Multiple Poliovirus Proteins Repress Cytoplasmic RNA Granules
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cell Culture, Transfections and Infections
2.2. Antibodies
2.3. Plasmids and Cloning
P1-Cherry: Forward: TGGAATTCTGCAGATATGGGTGCTCAGGTTTCATCACAGAAAGTGGG Reverse: GCCACTGTGCTGGATTTATATGTGGTCAGATCCTTGGTGGAGAGGGG PV 2A-Cherry: Forward: TGGAATTCTGCAGAT ATGGGATTCGGACACCAAAACAAAGCGGTG Reverse: GCCACTGTGCTGGATTTGCTCCATGGCTTCTTCTTCGTAGGCATACAA PV 3C-Cherry: Forward: TGGAATTCTGCAGATATGGGACCAGGGTTCGATTACGCAGT Reverse: GCCACTGTGCTGGATTTTTGACTCTGAGTGAAGTATGATCGC PV 3CD-Cherry Forward: TGGAATTCTGCAGATATGGGACCAGGGTTCGATTACGCAGT Reverse: GCCACTGTGCTGGATTTCCGTTGGCTTGACTCATTTTAG PV 3D-Cherry Forward: TGGAATTCTGCAGATATGGGTGAAATCCAGTGGATGAGA Reverse: GCCACTGTGCTGGATTTCCGTTGGCTTGACTCATTTTAG Cherry-PV-Vpg-3C: Forward: TGGAATTCTGCAGATACCATGGTGCCCACCATTCGGACAGCAAAGGTACAAGGACCAGGG Reverse: GCCACTGTGCTGGATTTATTGACTCTGAGTGAAGTATGATCGCTTCAGGGCCGCTGCAAA PV 2C-Cherry: Forward: TGGAATTCTGCAGATATGGGTGACAGTTGGTTGAAGAAGTTTACTGAAGCATGC Reverse: GCCACTGTGCTGGATTTGAAACAAAGCCTCCATACAATTGCCAATGTTGGATCTTCTGTT PV 2BC-Cherry: Forward: TGGAATTCTGCAGATATG GGCATCACCAATTACATAGAGTCACTTGGGGCC Reverse: GCCACTGTGCTGGATTTGAAACAAAGCCTCCATACAATTGCCAATGTTGGATCTTCTGTT PV 3A-Cherry: Forward: TGGAATTCTGCAGATATGGGACCACTCCAGTATAAAGACTTGAAAATTGACATCAAGACGAGT Reverse: GCCACTGTGCTGGATCTGGTGTCCAGCAAACAGTTTATACATGACATAGAC.
2.4. Immunofluorescence
3. Results
3.1. PV Proteins Drive Assembly or Disassembly of SGs
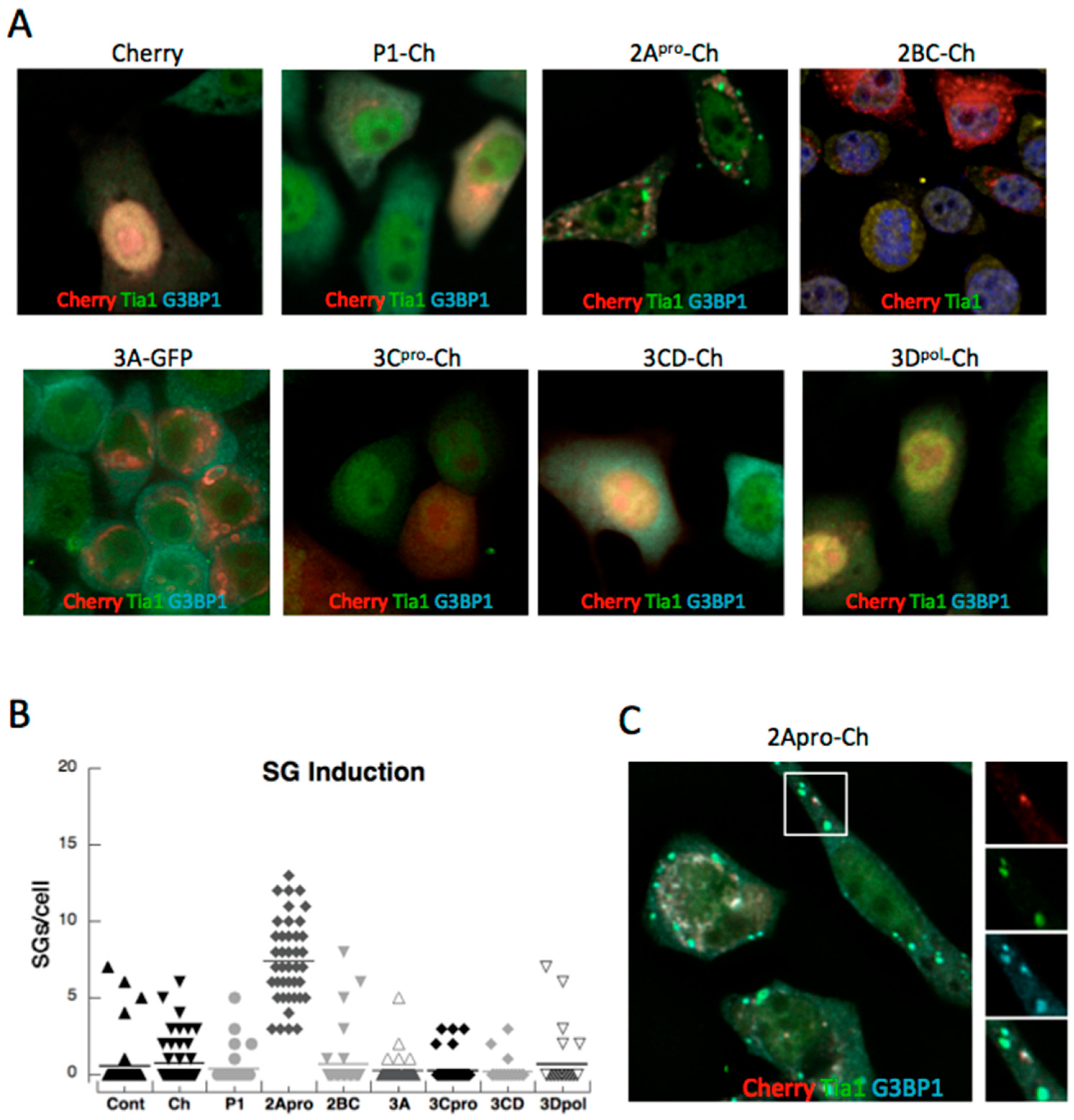
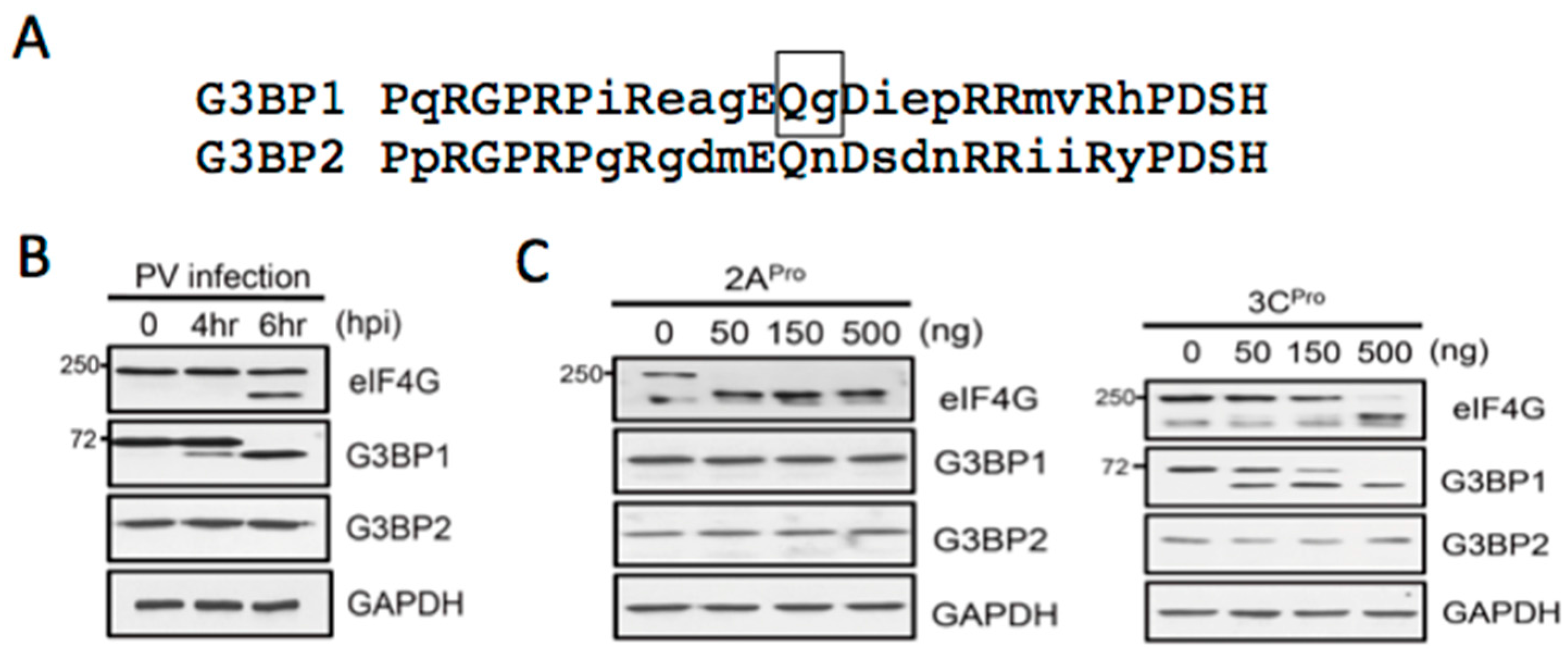
