Intranasal Administration of Maleic Anhydride-Modified Human Serum Albumin for Pre-Exposure Prophylaxis of Respiratory Syncytial Virus Infection
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Cells, Virus and Plasmid
2.3. Determination of RSV Titer
2.4. Chemical Modification of Proteins with Different Anhydrides under Variable Conditions
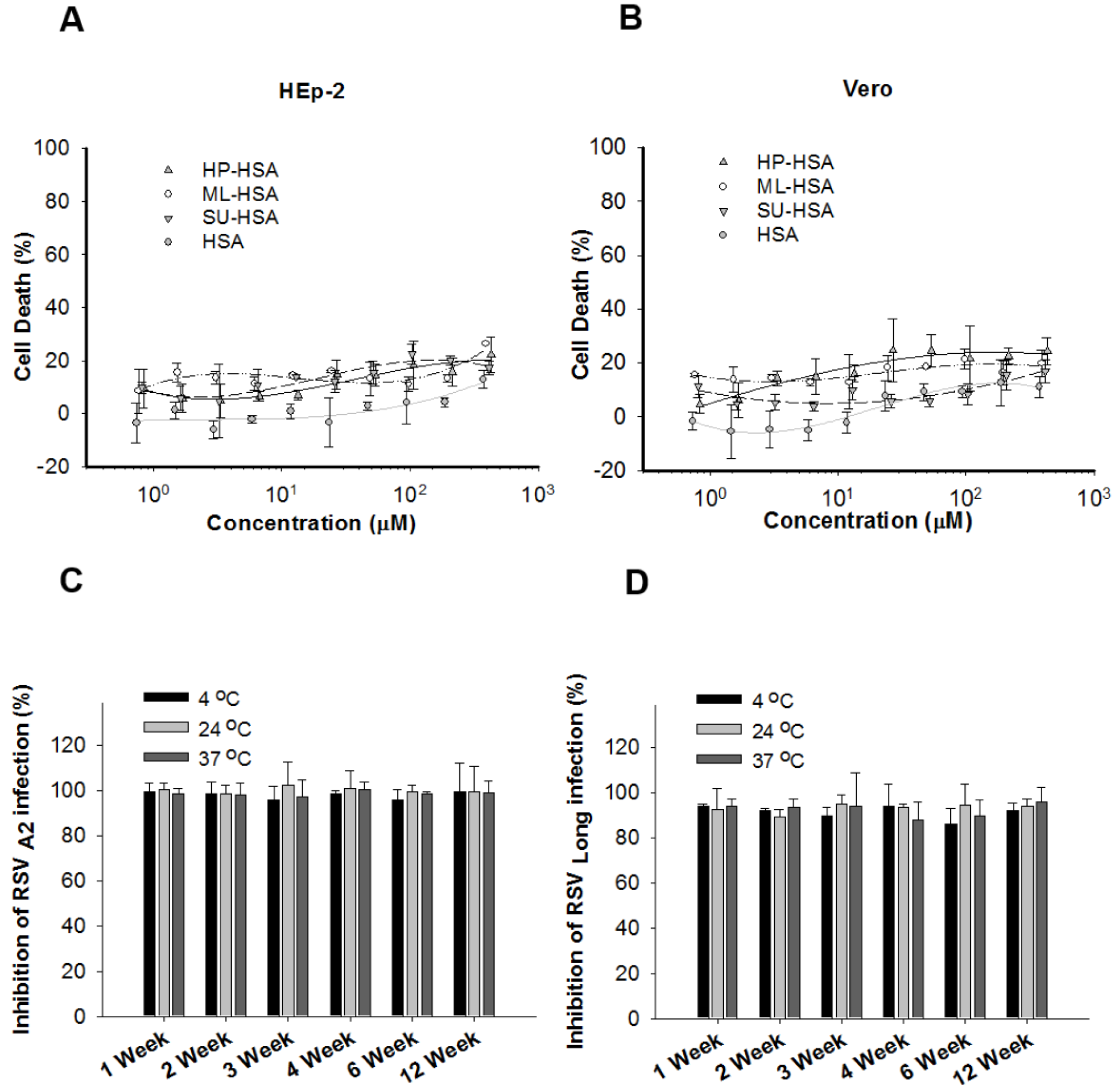
2.5. Cytotoxicity Assay
2.6. Assay for Cell Protection of Anhydride-Modified Proteins against RSV
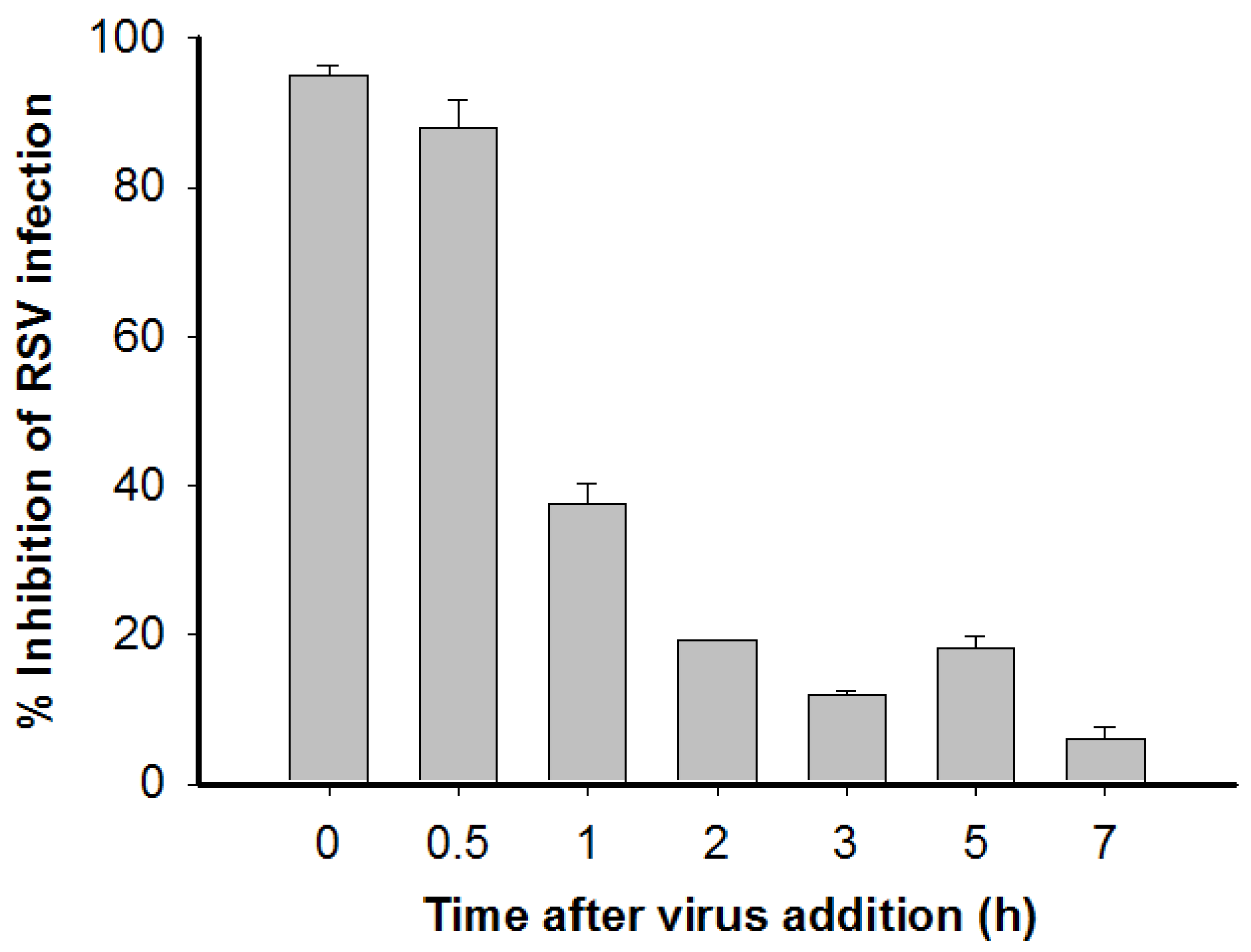
2.7. Time-of-Addition and Temperature Shift Assays
2.8. Cell-Cell Fusion Assay
2.9. ELISA Assay
2.10. Selection of Drug-Resistant Virus
2.11. RSV-RNA Extraction and RT-PCR Amplification
2.12. Mouse Models of RSV Infection
3. Results
3.1. Anhydride-Modified Proteins were Potent Inhibitors against RSV Infection
| Anhydride-Modified Protein | Inhibition of Infection by | |||
|---|---|---|---|---|
| RSV A2 Strain | RSV Long Strain | |||
| IC50 (μM) | IC90 (μM) | IC50 (μM) | IC90 (μM) | |
| HP | 3.142 ± 0.458 | >10 | 5.832 ± 0.753 | >10 |
| HP-β-LG | 0.062 ± 0.011 | 0.227 ± 0.029 | 0.153 ± 0.011 | 0.422 ± 0.021 |
| HP-OVA | 0.013 ± 0.002 | 0.052 ± 0.009 | 0.100 ± 0.007 | 0.517 ± 0.121 |
| HP-HSA | 0.006 ± 0.001 | 0.042 ± 0.022 | 0.011 ± 0.003 | 0.056 ± 0.010 |
| HP-BSA | 0.005 ± 0.001 | 0.025 ± 0.010 | 0.016 ± 0.004 | 0.099 ± 0.011 |
| ML | >10 | >10 | >10 | >10 |
| ML-β-LG | 0.283 ± 0.130 | 1.046 ± 0.263 | 0.173 ± 0.045 | 0.581 ± 0.091 |
| ML-OVA | 0.024 ± 0.011 | 0.077 ± 0.033 | 0.217 ± 0.005 | 1.425 ± 0.143 |
| ML-HSA | 0.012 ± 0.002 | 0.038 ± 0.003 | 0.002 ± 0.001 | 0.013 ± 0.005 |
| ML-BSA | 0.002 ± 0.000 | 0.026 ± 0.019 | 0.007 ± 0.002 | 0.025 ± 0.005 |
