Inflammatory Response Associated with West Nile Neuroinvasive Disease: A Systematic Review
Abstract
:1. Introduction
2. Methods
2.1. Eligibility Criteria
2.2. Information Sources and Search Strategy
2.3. Selection and Data Collection Process
2.4. Definitions
2.5. Data Items
- in vitro articles, the following items were additionally considered: the cellular line and viral strain used for the analysis, and viral replication data, including viral replication peak.
- in vivo articles, the following items were additionally considered: the animal species and viral strain used for the analysis, and the sample tested. Where possible, the entity and the timing of the cytokine/chemokine decreasing or increasing levels was specified.
- in human articles, the following items were additionally considered: the cohort dimension and the sample tested. The database was split into a first part regarding the analysis performed on patients’ sera (considering both the acute phase and the late phase of infection) and into a second part regarding the analysis performed on patients’ CSF. The control group used for the analysis in each article and the number of days post-infection on which the analysis was performed were specified.
2.6. Statistical Analysis
3. Results
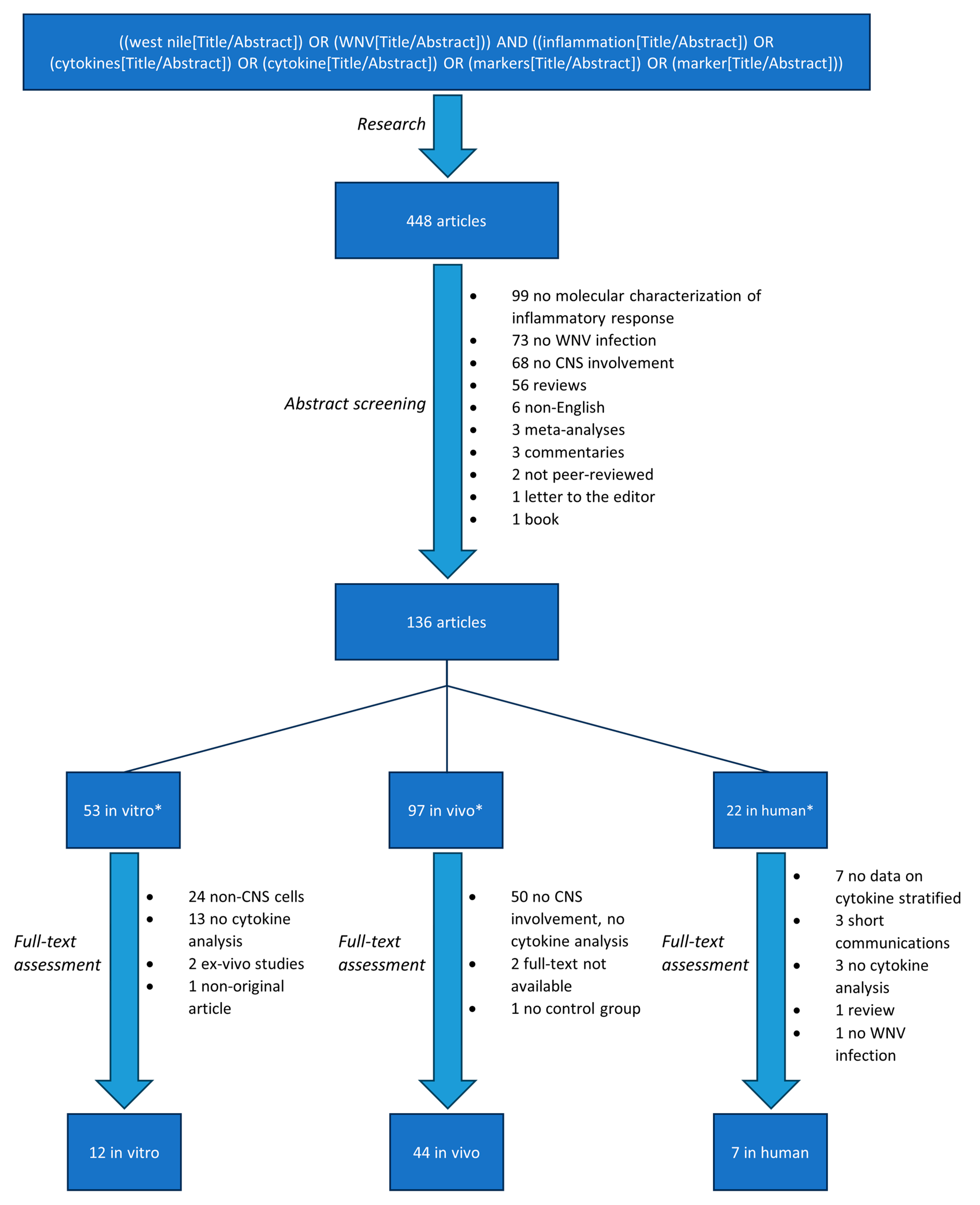
3.1. Search Results and Study Selection
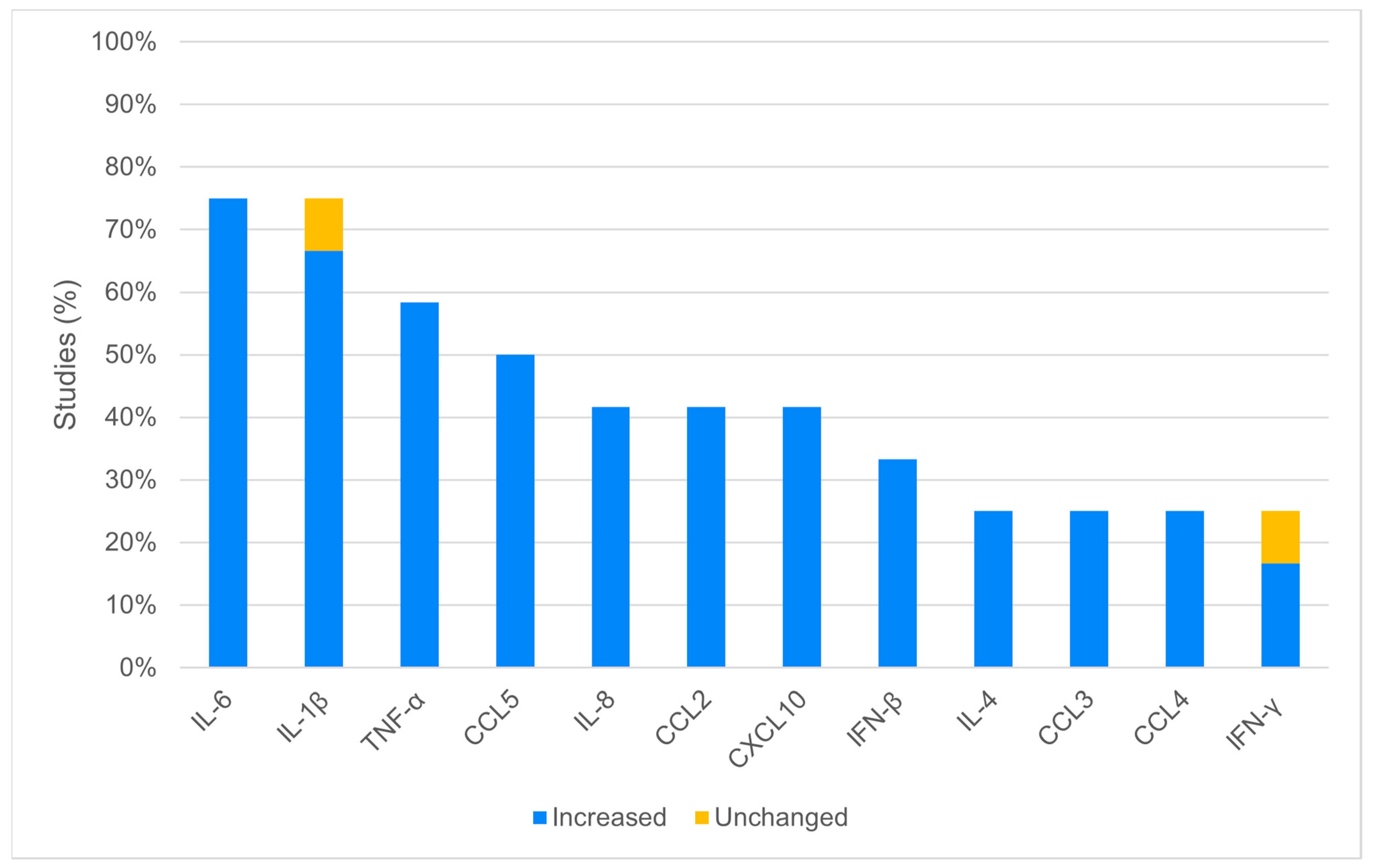
3.2. In Vitro Analysis
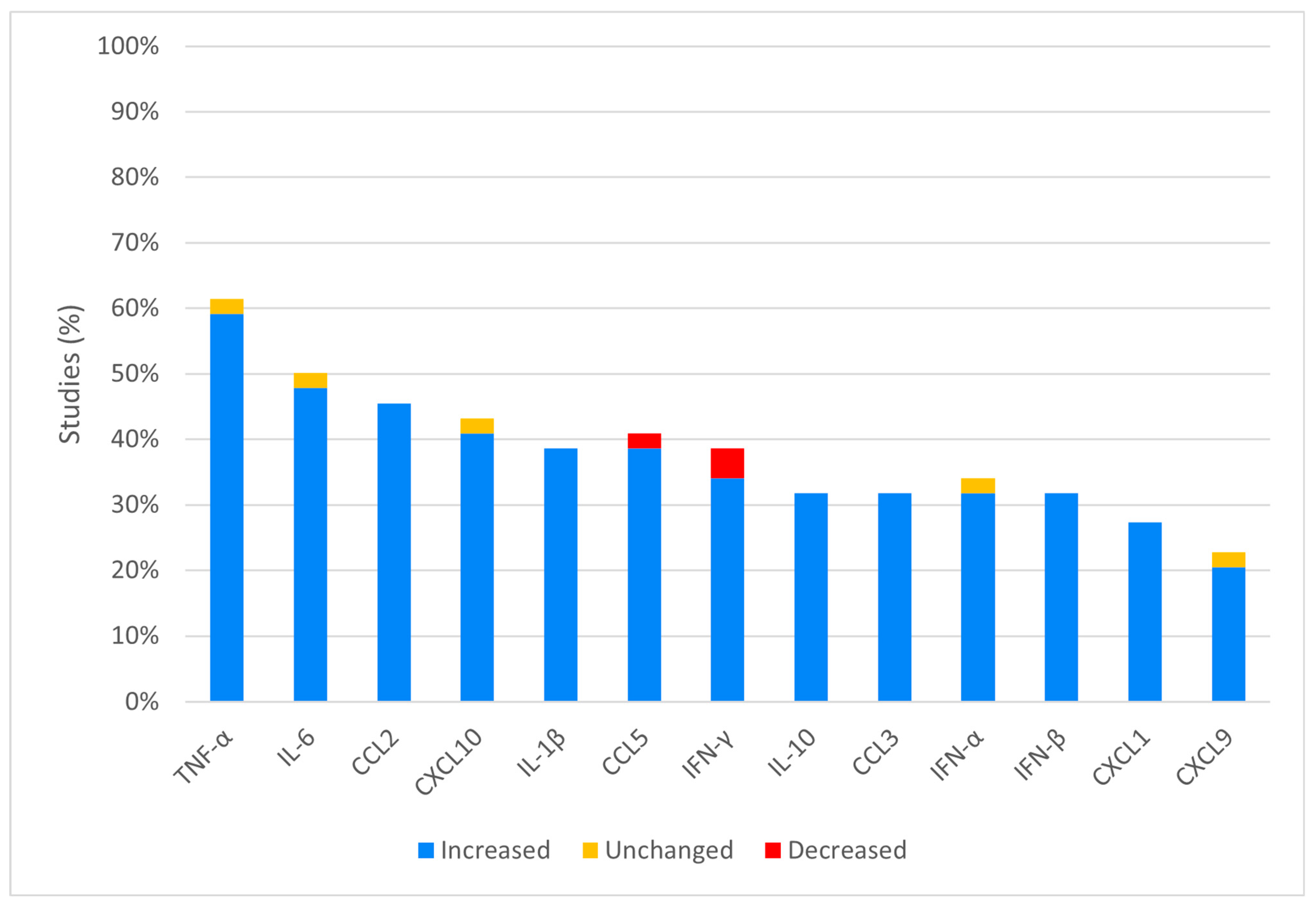
3.3. In Vivo Analysis
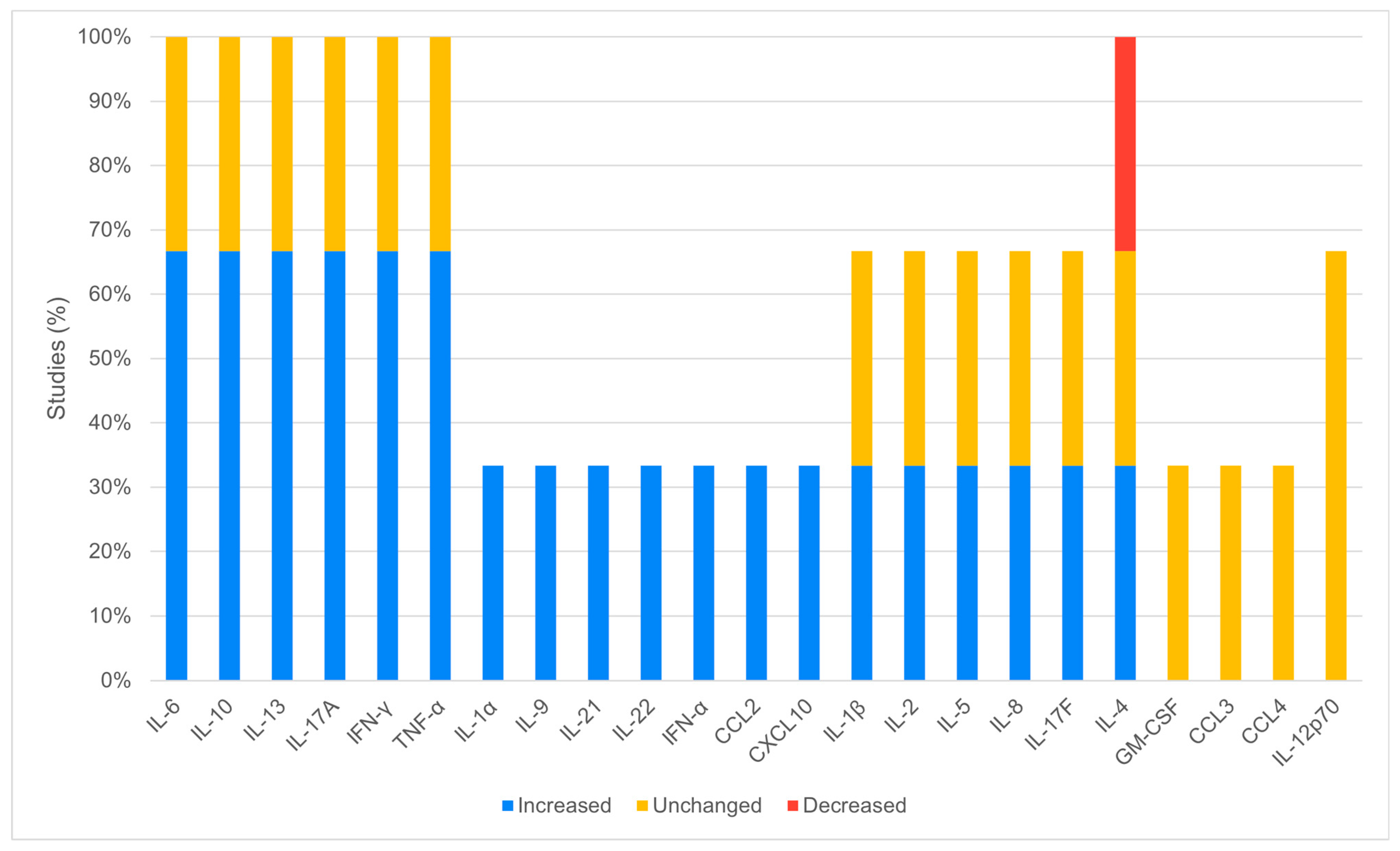
3.4. In Humans Analysis
4. Discussion
4.1. Increased Cytokines and Chemokines
4.2. Decreased Cytokines and Chemokines
4.3. Study Strengths and Limitations
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Habarugira, G.; Suen, W.W.; Hobson-Peters, J.; Hall, R.A.; Bielefeldt-Ohmann, H. West Nile Virus: An Update on Pathobiology, Epidemiology, Diagnostics, Control and “One Health” Implications. Pathogens 2020, 9, 589. [Google Scholar] [CrossRef] [PubMed]
- Kilpatrick, A.M.; LaDeau, S.L.; Marra, P.P. Ecology of West Nile Virus Transmission and its Impact on Birds in the Western Hemisphere. The Auk 2007, 124, 1121–1136. [Google Scholar] [CrossRef]
- Weekly Updates: 2023 West Nile Virus Transmission Season. Available online: https://www.ecdc.europa.eu/en/west-nile-fever/surveillance-and-disease-data/disease-data-ecdc (accessed on 17 July 2023).
- Moirano, G.; Richiardi, L.; Calzolari, M.; Merletti, F.; Maule, M. Recent rapid changes in the spatio-temporal distribution of West Nile Neuro-invasive Disease in Italy. Zoonoses Public Health 2020, 67, 54–61. [Google Scholar] [CrossRef] [PubMed]
- Moirano, G.; Gasparrini, A.; Acquaotta, F.; Fratianni, S.; Merletti, F.; Maule, M.; Richiardi, L. West Nile Virus infection in Northern Italy: Case-crossover study on the short-term effect of climatic parameters. Environ. Res. 2018, 167, 544–549. [Google Scholar] [CrossRef]
- One Health. Available online: https://www.who.int/health-topics/one-health (accessed on 30 July 2023).