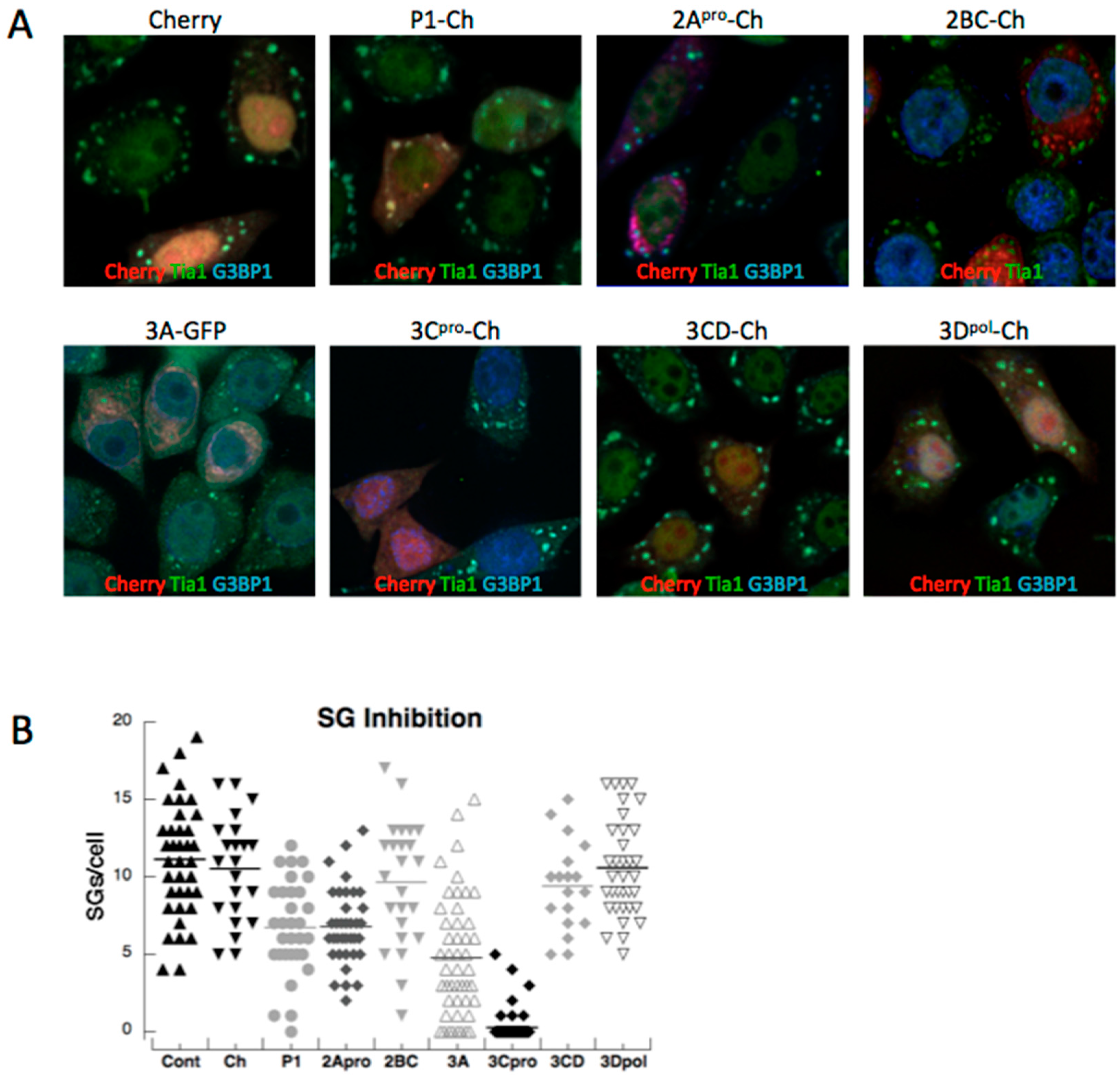
3.2. Both PV Proteases Mediate PB Disruption
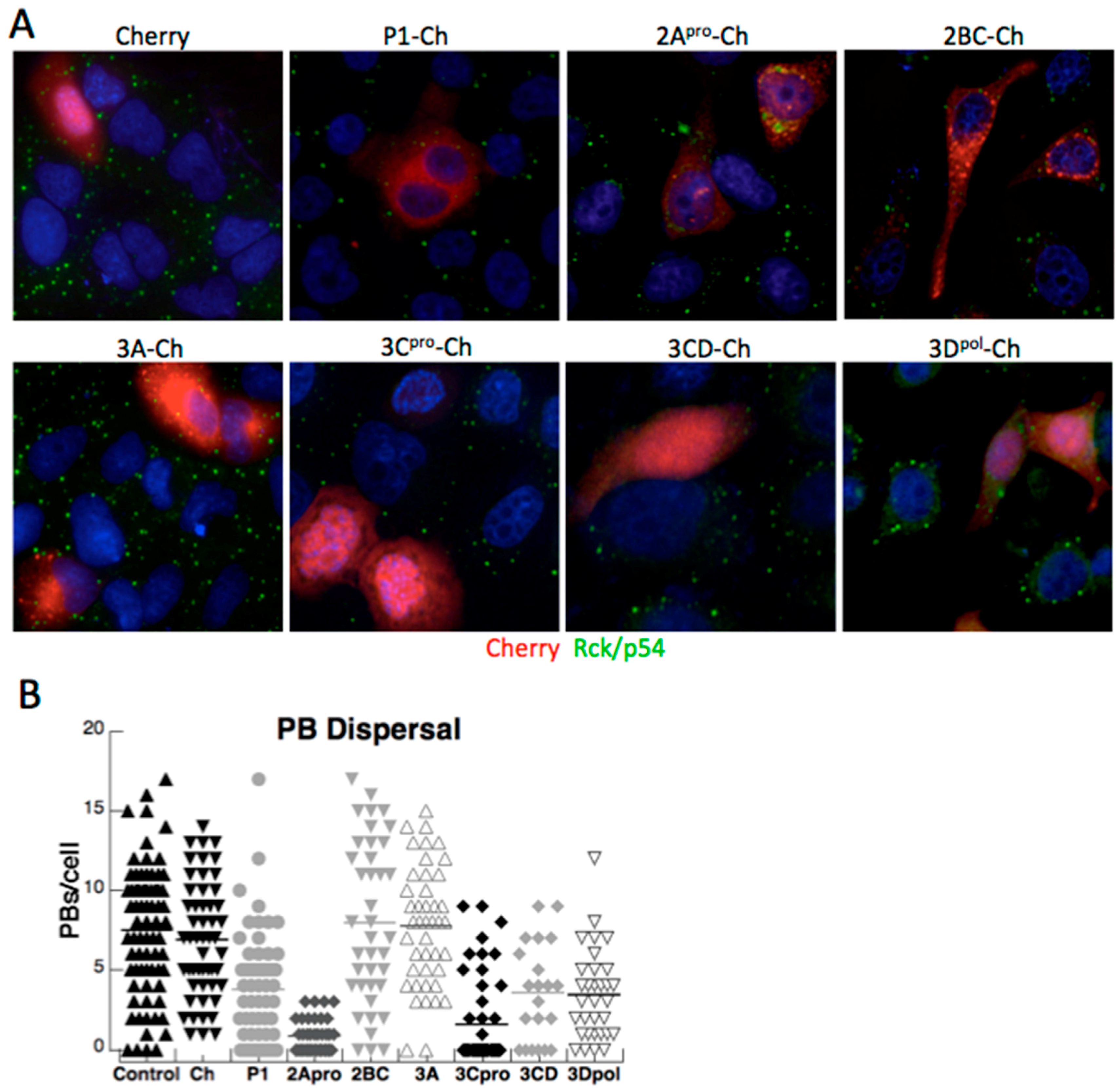
3.3. Exogenous Stress can Rescue PB Formation following Chemical Dispersal
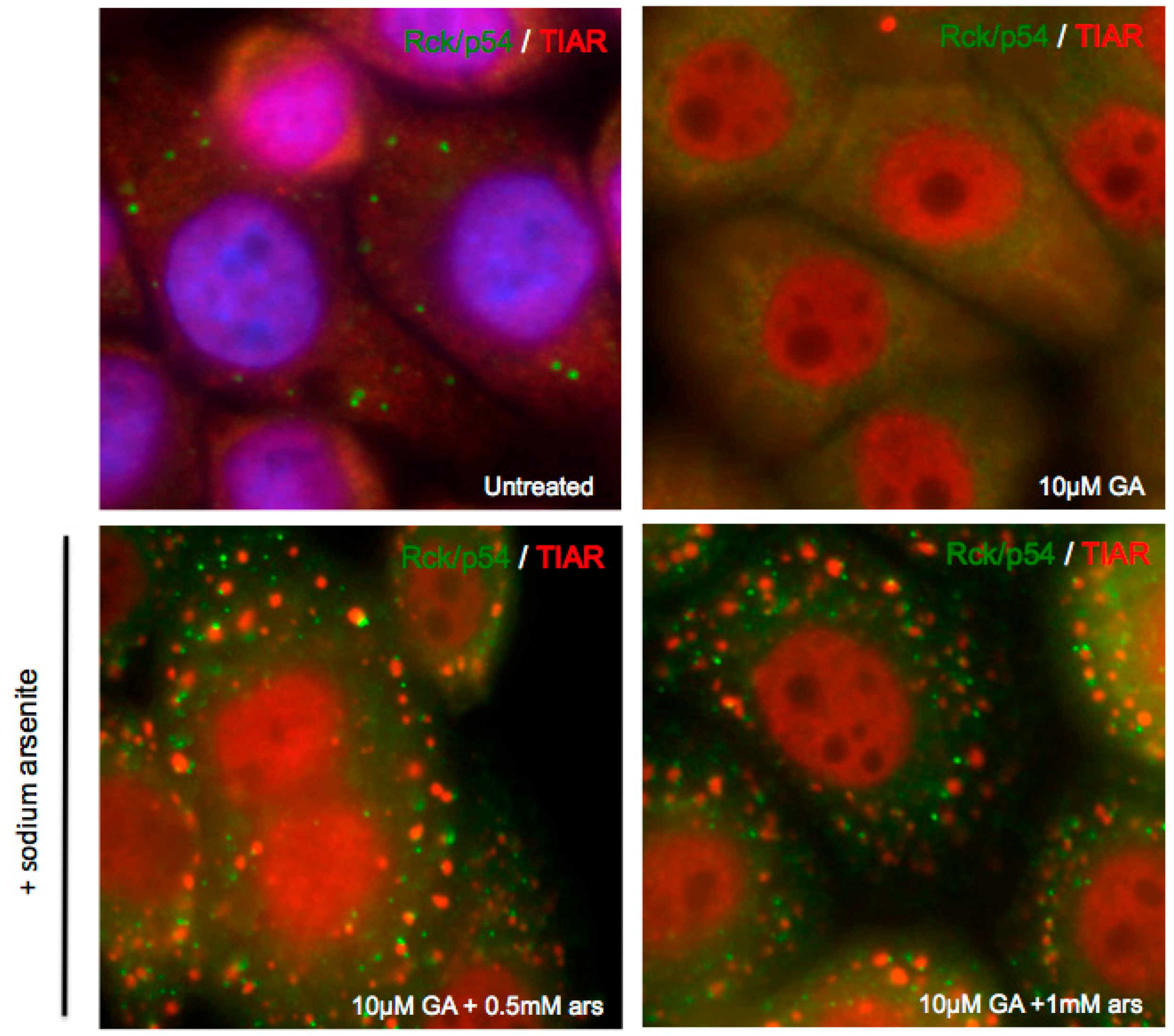
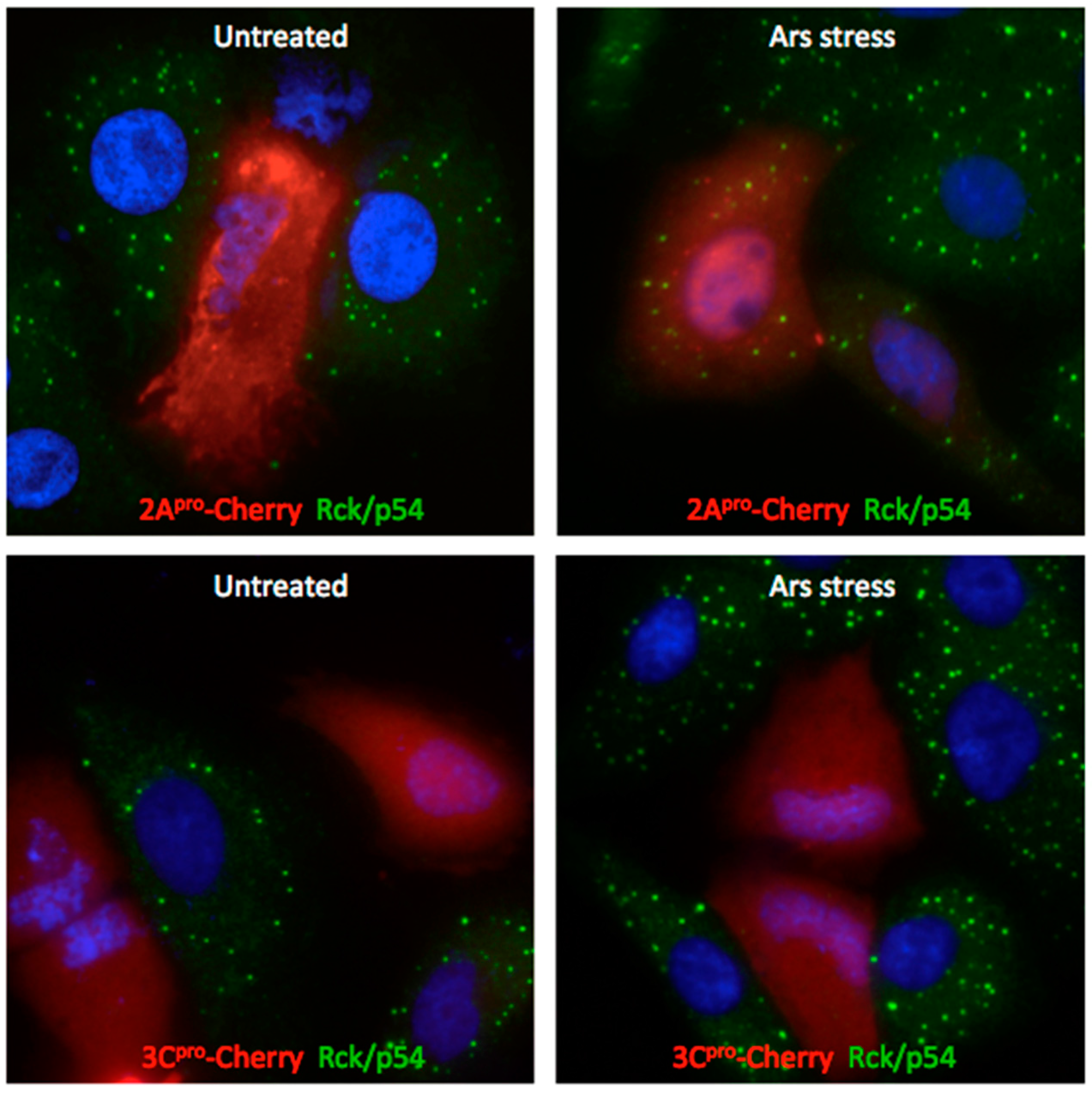
3.4. Exogenous Stress Rescues PBs Disrupted by PV 2Apro but not 3Cpro
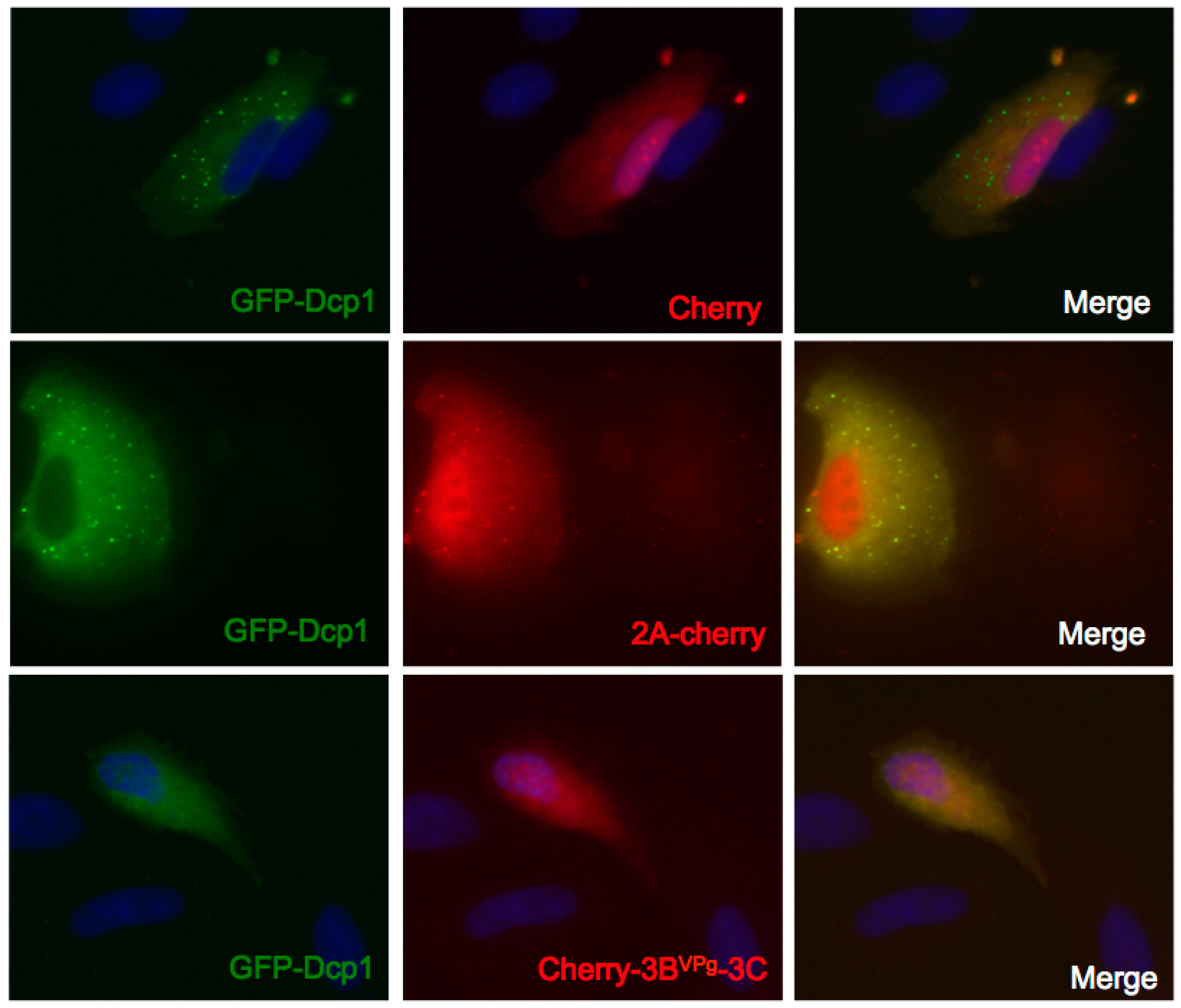
3.5. Expression of 3Cpro, but not 2Apro, Disrupts GFP-Dcp1a Containing PB
4. Discussion
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Kedersha, N.; Stoecklin, G.; Ayodele, M.; Yacono, P.; Lykke-Andersen, J.; Fritzler, M.J.; Scheuner, D.; Kaufman, R.J.; Golan, D.E.; Anderson, P. Stress granules and processing bodies are dynamically linked sites of mRNP remodeling. J. Cell Biol. 2005, 169, 871–884. [Google Scholar] [CrossRef] [PubMed]
- Anderson, P.; Kedersha, N. RNA granules. J. Cell Biol. 2006, 172, 803–808. [Google Scholar] [CrossRef] [PubMed]
- Kedersha, N.; Ivanov, P.; Anderson, P. Stress granules and cell signaling: More than just a passing phase? Trends Biochem. Sci. 2013, 38, 494–506. [Google Scholar] [CrossRef] [PubMed]
- Teixeira, D.; Sheth, U.; Valencia-Sanchez, M.A.; Brengues, M.; Parker, R. Processing bodies require RNA for assembly and contain nontranslating mRNAs. RNA 2005, 11, 371–382. [Google Scholar] [CrossRef] [PubMed]
- Eulalio, A.; Behm-Ansmant, I.; Izaurralde, E. P bodies: At the crossroads of post-transcriptional pathways. Nat. Rev. Mol. Cell Biol. 2007, 8, 9–22. [Google Scholar] [CrossRef] [PubMed]