| SU | >10 | >10 | >10 | >10 |
| SU-β-LG | 0.599 ± 0.079 | 1.388 ± 0.112 | 0.405 ± 0.100 | 1.093 ± 0.209 |
| SU-OVA | 0.046 ± 0.008 | 0.172 ± 0.024 | 0.277 ± 0.077 | 1.173 ± 0.321 |
| SU-HSA | 0.011 ± 0.005 | 0.049 ± 0.021 | 0.014 ± 0.005 | 0.110 ± 0.070 |
| SU-BSA | 0.006 ± 0.001 | 0.047 ± 0.011 | 0.026 ± 0.015 | 0.125 ± 0.020 |
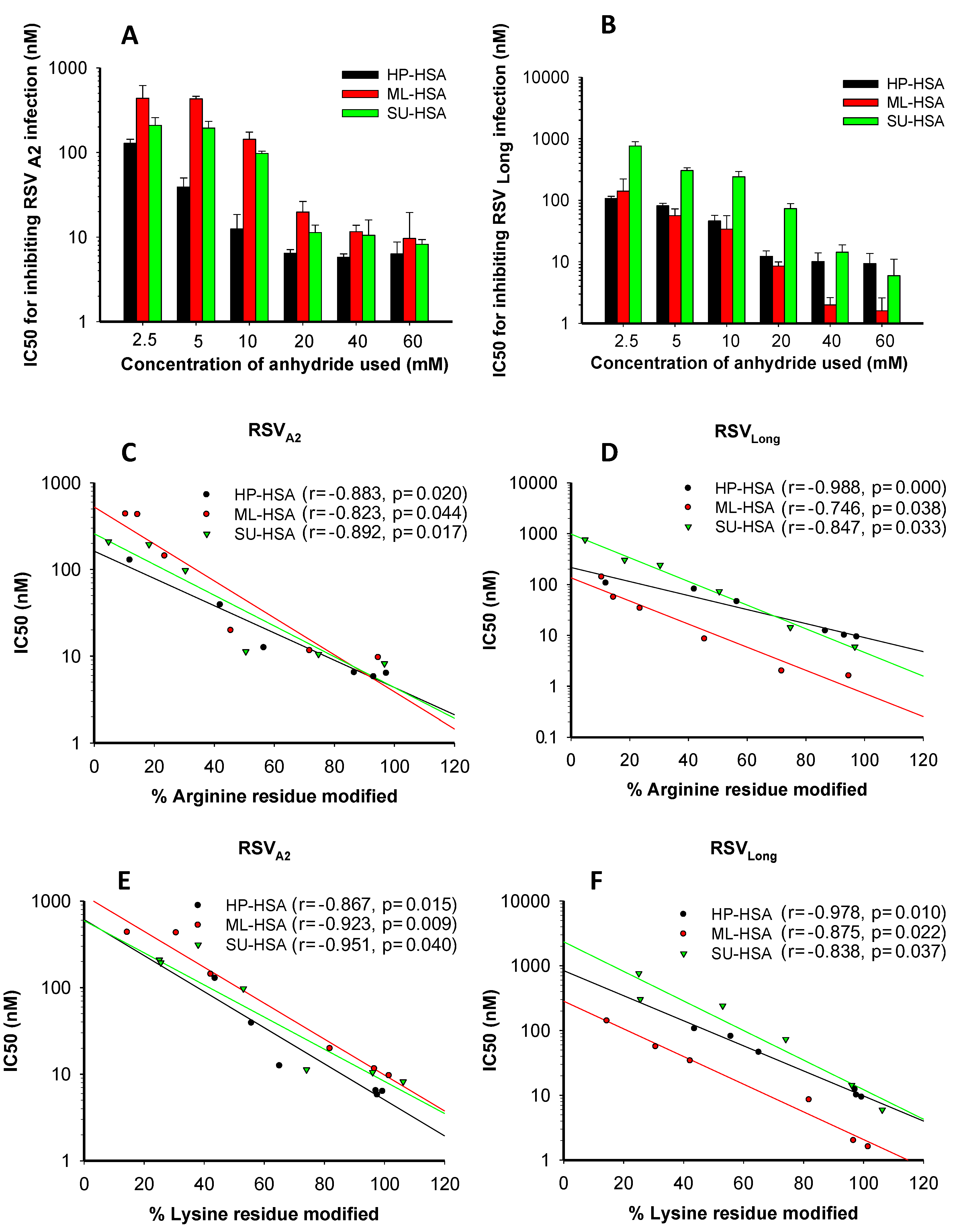
3.2. The Percentages of Modified Residues of arginine and Lysine Correlate with Anti-RSV Activity of Anhydride-Modified Proteins
3.3. Time-of-Addition and Temperature Shift Studies Suggest that ML-HSA Inhibits RSV Infection by Blocking RSV Attachment to the Target Cells

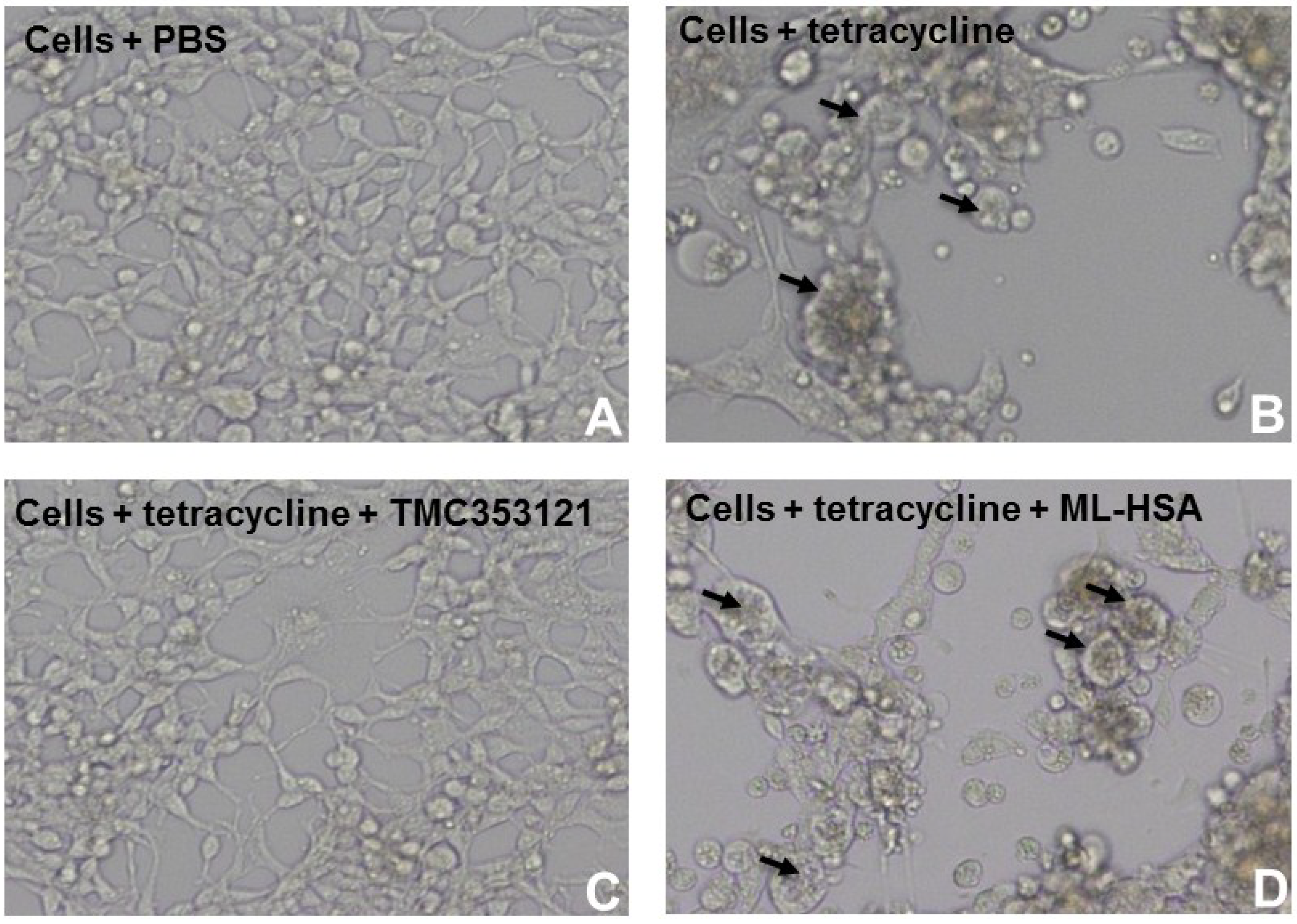
3.4. ML-HSA Interacted with G Protein of RSV
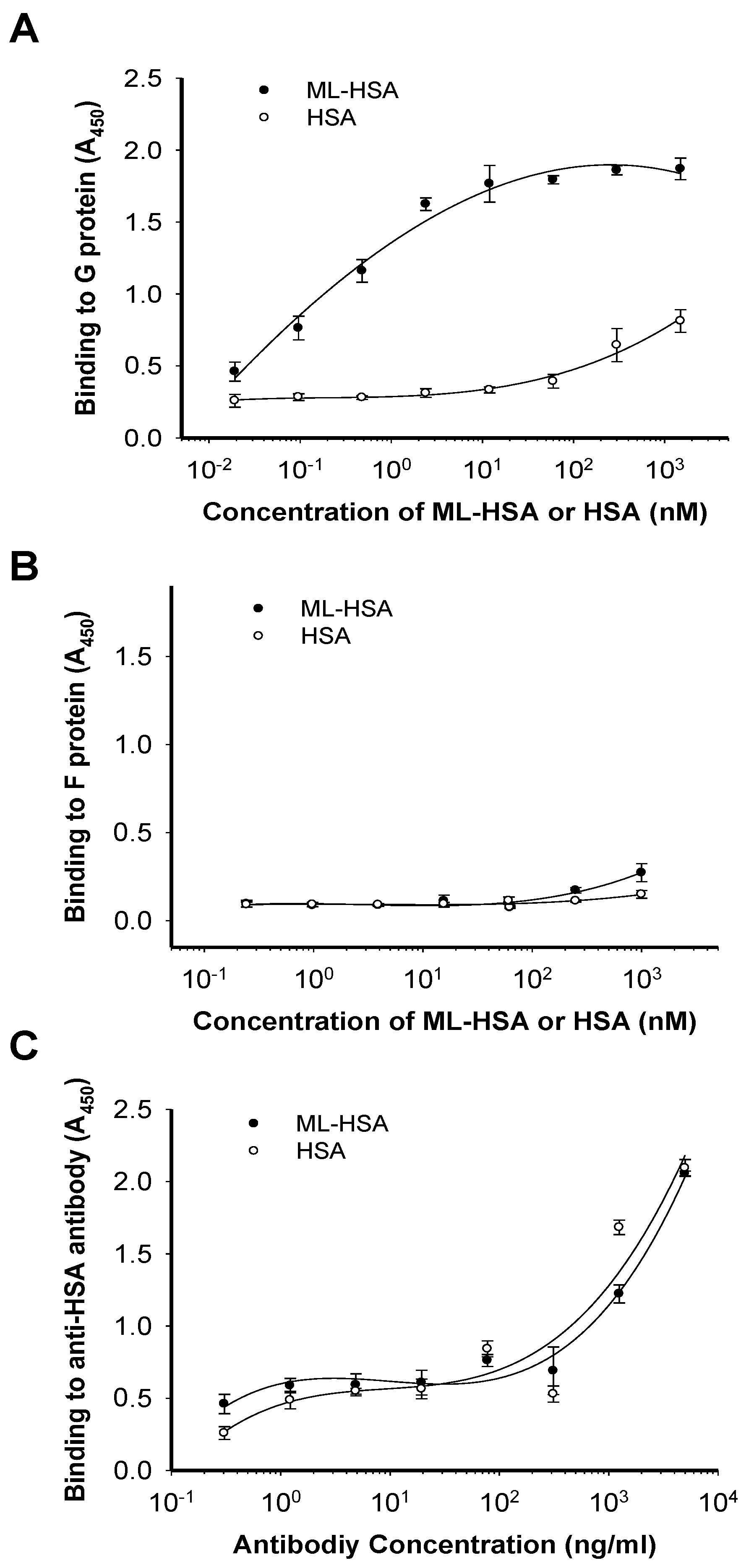
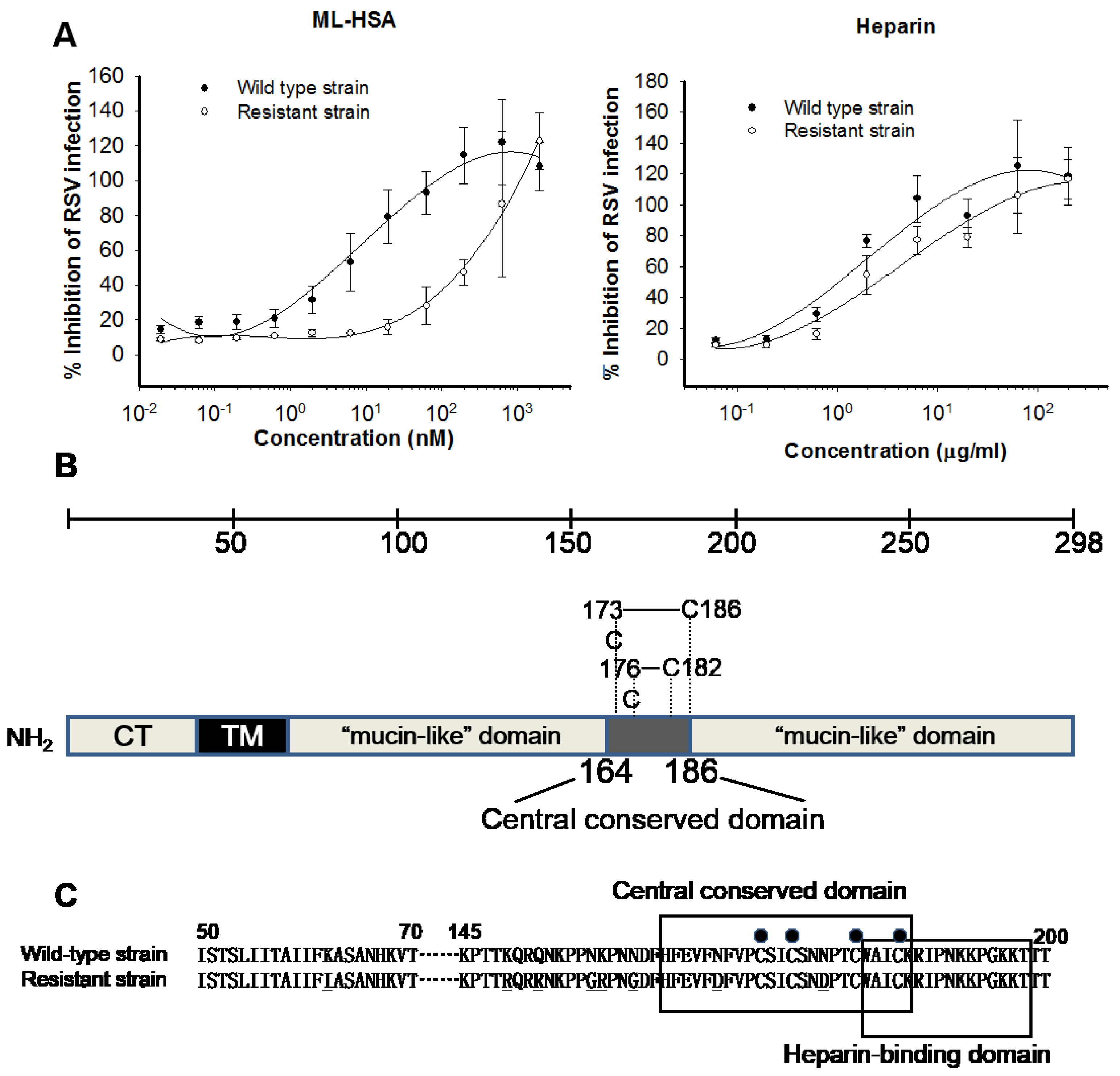
3.5. ML-HSA may Bind to the Middle Portion (Residues 145–186) of the G Protein Based on Drug-Resistance Study
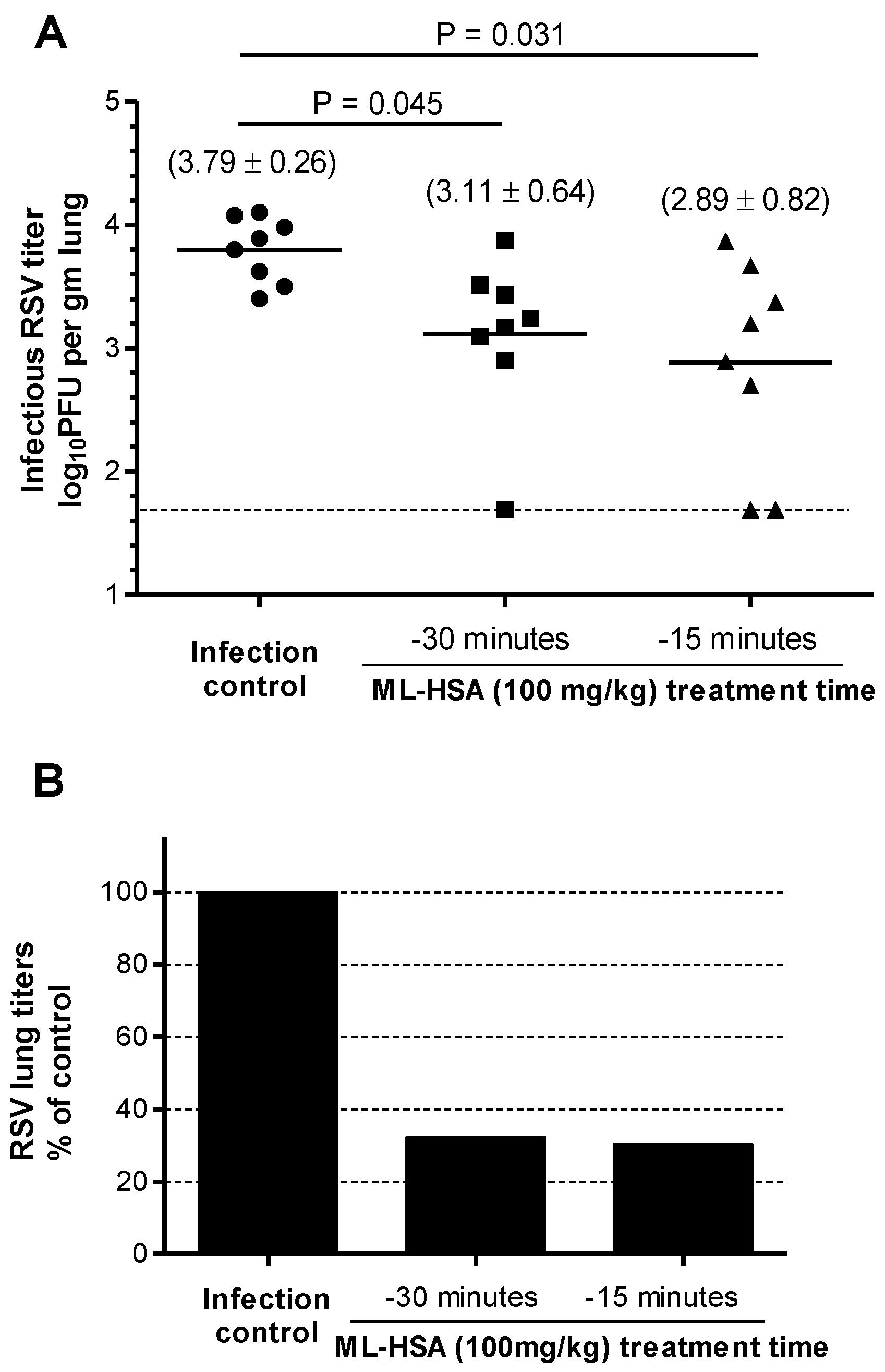
3.6. ML-HSA Inactivated RSV when RSV and ML-HSA Were Mixed before Intranasal Administration to Mice
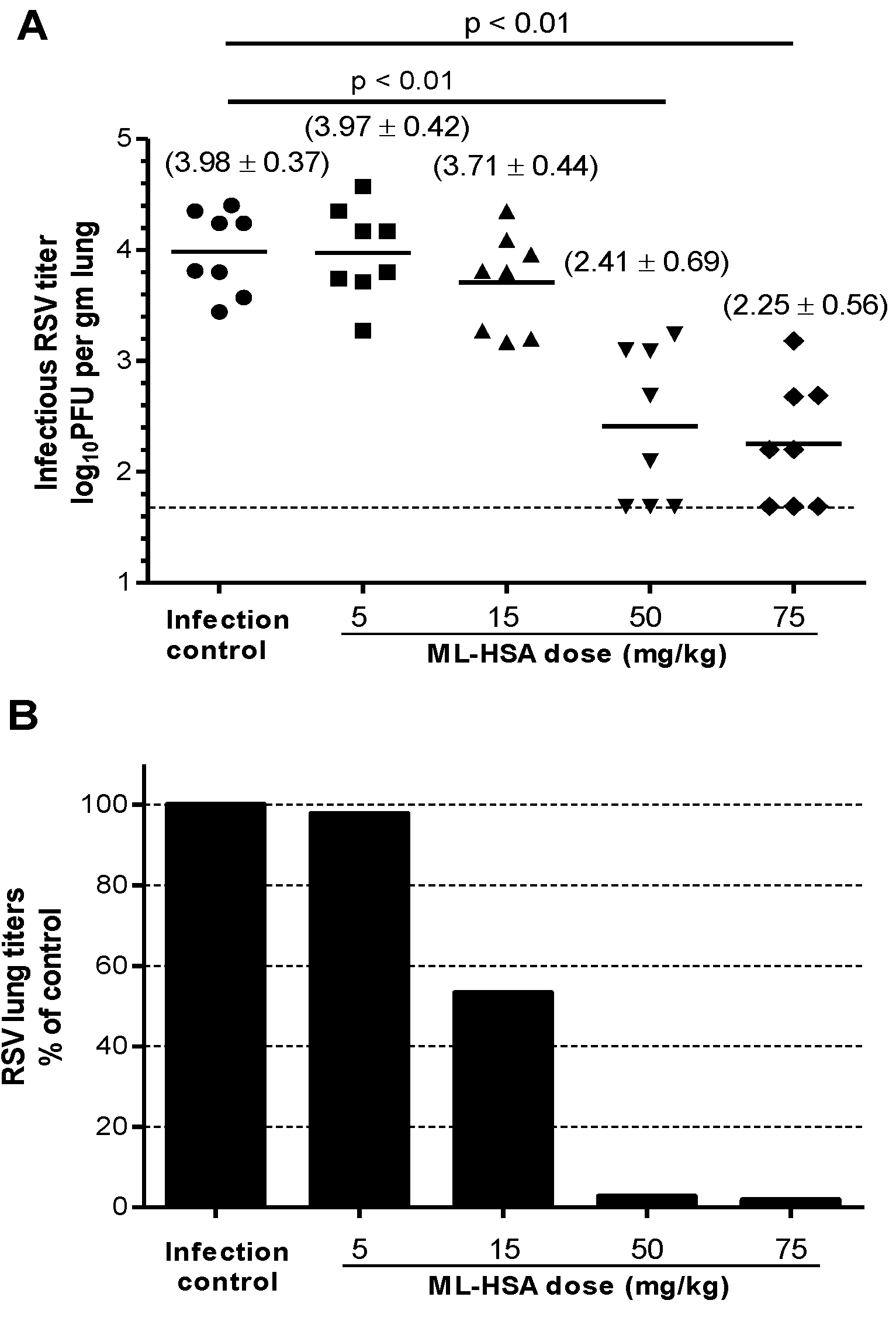
3.7. ML-HSA Exhibited Prophylactic Efficacy in the Mouse Model against RSV Infection
4. Discussion
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Falsey, A.R.; Hennessey, P.A.; Formica, M.A.; Cox, C.; Walsh, E.E. Respiratory syncytial virus infection in elderly and high-risk adults. N. Engl. J. Med. 2005, 352, 1749–1759. [Google Scholar] [CrossRef] [PubMed]
- Collins, P.L.; Crowe, J.E.J. Respiratory syncytial virus and Metapneumovirus. In Fields Virology, 5th ed.; Knipe, D.M., Howley, P.M., Griffin, D.E., Lamb, R.A., Martin, M.A., Roizman, B., Straus, S.E., Eds.; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2007; Volume 2, pp. 1601–1646. [Google Scholar]
