying, B.; Toth, K.; Spencer, J.F.; Meyer, J.; Tollefson, A.E.; Patra, D.; Dhar, D.; Shashkova, E.V.; Kuppuswamy, M.; Doronin, K.; Thomas, M.A.; Zumstein, L.A.; Wold, W.S.; Lichtenstein, D.L. INGN 007, an oncolytic adenovirus vector, replicates in Syrian hamsters but not mice: Comparison of biodistribution studies. Cancer Gene Ther. 2009, 16, 625–37. [Google Scholar]
- Echavarria, M. Adenoviruses in immunocompromised hosts. Clin. Microbiol. Rev. 2008, 21, 704–715. [Google Scholar]
- Ison, M.G. Adenovirus infections in transplant recipients. Clin. Infect. Dis. 2006, 43, 331–9. [Google Scholar]
- Lenaerts, L.; Kelchtermans, H.; Geboes, L.; Matthys, P.; Verbeken, E.; De Clercq, E.; Naesens, L. Recovery of humoral immunity is critical for successful antiviral therapy in disseminated mouse adenovirus type 1 infection. Antimicrob. Agents Chemother. 2008, 52, 1462–1471. [Google Scholar]
- Lindemans, C.A.; Leen, A.M.; Boelens, J.J. How I treat adenovirus in hematopoietic stem cell transplant recipients. Blood 2010, 116, 5476–5485. [Google Scholar]
- Ying, B.; Tollefson, A.E.; Spencer, J.F.; Balakrishnan, L.; Dewhurst, S.; Capella, C.; Buller, R.M.; Toth, K.; Wold, W.S. Ganciclovir inhibits human adenovirus replication and pathogenicity in permissive immunosuppressed Syrian hamsters. Antimicrob. Agents Chemother. 2014, 58, 7171–81. [Google Scholar]
- Levitsky, J.; Singh, N.; Wagener, M.M.; Stosor, V.; Abecassis, M.; Ison, M.G. A survey of CMV prevention strategies after liver transplantation. American journal of transplantation: Official journal of the American Society of Transplantation and the American Society of Transplant Surgeons 2008, 8, 158–61. [Google Scholar]
- Sugawara, M.; Huang, W.; Fei, Y.J.; Leibach, F.H.; Ganapathy, V.; Ganapathy, M.E. Transport of valganciclovir, a ganciclovir prodrug, via peptide transporters PEPT1 and PEPT2. J. Pharm. Sci. 2000, 89, 781–9. [Google Scholar]
- Lillie, J.W.; Loewenstein, P.M.; Green, M.R.; Green, M. Functional domains of adenovirus type 5 E1a proteins. Cell 1987, 50, 1091–1100. [Google Scholar]
- Cepko, C.L.; Whetstone, C.A.; Sharp, P.A. Adenovirus hexon monoclonal antibody that is group specific and potentially useful as a diagnostic reagent. J. Clin. Microbiol. 1983, 17, 360–4. [Google Scholar]
- Thomas, G.P.; Mathews, M.B. DNA replication and the early to late transition in adenovirus infection. Cell 1980, 22, 523–533. [Google Scholar]
- Sugawara, K.; Gilead, Z.; Wold, W.S. M.; Green, M. Immunofluorescence study of the adenovirus type 2 single- stranded DNA binding protein in infected and transformed cells. J. Virol. 1977, 22, 527–539. [Google Scholar]
- Voelkerding, K.; Klessig, D.F. Identification of two nuclear subclasses of the adenovirus type 5-encoded DNA-binding protein. J. Virol. 1986, 60, 353–362. [Google Scholar]
- Dhar, D.; Spencer, J.F.; Toth, K.; Wold, W.S. M. Pre-existing immunity and passive immunity to adenovirus 5 prevents toxicity caused by an oncolytic adenovirus vector in the Syrian hamster model. Mol. Ther 2009, 17, 1724–1732. [Google Scholar]
- Avivi, I.; Chakrabarti, S.; Milligan, D.W.; Waldmann, H.; Hale, G.; Osman, H.; Ward, K.N.; Fegan, C.D.; Yong, K.; Goldstone, A.H.; Linch, D.C.; Mackinnon, S. Incidence and outcome of adenovirus disease in transplant recipients after reduced-intensity conditioning with alemtuzumab. Biol. Blood Marrow Transplant. 2004, 10, 186–194. [Google Scholar]