- Centers for Disease Control and Prevention (CDC). Intrauterine West Nile virus infection—New York, 2002. MMWR Morb. Mortal. Wkly. Rep. 2002, 51, 1135–1136. [Google Scholar]
- Sejvar, J.J. Clinical Manifestations and Outcomes of West Nile Virus Infection. Viruses 2014, 6, 606–623. [Google Scholar] [CrossRef]
- Yeung, M.W.; Shing, E.; Nelder, M.; Sander, B. Epidemiologic and clinical parameters of West Nile virus infections in humans: A scoping review. BMC Infect. Dis. 2017, 17, 609. [Google Scholar] [CrossRef] [PubMed]
- Carson, P.J.; Borchardt, S.M.; Custer, B.; Prince, H.E.; Dunn-Williams, J.; Winkelman, V.; Tobler, L.; Biggerstaff, B.J.; Lanciotti, R.; Petersen, L.R.; et al. Neuroinvasive Disease and West Nile Virus Infection, North Dakota, USA, 1999–2008. Emerg. Infect. Dis. 2012, 18, 684–686. [Google Scholar] [CrossRef]
- Lindsey, N.P.; Staples, J.E.; Lehman, J.A.; Fischer, M. Medical Risk Factors for Severe West Nile Virus Disease, United States, 2008–2010. Am. J. Trop. Med. Hyg. 2012, 87, 179–184. [Google Scholar] [CrossRef]
- Murray, K.O.; Koers, E.; Baraniuk, S.; Herrington, E.; Carter, H.; Sierra, M.; Kilborn, C.; Arafat, R. Risk factors for encephalitis from West Nile Virus: A matched case-control study using hospitalized controls. Zoonoses Public Health 2009, 56, 370–375. [Google Scholar] [CrossRef]
- Sutinen, J.; Fell, D.B.; Sander, B.; Kulkarni, M.A. Comorbid conditions as risk factors for West Nile neuroinvasive disease in Ontario, Canada: A population-based cohort study. Epidemiol. Infect. 2022, 150, e103. [Google Scholar] [CrossRef] [PubMed]
- Gervais, A.; Rovida, F.; Avanzini, M.A.; Croce, S.; Marchal, A.; Lin, S.-C.; Ferrari, A.; Thorball, C.W.; Constant, O.; Le Voyer, T.; et al. Autoantibodies neutralizing type I IFNs underlie West Nile virus encephalitis in ∼40% of patients. J. Exp. Med. 2023, 220, e20230661. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.-C.; Zhao, F.R.; Janova, H.; Gervais, A.; Rucknagel, S.; Murray, K.O.; Casanova, J.-L.; Diamond, M.S. Blockade of interferon signaling decreases gut barrier integrity and promotes severe West Nile virus disease. Nat. Commun. 2023, 14, 5973. [Google Scholar] [CrossRef] [PubMed]
- Lindsey, N.P.; Staples, J.E.; Lehman, J.A.; Fischer, M. Centers for Disease Control and Prevention (CDC) Surveillance for human West Nile virus disease—United States, 1999–2008. Morb. Mortal. Wkly. Rep. Surveill. Summ. 2010, 59, 1–17. [Google Scholar]
- Yu, A.; Ferenczi, E.; Moussa, K.; Eliott, D.; Matiello, M. Clinical Spectrum of West Nile Virus Neuroinvasive Disease. Neurohospitalist 2020, 10, 43–47. [Google Scholar] [CrossRef]
- Colaneri, M.; Lissandrin, R.; Calia, M.; Bassoli, C.; Seminari, E.; Pavesi, A.; Rovida, F.; Baldanti, F.; Muzzi, A.; Chichino, G.; et al. The WEST Study: A Retrospective and Multicentric Study on the Impact of Steroid Therapy in West Nile Encephalitis. Open Forum Infect. Dis. 2023, 10, ofad092. [Google Scholar] [CrossRef]
- Saiz, J.-C. Animal and Human Vaccines against West Nile Virus. Pathogens 2020, 9, 1073. [Google Scholar] [CrossRef]
- Ulbert, S. West Nile virus vaccines—Current situation and future directions. Hum. Vaccines Immunother. 2019, 15, 2337–2342. [Google Scholar] [CrossRef]
- Ministero della Salute Piano Nazionale di Prevenzione, Sorveglianza e Risposta alle Arbovirosi (PNA), 2020–2025. Available online: https://www.salute.gov.it/portale/documentazione/p6_2_2_1.jsp?lingua=italiano&id=2947 (accessed on 22 May 2022).
- Fiacre, L.; Pagès, N.; Albina, E.; Richardson, J.; Lecollinet, S.; Gonzalez, G. Molecular Determinants of West Nile Virus Virulence and Pathogenesis in Vertebrate and Invertebrate Hosts. Int. J. Mol. Sci. 2020, 21, E9117. [Google Scholar] [CrossRef] [PubMed]
- Zidovec-Lepej, S.; Vilibic-Cavlek, T.; Barbic, L.; Ilic, M.; Savic, V.; Tabain, I.; Ferenc, T.; Grgic, I.; Gorenec, L.; Bogdanic, M.; et al. Antiviral Cytokine Response in Neuroinvasive and Non-Neuroinvasive West Nile Virus Infection. Viruses 2021, 13, 342. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef] [PubMed]
- Murray, K.O.; Garcia, M.N.; Yan, C.; Gorchakov, R. Persistence of Detectable Immunoglobulin M Antibodies Up to 8 Years After Infection with West Nile Virus. Am. J. Trop. Med. Hyg. 2013, 89, 996–1000. [Google Scholar] [CrossRef] [PubMed]
- Kumar, M.; Verma, S.; Nerurkar, V.R. Pro-inflammatory cytokines derived from West Nile virus (WNV)-infected SK-N-SH cells mediate neuroinflammatory markers and neuronal death. J. Neuroinflamm. 2010, 7, 73. [Google Scholar] [CrossRef] [PubMed]
- Constant, O.; Maarifi, G.; Barthelemy, J.; Martin, M.-F.; Tinto, B.; Savini, G.; Van de Perre, P.; Nisole, S.; Simonin, Y.; Salinas, S. Differential effects of Usutu and West Nile viruses on neuroinflammation, immune cell recruitment and blood-brain barrier integrity. Emerg. Microbes Infect. 2023, 12, 2156815. [Google Scholar] [CrossRef] [PubMed]