- Collins, P.L.; Melero, J.A. Progress in understanding and controlling respiratory syncytial virus: Still crazy after all these years. Virus Res. 2011, 162, 80–99. [Google Scholar] [CrossRef] [PubMed]
- Nair, H.; Noke, D.J.; Gessner, B.D.; Dherani, M.; Madhi, S.A.; Singleton, R.J.; O'Brien, K.L.; Roca, A.; Wright, P.F.; Bruce, N.; et al. Global burden of acute lower respiratory infections due to respiratory syncytial virus in young children: A systematic review and meta-analysis. Lancet 2010, 375, 1545–1555. [Google Scholar] [CrossRef] [PubMed]
- Hall, C.B.; Weinberg, G.A.; Iwane, M.K.; Blumkin, A.K.; Edwards, K.M.; Staat, M.A.; Auinger, P.; Griffin, M.R.; Poehling, K.A.; Erdman, D.; et al. The Burden of Respiratory Syncytial Virus Infection in Young Children. N. Engl. J. Med. 2009, 360, 588–598. [Google Scholar] [CrossRef] [PubMed]
- Hacking, D.; Hull, J. Respiratory syncytial virus - Viral biology and the host response. J. Infect. 2002, 45, 18–24. [Google Scholar] [CrossRef] [PubMed]
- Simoes, E.A. Respiratory syncytial virus infection. Lancet 1999, 354, 847–852. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Pfarr, D.S.; Losonsky, G.A.; Kiener, P.A. Immunoprophylaxis of RSV infection: Advancing from RSV-IGIV to palivizumab and motavizumab. Curr. Top Microbiol. Immunol. 2008, 317, 103–123. [Google Scholar] [PubMed]
- Wang, D.; Bayliss, S.; Meads, C. Palivizumab for immunoprophylaxis of respiratory syncytial virus (RSV) bronchiolitis in high-risk infants and young children: A systematic review and additional economic modelling of subgroup analyses. Health Technol. Assess 2011, 15, i–iii. [Google Scholar] [CrossRef] [PubMed]