- Kedersha, N.; Anderson, P. Mammalian stress granules and processing bodies. Methods Enzymol. 2007, 431, 61–81. [Google Scholar] [PubMed]
- Anderson, P.; Kedersha, N.; Ivanov, P. Stress granules, P-bodies and cancer. Biochim. Biophys. Acta 2015, 1849, 861–870. [Google Scholar] [CrossRef] [PubMed]
- Weil, T.T.; Parton, R.M.; Herpers, B.; Soetaert, J.; Veenendaal, T.; Xanthakis, D.; Dobbie, I.M.; Halstead, J.M.; Hayashi, R.; Rabouille, C.; et al. Drosophila patterning is established by differential association of mRNAs with P bodies. Nat. Cell Biol. 2012, 14, 1305–1315. [Google Scholar] [CrossRef] [PubMed]
- Wolozin, B. Physiological protein aggregation run amuck: Stress granules and the genesis of neurodegenerative disease. Discov. Med. 2014, 17, 47–52. [Google Scholar] [PubMed]
- Buchan, J.R. mRNP granules. Assembly, function, and connections with disease. RNA Biol. 2014, 11, 1019–1030. [Google Scholar] [CrossRef] [PubMed]
- Tsai, W.C.; Lloyd, R.E. Cytoplasmic RNA granules and viral infection. Annu. Rev. Virol. 2014, 1, 147–170. [Google Scholar] [CrossRef]
- Reineke, L.C.; Lloyd, R.E. Diversion of stress granules and P-bodies during viral infection. Virology 2013, 436, 255–267. [Google Scholar] [CrossRef] [PubMed]
- Dougherty, J.D.; White, J.P.; Lloyd, R.E. Poliovirus-mediated disruption of cytoplasmic processing bodies. J. Virol. 2011, 85, 64–75. [Google Scholar] [CrossRef] [PubMed]
- White, J.P.; Cardenas, A.M.; Marissen, W.E.; Lloyd, R.E. Inhibition of cytoplasmic mRNA stress granule formation by a viral proteinase. Cell Host Microbe 2007, 2, 295–305. [Google Scholar] [CrossRef] [PubMed]