- Verma, S.; Kumar, M.; Nerurkar, V.R. Cyclooxygenase-2 inhibitor blocks the production of West Nile virus-induced neuroinflammatory markers in astrocytes. J. Gen. Virol. 2011, 92 Pt 3, 507–515. [Google Scholar] [CrossRef] [PubMed]
- Huang, B.; West, N.; Vider, J.; Zhang, P.; Griffiths, R.E.; Wolvetang, E.; Burtonclay, P.; Warrilow, D. Inflammatory responses to a pathogenic West Nile virus strain. BMC Infect. Dis. 2019, 19, 912. [Google Scholar] [CrossRef] [PubMed]
- Bhide, K.; Mochnáčová, E.; Tkáčová, Z.; Petroušková, P.; Kulkarni, A.; Bhide, M. Signaling events evoked by domain III of envelop glycoprotein of tick-borne encephalitis virus and West Nile virus in human brain microvascular endothelial cells. Sci. Rep. 2022, 12, 8863. [Google Scholar] [CrossRef] [PubMed]
- Cheeran, M.C.-J.; Hu, S.; Sheng, W.S.; Rashid, A.; Peterson, P.K.; Lokensgard, J.R. Differential responses of human brain cells to West Nile virus infection. J. Neurovirol. 2005, 11, 512–524. [Google Scholar] [CrossRef]
- Nelson, J.; Ochoa, L.; Villareal, P.; Dunn, T.; Wu, P.; Vargas, G.; Freiberg, A.N. Powassan Virus Induces Structural Changes in Human Neuronal Cells In Vitro and Murine Neurons In Vivo. Pathogens 2022, 11, 1218. [Google Scholar] [CrossRef]
- Zhang, H.; Sun, J.; Ye, J.; Ashraf, U.; Chen, Z.; Zhu, B.; He, W.; Xu, Q.; Wei, Y.; Chen, H.; et al. Quantitative Label-Free Phosphoproteomics Reveals Differentially Regulated Protein Phosphorylation Involved in West Nile Virus-Induced Host Inflammatory Response. J. Proteome Res. 2015, 14, 5157–5168. [Google Scholar] [CrossRef]
- Durrant, D.M.; Robinette, M.L.; Klein, R.S. IL-1R1 is required for dendritic cell-mediated T cell reactivation within the CNS during West Nile virus encephalitis. J. Exp. Med. 2013, 210, 503–516. [Google Scholar] [CrossRef]
- Getts, D.R.; Matsumoto, I.; Müller, M.; Getts, M.T.; Radford, J.; Shrestha, B.; Campbell, I.L.; King, N.J.C. Role of IFN-gamma in an experimental murine model of West Nile virus-induced seizures. J. Neurochem. 2007, 103, 1019–1030. [Google Scholar] [CrossRef] [PubMed]
- Stonedahl, S.; Leser, J.S.; Clarke, P.; Tyler, K.L. Depletion of Microglia in an Ex Vivo Brain Slice Culture Model of West Nile Virus Infection Leads to Increased Viral Titers and Cell Death. Microbiol. Spectr. 2022, 10, e0068522. [Google Scholar] [CrossRef]
- Daniels, B.P.; Holman, D.W.; Cruz-Orengo, L.; Jujjavarapu, H.; Durrant, D.M.; Klein, R.S. Viral pathogen-associated molecular patterns regulate blood-brain barrier integrity via competing innate cytokine signals. mBio 2014, 5, e01476-14. [Google Scholar] [CrossRef]
- Patel, S.; Sinigaglia, A.; Barzon, L.; Fassan, M.; Sparber, F.; LeibundGut-Landmann, S.; Ackermann, M. Role of NS1 and TLR3 in Pathogenesis and Immunity of WNV. Viruses 2019, 11, 603. [Google Scholar] [CrossRef]
- Luo, H.; Winkelmann, E.R.; Zhu, S.; Ru, W.; Mays, E.; Silvas, J.A.; Vollmer, L.L.; Gao, J.; Peng, B.-H.; Bopp, N.E.; et al. Peli1 facilitates virus replication and promotes neuroinflammation during West Nile virus infection. J. Clin. Investig. 2018, 128, 4980–4991. [Google Scholar] [CrossRef] [PubMed]
- Uddin, M.J.; Suen, W.W.; Bosco-Lauth, A.; Hartwig, A.-E.; Hall, R.A.; Bowen, R.A.; Bielefeldt-Ohmann, H. Kinetics of the West Nile virus induced transcripts of selected cytokines and Toll-like receptors in equine peripheral blood mononuclear cells. Vet. Res. 2016, 47, 61. [Google Scholar] [CrossRef] [PubMed]
- Natekar, J.P.; Rothan, H.A.; Arora, K.; Strate, P.G.; Kumar, M. Cellular microRNA-155 Regulates Virus-Induced Inflammatory Response and Protects against Lethal West Nile Virus Infection. Viruses 2019, 12, 9. [Google Scholar] [CrossRef]
- Krause, K.; Azouz, F.; Nakano, E.; Nerurkar, V.R.; Kumar, M. Deletion of Pregnancy Zone Protein and Murinoglobulin-1 Restricts the Pathogenesis of West Nile Virus Infection in Mice. Front. Microbiol. 2019, 10, 259. [Google Scholar] [CrossRef]
- Saxena, V.; Xie, G.; Li, B.; Farris, T.; Welte, T.; Gong, B.; Boor, P.; Wu, P.; Tang, S.-J.; Tesh, R.; et al. A hamster-derived West Nile virus isolate induces persistent renal infection in mice. PLoS Negl. Trop. Dis. 2013, 7, e2275. [Google Scholar] [CrossRef]
- Rothan, H.A.; Arora, K.; Natekar, J.P.; Strate, P.G.; Brinton, M.A.; Kumar, M. Z-DNA-Binding Protein 1 Is Critical for Controlling Virus Replication and Survival in West Nile Virus Encephalitis. Front. Microbiol. 2019, 10, 2089. [Google Scholar] [CrossRef]
- Wang, P.; Bai, F.; Zenewicz, L.A.; Dai, J.; Gate, D.; Cheng, G.; Yang, L.; Qian, F.; Yuan, X.; Montgomery, R.R.; et al. IL-22 signaling contributes to West Nile encephalitis pathogenesis. PLoS ONE 2012, 7, e44153. [Google Scholar] [CrossRef] [PubMed]