- Committee on infectious diseases and bronchiolitis gudelines committee. Updated guidance for palivizumab prophylaxis among infants and young children at increased risk of hospitalization for respiratory syncytial virus. Pediatrics 2014, 134, 415–420. [Google Scholar]
- Meissner, H.C.; Kimberlin, D.W. RSV Immunoprophylaxis: Does the Benefit Justify the Cost? Pediatrics 2013, 132, 915–918. [Google Scholar] [CrossRef] [PubMed]
- Lu, L.; Yang, X.; Li, Y.; Jiang, S. Chemically modified bovine beta-lactoglobulin inhibits human papillomavirus infection. Microbes Infect. 2013, 15, 506–510. [Google Scholar] [CrossRef] [PubMed]
- Neurath, A.R.; Jiang, S.; Strick, N.; Lin, K.; Li, Y.Y.; Debnath, A.K. Bovine beta-lactoglobulin modified by 3-hydroxyphthalic anhydride blocks the CD4 cell receptor for HIV. Nat. Med. 1996, 2, 230–234. [Google Scholar] [CrossRef] [PubMed]
- Neurath, A.R.; Debnath, A.K.; Strick, N.; Li, Y.Y.; Lin, K.; Jiang, S. Blocking of CD4 cell receptors for the human immunodeficiency virus type 1 (HIV-1) by chemically modified bovine milk proteins: Potential for AIDS prophylaxis. J. Mol. Recognit. 1995, 8, 304–316. [Google Scholar] [CrossRef] [PubMed]
- Razinkov, V.; Gazumyan, A.; Nikitenko, A.; Ellestad, G.; Krishnamurthy, G. RFI-641 inhibits entry of respiratory syncytial virus via interactions with fusion protein. Chem. Biol. 2001, 8, 645–659. [Google Scholar] [CrossRef] [PubMed]
- Kluwe, W.M. Carcinogenic Potential of Phthalic Acid Esters and Related-Compounds: Structure-Activity-Relationships. Environ. Health Perspect 1986, 65, 271–278. [Google Scholar] [PubMed]