- White, J.P.; Lloyd, R.E. Poliovirus unlinks TIA1 aggregation and mRNA stress granule formation. J. Virol. 2011, 85, 12442–12454. [Google Scholar] [CrossRef] [PubMed]
- Lloyd, R.E.; Grubman, M.J.; Ehrenfeld, E. Relationship of p220 cleavage during picornavirus infection to 2A proteinase sequencing. J. Virol. 1988, 62, 4216–4223. [Google Scholar] [PubMed]
- Mazroui, R.; Sukarieh, R.; Bordeleau, M.-E.; Kaufman, R.J.; Northcote, P.; Tanaka, J.; Gallouzi, I.; Pelletier, J. Inhibition of ribosome recruitment induces stress granule formation independently of eukaryotic initiation factor 2alpha phosphorylation. Mol. Biol. Cell 2006, 17, 4212–4219. [Google Scholar] [CrossRef] [PubMed]
- Bidet, K.; Dadlani, D.; Garcia-Blanco, M.A. G3BP1, G3BP2 and CAPRIN1 are required for translation of interferon stimulated mRNAs and are targeted by a dengue virus non-coding RNA. PLoS Pathog. 2014, 10, e1004242. [Google Scholar] [CrossRef] [PubMed]
- Matsuki, H.; Takahashi, M.; Higuchi, M.; Makokha, G.N.; Oie, M.; Fujii, M. Both G3BP1 and G3BP2 contribute to stress granule formation. Genes Cells 2013, 18, 135–146. [Google Scholar] [CrossRef] [PubMed]
- Ypma-Wong, M.F.; Dewalt, P.G.; Johnson, V.H.; Lamb, J.G.; Semler, B.L. Protein 3CD is the major poliovirus proteinase responsible for cleavage of the P1 capsid precursor. Virology 1988, 166, 265–270. [Google Scholar] [CrossRef]