- Welte, T.; Aronson, J.; Gong, B.; Rachamallu, A.; Mendell, N.; Tesh, R.; Paessler, S.; Born, W.K.; O’Brien, R.L.; Wang, T. Vγ4+ T cells regulate host immune response to West Nile virus infection. FEMS Immunol. Med. Microbiol. 2011, 63, 183–192. [Google Scholar] [CrossRef] [PubMed]
- Michlmayr, D.; McKimmie, C.S.; Pingen, M.; Haxton, B.; Mansfield, K.; Johnson, N.; Fooks, A.R.; Graham, G.J. Defining the chemokine basis for leukocyte recruitment during viral encephalitis. J. Virol. 2014, 88, 9553–9567. [Google Scholar] [CrossRef] [PubMed]
- Durrant, D.M.; Daniels, B.P.; Klein, R.S. IL-1R1 signaling regulates CXCL12-mediated T cell localization and fate within the central nervous system during West Nile Virus encephalitis. J. Immunol. 2014, 193, 4095–4106. [Google Scholar] [CrossRef] [PubMed]
- Peña, J.; Plante, J.A.; Carillo, A.C.; Roberts, K.K.; Smith, J.K.; Juelich, T.L.; Beasley, D.W.C.; Freiberg, A.N.; Labute, M.X.; Naraghi-Arani, P. Multiplexed digital mRNA profiling of the inflammatory response in the West Nile Swiss Webster mouse model. PLoS Negl. Trop. Dis. 2014, 8, e3216. [Google Scholar] [CrossRef] [PubMed]
- Seitz, S.; Clarke, P.; Tyler, K.L. Pharmacologic Depletion of Microglia Increases Viral Load in the Brain and Enhances Mortality in Murine Models of Flavivirus-Induced Encephalitis. J. Virol. 2018, 92, e00525-18. [Google Scholar] [CrossRef] [PubMed]
- Maximova, O.A.; Sturdevant, D.E.; Kash, J.C.; Kanakabandi, K.; Xiao, Y.; Minai, M.; Moore, I.N.; Taubenberger, J.; Martens, C.; Cohen, J.I.; et al. Virus infection of the CNS disrupts the immune-neural-synaptic axis via induction of pleiotropic gene regulation of host responses. eLife 2021, 10, e62273. [Google Scholar] [CrossRef]
- Clarke, P.; Leser, J.S.; Bowen, R.A.; Tyler, K.L. Virus-induced transcriptional changes in the brain include the differential expression of genes associated with interferon, apoptosis, interleukin 17 receptor A, and glutamate signaling as well as flavivirus-specific upregulation of tRNA synthetases. mBio 2014, 5, e00902–e00914. [Google Scholar] [CrossRef]
- Rosen, S.F.; Soung, A.L.; Yang, W.; Ai, S.; Kanmogne, M.; Davé, V.A.; Artyomov, M.; Magee, J.A.; Klein, R.S. Single-cell RNA transcriptome analysis of CNS immune cells reveals CXCL16/CXCR6 as maintenance factors for tissue-resident T cells that drive synapse elimination. Genome Med. 2022, 14, 108. [Google Scholar] [CrossRef]
- Clarke, P.; Leser, J.S.; Tyler, K.L. Intrinsic Innate Immune Responses Control Viral Growth and Protect against Neuronal Death in an Ex Vivo Model of West Nile Virus-Induced Central Nervous System Disease. J. Virol. 2021, 95, e0083521. [Google Scholar] [CrossRef]
- Quick, E.D.; Seitz, S.; Clarke, P.; Tyler, K.L. Minocycline Has Anti-inflammatory Effects and Reduces Cytotoxicity in an Ex Vivo Spinal Cord Slice Culture Model of West Nile Virus Infection. J. Virol. 2017, 91, e00569-17. [Google Scholar] [CrossRef]
- Quick, E.D.; Leser, J.S.; Clarke, P.; Tyler, K.L. Activation of intrinsic immune responses and microglial phagocytosis in an ex vivo spinal cord slice culture model of West Nile virus infection. J. Virol. 2014, 88, 13005–13014. [Google Scholar] [CrossRef]
- Garber, C.; Vasek, M.J.; Vollmer, L.L.; Sun, T.; Jiang, X.; Klein, R.S. Astrocytes decrease adult neurogenesis during virus-induced memory dysfunction via IL-1. Nat. Immunol. 2018, 19, 151–161. [Google Scholar] [CrossRef]
- Wang, T.; Town, T.; Alexopoulou, L.; Anderson, J.F.; Fikrig, E.; Flavell, R.A. Toll-like receptor 3 mediates West Nile virus entry into the brain causing lethal encephalitis. Nat. Med. 2004, 10, 1366–1373. [Google Scholar] [CrossRef] [PubMed]
- Arjona, A.; Foellmer, H.G.; Town, T.; Leng, L.; McDonald, C.; Wang, T.; Wong, S.J.; Montgomery, R.R.; Fikrig, E.; Bucala, R. Abrogation of macrophage migration inhibitory factor decreases West Nile virus lethality by limiting viral neuroinvasion. J. Clin. Investig. 2007, 117, 3059–3066. [Google Scholar] [CrossRef] [PubMed]
- Lim, S.M.; van den Ham, H.-J.; Oduber, M.; Martina, E.; Zaaraoui-Boutahar, F.; Roose, J.M.; van IJcken, W.F.J.; Osterhaus, A.D.M.E.; Andeweg, A.C.; Koraka, P.; et al. Transcriptomic Analyses Reveal Differential Gene Expression of Immune and Cell Death Pathways in the Brains of Mice Infected with West Nile Virus and Chikungunya Virus. Front. Microbiol. 2017, 8, 1556. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Dai, J.; Bai, F.; Kong, K.-F.; Wong, S.J.; Montgomery, R.R.; Madri, J.A.; Fikrig, E. Matrix metalloproteinase 9 facilitates West Nile virus entry into the brain. J. Virol. 2008, 82, 8978–8985. [Google Scholar] [CrossRef] [PubMed]