- Li, L.; He, L.; Tan, S.; Guo, X.; Lu, H.; Qi, Z.; Pan, C.; An, X.; Jiang, S.; Liu, S. 3-Hydroxyphthalic Anhydride-Modified Chicken Ovalbumin Exhibits Potent and Broad Anti-HIV-1 Activity: A Potential Microbicide for Preventing Sexual Transmission of HIV-1. Antimicrob. Agents Chemother. 2010, 54, 1700–1711. [Google Scholar] [CrossRef] [PubMed]
- Yamasaki, R.B.; Vega, A.; Feeney, R.E. Modification of Available Arginine Residues in Proteins by Para-Hydroxyphenylglyoxal. Anal. Biochem. 1980, 109, 32–40. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Qiao, P.; Yang, J.; Lu, L.; Tan, S.; Lu, H.; Zhang, X.; Chen, X.; Wu, S.; Jiang, S.; et al. Maleic anhydride-modified chicken ovalbumin as an effective and inexpensive anti-HIV microbicide candidate for prevention of HIV sexual transmission. Retrovirology 2010, 7. [Google Scholar] [CrossRef]
- Lu, L.; Liu, Q.; Zhu, Y.; Chan, K.H.; Qin, L.; Li, Y.; Wang, Q.; Chan, J.F.; Du, L.; Yu, F.; et al. Structure-based discovery of Middle East respiratory syndrome coronavirus fusion inhibitor. Nat. Commun. 2014, 5. [Google Scholar] [CrossRef] [PubMed]
- Cianci, C.; Yu, K.L.; Combrink, K.; Sin, N.; Pearce, B.; Wang, A.; Civiello, R.; Voss, S.; Luo, G.; Kadow, K.; et al. Orally active fusion inhibitor of respiratory syncytial virus. Antimicro. Agents Chemother. 2004, 48, 413–422. [Google Scholar] [CrossRef]
- Huntley, C.C.; Weiss, W.J.; Gazumyan, A.; Buklan, A.; Feld, B.; Hu, W.; Jones, T.R.; Murphy, T.; Nikitenko, A.A.; O'Hara, B.; et al. RFI-641, a potent respiratory syncytial virus inhibitor. Antimicro. Agents Chemother. 2002, 46, 841–847. [Google Scholar] [CrossRef]