- Blair, W.S.; Nguyen, J.H.; Parsley, T.B.; Semler, B.L. Mutations in the poliovirus 3CD proteinase S1-specificity pocket affect substrate recognition and RNA binding. Virology 1996, 218, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Johnston, M.; Geoffroy, M.-C.; Sobala, A.; Hay, R.; Hutvagner, G. HSP90 protein stabilizes unloaded argonaute complexes and microscopic P-bodies in human cells. Mol. Biol. Cell 2010, 21, 1462–1469. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, K.; Minami, M.; Shinozaki, F.; Suzuki, Y.; Abe, K.; Zenno, S.; Matsumoto, S.; Minami, Y. Hsp90 is involved in the formation of P-bodies and stress granules. Biochem. Biophys. Res. Commun. 2011, 407, 720–724. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, Y.; Minami, M.; Suzuki, M.; Abe, K.; Zenno, S.; Tsujimoto, M.; Matsumoto, K.; Minami, Y. The Hsp90 inhibitor geldanamycin abrogates colocalization of eIF4E and eIF4E-transporter into stress granules and association of eIF4E with eIF4G. J. Biol. Chem. 2009, 284, 35597–35604. [Google Scholar] [CrossRef] [PubMed]
- Dougherty, J.D.; Reineke, L.C.; Lloyd, R.E. mRNA decapping enzyme 1a (Dcp1a)-induced translational arrest through protein kinase R (PKR) activation requires the N-terminal enabled vasodilator-stimulated protein homology 1 (EVH1) domain. J. Biol. Chem. 2014, 289, 3936–3949. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Wang, Y.; Lin, L.; Si, X.; Wang, T.; Zhong, X.; Tong, L.; Luan, Y.; Chen, Y.; Li, X.; et al. Protease 2A induces stress granule formation during coxsackievirus B3 and enterovirus 71 infections. Virol. J. 2014, 11, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, T.; Winslow, S.; Sunesson, L.; Hellman, U.; Larsson, C. PKCα Binds G3BP2 and Regulates Stress Granule Formation Following Cellular Stress. PLoS ONE 2012, 7, e35820. [Google Scholar] [CrossRef] [PubMed]