- Town, T.; Bai, F.; Wang, T.; Kaplan, A.T.; Qian, F.; Montgomery, R.R.; Anderson, J.F.; Flavell, R.A.; Fikrig, E. Toll-like receptor 7 mitigates lethal West Nile encephalitis via interleukin 23-dependent immune cell infiltration and homing. Immunity 2009, 30, 242–253. [Google Scholar] [CrossRef]
- Bai, F.; Town, T.; Qian, F.; Wang, P.; Kamanaka, M.; Connolly, T.M.; Gate, D.; Montgomery, R.R.; Flavell, R.A.; Fikrig, E. IL-10 signaling blockade controls murine West Nile virus infection. PLoS Pathog. 2009, 5, e1000610. [Google Scholar] [CrossRef]
- Kumar, M.; Roe, K.; O’Connell, M.; Nerurkar, V.R. Induction of virus-specific effector immune cell response limits virus replication and severe disease in mice infected with non-lethal West Nile virus Eg101 strain. J. Neuroinflamm. 2015, 12, 178. [Google Scholar] [CrossRef]
- Ramos, H.J.; Lanteri, M.C.; Blahnik, G.; Negash, A.; Suthar, M.S.; Brassil, M.M.; Sodhi, K.; Treuting, P.M.; Busch, M.P.; Norris, P.J.; et al. IL-1β signaling promotes CNS-intrinsic immune control of West Nile virus infection. PLoS Pathog. 2012, 8, e1003039. [Google Scholar] [CrossRef]
- Kumar, M.; Belcaid, M.; Nerurkar, V.R. Identification of host genes leading to West Nile virus encephalitis in mice brain using RNA-seq analysis. Sci. Rep. 2016, 6, 26350. [Google Scholar] [CrossRef]
- Kumar, M.; Nerurkar, V.R. Integrated analysis of microRNAs and their disease related targets in the brain of mice infected with West Nile virus. Virology 2014, 452–453, 143–151. [Google Scholar] [CrossRef] [PubMed]
- Kumar, M.; Roe, K.; Nerurkar, P.V.; Orillo, B.; Thompson, K.S.; Verma, S.; Nerurkar, V.R. Reduced immune cell infiltration and increased pro-inflammatory mediators in the brain of Type 2 diabetic mouse model infected with West Nile virus. J. Neuroinflamm. 2014, 11, 80. [Google Scholar] [CrossRef] [PubMed]
- Kumar, M.; Roe, K.; Orillo, B.; Muruve, D.A.; Nerurkar, V.R.; Gale, M.; Verma, S. Inflammasome adaptor protein Apoptosis-associated speck-like protein containing CARD (ASC) is critical for the immune response and survival in west Nile virus encephalitis. J. Virol. 2013, 87, 3655–3667. [Google Scholar] [CrossRef] [PubMed]
- Sabouri, A.H.; Marcondes, M.C.G.; Flynn, C.; Berger, M.; Xiao, N.; Fox, H.S.; Sarvetnick, N.E. TLR signaling controls lethal encephalitis in WNV-infected brain. Brain Res. 2014, 1574, 84–95. [Google Scholar] [CrossRef] [PubMed]
- Daffis, S.; Samuel, M.A.; Suthar, M.S.; Keller, B.C.; Gale, M.; Diamond, M.S. Interferon regulatory factor IRF-7 induces the antiviral alpha interferon response and protects against lethal West Nile virus infection. J. Virol. 2008, 82, 8465–8475. [Google Scholar] [CrossRef] [PubMed]
- Paul, A.M.; Acharya, D.; Le, L.; Wang, P.; Stokic, D.S.; Leis, A.A.; Alexopoulou, L.; Town, T.; Flavell, R.A.; Fikrig, E.; et al. TLR8 Couples SOCS-1 and Restrains TLR7-Mediated Antiviral Immunity, Exacerbating West Nile Virus Infection in Mice. J. Immunol. 2016, 197, 4425–4435. [Google Scholar] [CrossRef] [PubMed]
- Xie, G.; Luo, H.; Pang, L.; Peng, B.-H.; Winkelmann, E.; McGruder, B.; Hesse, J.; Whiteman, M.; Campbell, G.; Milligan, G.N.; et al. Dysregulation of Toll-Like Receptor 7 Compromises Innate and Adaptive T Cell Responses and Host Resistance to an Attenuated West Nile Virus Infection in Old Mice. J. Virol. 2016, 90, 1333–1344. [Google Scholar] [CrossRef] [PubMed]
- Bourgeois, M.A.; Denslow, N.D.; Seino, K.S.; Barber, D.S.; Long, M.T. Gene expression analysis in the thalamus and cerebrum of horses experimentally infected with West Nile virus. PLoS ONE 2011, 6, e24371. [Google Scholar] [CrossRef]
- Suen, W.W.; Uddin, M.J.; Prow, N.A.; Bowen, R.A.; Hall, R.A.; Bielefeldt-Ohmann, H. Tissue-specific transcription profile of cytokine and chemokine genes associated with flavivirus control and non-lethal neuropathogenesis in rabbits. Virology 2016, 494, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Acharya, D.; Wang, P.; Paul, A.M.; Dai, J.; Gate, D.; Lowery, J.E.; Stokic, D.S.; Leis, A.A.; Flavell, R.A.; Town, T.; et al. Interleukin-17A Promotes CD8+ T Cell Cytotoxicity To Facilitate West Nile Virus Clearance. J. Virol. 2017, 91, e01529-16. [Google Scholar] [CrossRef] [PubMed]
- Lino, A.; Erickson, T.A.; Nolan, M.S.; Murray, K.O.; Ronca, S.E. A Preliminary Study of Proinflammatory Cytokines and Depression Following West Nile Virus Infection. Pathogens 2022, 11, 650. [Google Scholar] [CrossRef] [PubMed]
- Constant, O.; Barthelemy, J.; Nagy, A.; Salinas, S.; Simonin, Y. West Nile Virus Neuroinfection in Humans: Peripheral Biomarkers of Neuroinflammation and Neuronal Damage. Viruses 2022, 14, 756. [Google Scholar] [CrossRef] [PubMed]