- Andries, K.; Moeremans, M.; Gevers, T.; Willebrords, R.; Sommen, C.; Lacrampe, J.; Janssens, F.; Wyde, P.R. Substituted benzimidazoles with nanomolar activity against respiratory syncytial virus. Antivir. Res. 2003, 60, 209–219. [Google Scholar] [CrossRef] [PubMed]
- Douglas, J.L.; Panis, M.L.; Ho, E.; Lin, K.Y.; Krawczyk, S.H.; Grant, D.M.; Cai, R.; Swaminathan, S.; Cihlar, T. Inhibition of respiratory syncytial virus fusion by the small molecule VP-14637 via specific interactions with F protein. J. Virol. 2003, 77, 5054–5064. [Google Scholar] [CrossRef] [PubMed]
- Kahn, J.S.; Schnell, M.J.; Buonocore, L.; Rose, J.R. Recombinant vesicular stomatitis virus expressing respiratory syncytial virus (RSV) glycoproteins: RSV fusion protein can mediate infection and cell fusion. Virology 1999, 254, 81–89. [Google Scholar] [CrossRef] [PubMed]
- Roymans, D.; de Bondt, H.L.; Arnoult, E.; Geluykens, P.; Gevers, T.; van Ginderen, M.; Verheyen, N.; Kim, H.; Willebrords, R.; Bonfanti, J.F.; et al. Binding of a potent small-molecule inhibitor of six-helix bundle formation requires interactions with both heptad-repeats of the RSV fusion protein. Proc. Natl. Acad. Sci. USA 2010, 107, 308–313. [Google Scholar] [CrossRef] [PubMed]
- Lu, L.; Pan, C.; Li, Y.; Lu, H.; He, W.; Jiang, S. A bivalent recombinant protein inactivates HIV-1 by targeting the gp41 prehairpin fusion intermediate induced by CD4 D1D2 domains. Retrovirology 2012, 9. [Google Scholar] [CrossRef] [PubMed]
- Kimura, K.; Ishioka, K.; Hashimoto, K.; Mori, S.; Suzutani, T.; Bowlin, T.L.; Shigeta, S. Isolation and characterization of NMSO3-resistant mutants of respiratory syncytial virus. Antivir. Res. 2004, 61, 165–171. [Google Scholar] [CrossRef] [PubMed]