- Reineke, L.C.; Lloyd, R.E. The Stress Granule Protein G3BP1 Recruits Protein Kinase R to Promote Multiple Innate Immune Antiviral Responses. J. Virol. 2015, 89, 2575–2589. [Google Scholar] [CrossRef] [PubMed]
- Reineke, L.C.; Kedersha, N.; Langereis, M.A.; van Kuppeveld, F.J.M.; Lloyd, R.E. Stress granules regulate double-stranded RNA-dependent protein kinase activation through a complex containing G3BP1 and Caprin1. MBio 2015, 6, e02486. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Mollet, S.; Souquere, S.; Le Roy, F.; Ernoult-Lange, M.; Pierron, G.; Dautry, F.; Weil, D. Mitochondria associate with P-bodies and modulate microRNA-mediated RNA interference. J. Biol. Chem. 2011, 286, 24219–24230. [Google Scholar] [CrossRef] [PubMed]
- Ernoult-Lange, M.; Baconnais, S.; Harper, M.; Minshall, N.; Souquere, S.; Boudier, T.; Bénard, M.; Andrey, P.; Pierron, G.; Kress, M.; et al. Multiple binding of repressed mRNAs by the P-body protein Rck/p54. RNA 2012, 18, 1702–1715. [Google Scholar] [CrossRef] [PubMed]
- Minshall, N.; Kress, M.; Weil, D.; Standart, N. Role of p54 RNA helicase activity and its C-terminal domain in translational repression, P-body localization and assembly. Mol. Biol. Cell 2009, 20, 2464–2472. [Google Scholar] [CrossRef] [PubMed]
- Zheng, D.; Ezzeddine, N.; Chen, C.-Y.A.; Zhu, W.; He, X.; Shyu, A.-B. Deadenylation is prerequisite for P-body formation and mRNA decay in mammalian cells. J. Cell Biol. 2008, 182, 89–101. [Google Scholar] [CrossRef] [PubMed]