- Blohme, I.; Nyberg, G.; Jeansson, S.; Svalander, C. Adenovirus infection in a renal transplant patient. Transplant. Proc. 1992, 24, 295. [Google Scholar]
- Bruno, B.; Gooley, T.; Hackman, R.C.; Davis, C.; Corey, L.; Boeckh, M. Adenovirus infection in hematopoietic stem cell transplantation: Effect of ganciclovir and impact on survival. Biol. Blood Marrow Transplant. 2003, 9, 341–352. [Google Scholar]
- Chen, F.E.; Liang, R.H.; Lo, J.Y.; Yuen, K.Y.; Chan, T.K.; Peiris, M. Treatment of adenovirus-associated haemorrhagic cystitis with ganciclovir. Bone Marrow Transplant. 1997, 20, 997–9. [Google Scholar]
- Duggan, J.M.; Farrehi, J.; Duderstadt, S.; Turner, N.J.; Fekety, R. Treatment with ganciclovir of adenovirus pneumonia in a cardiac transplant patient. Am. J. Med. 1997, 103, 439–40. [Google Scholar]
- Lenaerts, L.; De Clercq, E.; Naesens, L. Clinical features and treatment of adenovirus infections. Rev.Med.Virol. 2008, 18, 357–374. [Google Scholar]
- Yabiku, S.T.; Yabiku, M.M.; Bottos, K.M.; Araujo, A.L.; Freitas, D.; Belfort, R., Jr. [Ganciclovir 0.15% ophthalmic gel in the treatment of adenovirus keratoconjunctivitis]. Arq. Bras. Oftalmol. 2011, 74, 417–21. [Google Scholar]
- Asberg, A.; Humar, A.; Rollag, H.; Jardine, A.G.; Mouas, H.; Pescovitz, M.D.; Sgarabotto, D.; Tuncer, M.; Noronha, I.L.; Hartmann, A.; Group, V.S. Oral valganciclovir is noninferior to intravenous ganciclovir for the treatment of cytomegalovirus disease in solid organ transplant recipients. American journal of transplantation: Official journal of the American Society of Transplantation and the American Society of Transplant Surgeons 2007, 7, 2106–13. [Google Scholar]
- Guidance for Industry Estimating the Maximum Safe Starting Dose in Initial Clinical Trials for Therapeutics in Adult Healthy Volunteers. Available online: www.fda.gov/downloads/drugs/guidancecomplianceregulatoryinformation/guidances/ucm078932.pdf (accessed on 19 March 2015).
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Toth, K.; Ying, B.; Tollefson, A.E.; Spencer, J.F.; Balakrishnan, L.; Sagartz, J.E.; Buller, R.M.L.; Wold, W.S.M. Valganciclovir Inhibits Human Adenovirus Replication and Pathology in Permissive Immunosuppressed Female and Male Syrian Hamsters. Viruses 2015, 7, 1409-1428. https://doi.org/10.3390/v7031409
Toth K, Ying B, Tollefson AE, Spencer JF, Balakrishnan L, Sagartz JE, Buller RML, Wold WSM. Valganciclovir Inhibits Human Adenovirus Replication and Pathology in Permissive Immunosuppressed Female and Male Syrian Hamsters. Viruses. 2015; 7(3):1409-1428. https://doi.org/10.3390/v7031409
Chicago/Turabian StyleToth, Karoly, Baoling Ying, Ann E. Tollefson, Jacqueline F. Spencer, Lata Balakrishnan, John E. Sagartz, Robert Mark L. Buller, and William S. M. Wold. 2015. "Valganciclovir Inhibits Human Adenovirus Replication and Pathology in Permissive Immunosuppressed Female and Male Syrian Hamsters" Viruses 7, no. 3: 1409-1428. https://doi.org/10.3390/v7031409
APA StyleToth, K., Ying, B., Tollefson, A. E., Spencer, J. F., Balakrishnan, L., Sagartz, J. E., Buller, R. M. L., & Wold, W. S. M. (2015). Valganciclovir Inhibits Human Adenovirus Replication and Pathology in Permissive Immunosuppressed Female and Male Syrian Hamsters. Viruses, 7(3), 1409-1428. https://doi.org/10.3390/v7031409