- Morton, C.J.; Cameron, R.; Lawrence, L.J.; Lin, B.; Lowe, W.; Luttick, A.; Mason, A.; Kimm-Breschkin, J.; Parker, M.W.; Ryan, J.; et al. Structural characterization of respiratory syncytial virus fusion inhibitor escape mutants: Homology model of the F protein and a syncytium formation assay. Virology 2003, 311, 275–288. [Google Scholar] [CrossRef] [PubMed]
- Cianci, C.; Genovesi, E.V.; Lamb, L.; Medina, I.; Yang, Z.; Zadjura, L.; Yang, H.; D'Arienzo, C.; Sin, N.; Yu, K.L.; et al. Oral efficacy of a respiratory syncytial virus inhibitor in rodent models of infection. Antimicrob. Agents Chemother. 2004, 48, 2448–2454. [Google Scholar] [CrossRef] [PubMed]
- Sudo, K.; Watanabe, W.; Mori, S.; Konno, K.; Shigeta, S.; Yokota, T. Mouse model of respiratory syncytial virus infection to evaluate antiviral activity in vivo. Antivir. Chem. Chemother. 1999, 10, 135–139. [Google Scholar] [PubMed]
- Graham, B.S.; Perkins, M.D.; Wright, P.F.; Karzon, D.T. Primary Respiratory Syncytial Virus-Infection in Mice. J. Med. Virol. 1988, 26, 153–162. [Google Scholar] [CrossRef] [PubMed]
- Srinivasakumar, N.; Ogra, P.L.; Flanagan, T.D. Characteristics of Fusion of Respiratory Syncytial Virus with Hep-2 Cells as Measured by R18 Fluorescence Dequenching Assay. J. Virol. 1991, 65, 4063–4069. [Google Scholar] [PubMed]
- Mccormick, D.L.; Becci, P.J.; Moon, R.C. Inhibition of Mammary and Urinary-Bladder Carcinogenesis by A Retinoid and A Maleic Anhydride-Divinyl Ether Co-Polymer (Mve-2). Carcinogenesis 1982, 3, 1473–1477. [Google Scholar] [CrossRef] [PubMed]
- Xie, D.; Yao, C.; Wang, L.; Min, W.; Xu, J.; Xiao, J.; Huang, M.; Chen, B.; Liu, B.; Li, X.; et al. An Albumin-Conjugated Peptide Exhibits Potent Anti-HIV Activity and Long In Vivo Half-Life. Antimicro. Agents Chemother. 2010, 54, 191–196. [Google Scholar] [CrossRef]