- Almstead, L.L.; Sarnow, P. Inhibition of U snRNP assembly by a virus-encoded proteinase. Genes Dev. 2007, 21, 1086–1097. [Google Scholar] [CrossRef] [PubMed]
- Parker, R.; Sheth, U. P bodies and the control of mRNA translation and degradation. Mol. Cell 2007, 25, 635–646. [Google Scholar] [CrossRef] [PubMed]
- Pare, J.M.; Tahbaz, N.; López-Orozco, J.; LaPointe, P.; Lasko, P.; Hobman, T.C. Hsp90 regulates the function of argonaute 2 and its recruitment to stress granules and P-bodies. Mol. Biol. Cell 2009, 20, 3273–3284. [Google Scholar] [CrossRef] [PubMed]
- Roe, S.M.; Prodromou, C.; O’Brien, R.; Ladbury, J.E.; Piper, P.W.; Pearl, L.H. Structural basis for inhibition of the Hsp90 molecular chaperone by the antitumor antibiotics radicicol and geldanamycin. J. Med. Chem. 1999, 42, 260–266. [Google Scholar] [CrossRef] [PubMed]
- Joachims, M.; Van Breugel, P.C.; Lloyd, R.E. Cleavage of poly(A)-binding protein by enterovirus proteases concurrent with inhibition of translation in vitro. J. Virol. 1999, 73, 718–727. [Google Scholar] [PubMed]
- Kuyumcu-Martinez, N.M.; Van Eden, M.E.; Younan, P.; Lloyd, R.E. Cleavage of poly(A)-binding protein by poliovirus 3C protease inhibits host cell translation: A novel mechanism for host translation shutoff. Mol. Cell. Biol. 2004, 24, 1779–1790. [Google Scholar] [CrossRef] [PubMed]
- Fabian, M.R.; Cieplak, M.K.; Frank, F.; Morita, M.; Green, J.; Srikumar, T.; Nagar, B.; Yamamoto, T.; Raught, B.; Duchaine, T.F.; et al. miRNA-mediated deadenylation is orchestrated by GW182 through two conserved motifs that interact with CCR4-NOT. Nat. Struct. Mol. Biol. 2011, 18, 1211–1217. [Google Scholar] [CrossRef] [PubMed]
- Fabian, M.R.; Mathonnet, G.; Sundermeier, T.; Mathys, H.; Zipprich, J.T.; Svitkin, Y.V.; Rivas, F.; Jinek, M.; Wohlschlegel, J.; Doudna, J.A.; et al. Mammalian miRNA RISC recruits CAF1 and PABP to affect PABP-dependent deadenylation. Mol. Cell 2009, 35, 868–880. [Google Scholar] [CrossRef] [PubMed]
- Funakoshi, Y.; Doi, Y.; Hosoda, N.; Uchida, N.; Osawa, M.; Shimada, I.; Tsujimoto, M.; Suzuki, T.; Katada, T.; Hoshino, S.-I. Mechanism of mRNA deadenylation: Evidence for a molecular interplay between translation termination factor eRF3 and mRNA deadenylases. Genes Dev. 2007, 21, 3135–3148. [Google Scholar] [CrossRef] [PubMed]
- Rivera, C.I.; Lloyd, R.E. Modulation of enteroviral proteinase cleavage of poly(A)-binding protein (PABP) by conformation and PABP-associated factors. Virology 2008, 375, 59–72. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dougherty, J.D.; Tsai, W.-C.; Lloyd, R.E. Multiple Poliovirus Proteins Repress Cytoplasmic RNA Granules. Viruses 2015, 7, 6127-6140. https://doi.org/10.3390/v7122922
Dougherty JD, Tsai W-C, Lloyd RE. Multiple Poliovirus Proteins Repress Cytoplasmic RNA Granules. Viruses. 2015; 7(12):6127-6140. https://doi.org/10.3390/v7122922
Chicago/Turabian StyleDougherty, Jonathan D., Wei-Chih Tsai, and Richard E. Lloyd. 2015. "Multiple Poliovirus Proteins Repress Cytoplasmic RNA Granules" Viruses 7, no. 12: 6127-6140. https://doi.org/10.3390/v7122922