- Li, L.; Qiu, J.; Lu, L.; An, S.; Qiao, P.; Jiang, S.; Liu, W. 3-Hydroxyphthalic anhydride-modified human serum albumin as a microbicide candidate inhibits HIV infection by blocking viral entry. J. Antimicrob. Chemother. 2013, 68, 573–576. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.; Pan, Y.; Jiang, S.; Lu, L. Respiratory Syncytial Virus Entry Inhibitors Targeting the F Protein. Viruses 2013, 5, 211–225. [Google Scholar] [CrossRef] [PubMed]
- SYNAGIS® (PALIVIZUMAB) Instructions for Intramuscular Administration. Available online: http://www.accessdata.fda.gov/drugsatfda_docs/label/2002/palimed102302LB.pdf (accessed on 2 December 1999).
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, Z.; Wang, Q.; Jia, R.; Xia, S.; Li, Y.; Liu, Q.; Xu, W.; Xu, J.; Du, L.; Lu, L.; et al. Intranasal Administration of Maleic Anhydride-Modified Human Serum Albumin for Pre-Exposure Prophylaxis of Respiratory Syncytial Virus Infection. Viruses 2015, 7, 798-819. https://doi.org/10.3390/v7020798
Sun Z, Wang Q, Jia R, Xia S, Li Y, Liu Q, Xu W, Xu J, Du L, Lu L, et al. Intranasal Administration of Maleic Anhydride-Modified Human Serum Albumin for Pre-Exposure Prophylaxis of Respiratory Syncytial Virus Infection. Viruses. 2015; 7(2):798-819. https://doi.org/10.3390/v7020798
Chicago/Turabian StyleSun, Zhiwu, Qian Wang, Ran Jia, Shuai Xia, Yuan Li, Qi Liu, Wei Xu, Jin Xu, Lanying Du, Lu Lu, and et al. 2015. "Intranasal Administration of Maleic Anhydride-Modified Human Serum Albumin for Pre-Exposure Prophylaxis of Respiratory Syncytial Virus Infection" Viruses 7, no. 2: 798-819. https://doi.org/10.3390/v7020798