- Qian, F.; Thakar, J.; Yuan, X.; Nolan, M.; Murray, K.O.; Lee, W.T.; Wong, S.J.; Meng, H.; Fikrig, E.; Kleinstein, S.H.; et al. Immune markers associated with host susceptibility to infection with West Nile virus. Viral Immunol. 2014, 27, 39–47. [Google Scholar] [CrossRef] [PubMed]
- Qian, F.; Goel, G.; Meng, H.; Wang, X.; You, F.; Devine, L.; Raddassi, K.; Garcia, M.N.; Murray, K.O.; Bolen, C.R.; et al. Systems immunology reveals markers of susceptibility to West Nile virus infection. Clin. Vaccine Immunol. 2015, 22, 6–16. [Google Scholar] [CrossRef] [PubMed]
- Normandin, E.; Holroyd, K.B.; Collens, S.I.; Shaw, B.M.; Siddle, K.J.; Adams, G.; Rudy, M.; Solomon, I.H.; Anahtar, M.N.; Lemieux, J.E.; et al. Intrathecal inflammatory responses in the absence of SARS-CoV-2 nucleic acid in the CSF of COVID-19 hospitalized patients. J. Neurol. Sci. 2021, 430, 120023. [Google Scholar] [CrossRef] [PubMed]
- Leis, A.A.; Grill, M.F.; Goodman, B.P.; Sadiq, S.B.; Sinclair, D.J.; Vig, P.J.S.; Bai, F. Tumor Necrosis Factor-Alpha Signaling May Contribute to Chronic West Nile Virus Post-infectious Proinflammatory State. Front. Med. 2020, 7, 164. [Google Scholar] [CrossRef]
- Quicke, K.M.; Suthar, M.S. The innate immune playbook for restricting West Nile virus infection. Viruses 2013, 5, 2643–2658. [Google Scholar] [CrossRef]
- Montazersaheb, S.; Hosseiniyan Khatibi, S.M.; Hejazi, M.S.; Tarhriz, V.; Farjami, A.; Ghasemian Sorbeni, F.; Farahzadi, R.; Ghasemnejad, T. COVID-19 infection: An overview on cytokine storm and related interventions. Virol. J. 2022, 19, 92. [Google Scholar] [CrossRef] [PubMed]
- Sejvar, J.J. The long-term outcomes of human West Nile virus infection. Clin. Infect. Dis. 2007, 44, 1617–1624. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Chu, D.; Kalantar-Zadeh, K.; George, J.; Young, H.A.; Liu, G. Cytokines: From Clinical Significance to Quantification. Adv. Sci. 2021, 8, e2004433. [Google Scholar] [CrossRef] [PubMed]
- Mootoo, A.; Stylianou, E.; Arias, M.A.; Reljic, R. TNF-alpha in tuberculosis: A cytokine with a split personality. Inflamm. Allergy Drug Targets 2009, 8, 53–62. [Google Scholar] [CrossRef]
- Lan, T.; Chang, L.; Wu, L.; Yuan, Y.-F. IL-6 Plays a Crucial Role in HBV Infection. J. Clin. Transl. Hepatol. 2015, 3, 271–276. [Google Scholar] [CrossRef]
- Potere, N.; Batticciotto, A.; Vecchié, A.; Porreca, E.; Cappelli, A.; Abbate, A.; Dentali, F.; Bonaventura, A. The role of IL-6 and IL-6 blockade in COVID-19. Expert Rev. Clin. Immunol. 2021, 17, 601–618. [Google Scholar] [CrossRef]
- Gogos, C.A.; Drosou, E.; Bassaris, H.P.; Skoutelis, A. Pro- versus anti-inflammatory cytokine profile in patients with severe sepsis: A marker for prognosis and future therapeutic options. J. Infect. Dis. 2000, 181, 176–180. [Google Scholar] [CrossRef]
- Lee, A.J.; Ashkar, A.A. The Dual Nature of Type I and Type II Interferons. Front. Immunol. 2018, 9, 2061. [Google Scholar] [CrossRef]
- Quiros-Roldan, E.; Sottini, A.; Signorini, S.G.; Serana, F.; Tiecco, G.; Imberti, L. Autoantibodies to Interferons in Infectious Diseases. Viruses 2023, 15, 1215. [Google Scholar] [CrossRef]
- Sokol, C.L.; Luster, A.D. The chemokine system in innate immunity. Cold Spring Harb. Perspect. Biol. 2015, 7, a016303. [Google Scholar] [CrossRef] [PubMed]
- Gudowska-Sawczuk, M.; Mroczko, B. What Is Currently Known about the Role of CXCL10 in SARS-CoV-2 Infection? Int. J. Mol. Sci. 2022, 23, 3673. [Google Scholar] [CrossRef] [PubMed]
- Ranjbar, M.; Rahimi, A.; Baghernejadan, Z.; Ghorbani, A.; Khorramdelazad, H. Role of CCL2/CCR2 axis in the pathogenesis of COVID-19 and possible Treatments: All options on the Table. Int. Immunopharmacol. 2022, 113 Pt A, 109325. [Google Scholar] [CrossRef]
- Sakthivel, S.K.; Singh, U.P.; Singh, S.; Taub, D.D.; Igietseme, J.U.; Lillard, J.W. CCL5 regulation of mucosal chlamydial immunity and infection. BMC Microbiol. 2008, 8, 136. [Google Scholar] [CrossRef]
- Senft, A.P.; Taylor, R.H.; Lei, W.; Campbell, S.A.; Tipper, J.L.; Martinez, M.J.; Witt, T.L.; Clay, C.C.; Harrod, K.S. Respiratory syncytial virus impairs macrophage IFN-alpha/beta- and IFN-gamma-stimulated transcription by distinct mechanisms. Am. J. Respir. Cell Mol. Biol. 2010, 42, 404–414. [Google Scholar] [CrossRef] [PubMed]
- Graziosi, C.; Gantt, K.R.; Vaccarezza, M.; Demarest, J.F.; Daucher, M.; Saag, M.S.; Shaw, G.M.; Quinn, T.C.; Cohen, O.J.; Welbon, C.C.; et al. Kinetics of cytokine expression during primary human immunodeficiency virus type 1 infection. Proc. Natl. Acad. Sci. USA 1996, 93, 4386–4391. [Google Scholar] [CrossRef] [PubMed]
- Yoshikawa, T.; Kato, Y.; Ihira, M.; Nishimura, N.; Ozaki, T.; Kumagai, T.; Asano, Y. Kinetics of cytokine and chemokine responses in patients with primary human herpesvirus 6 infection. J. Clin. Virol. 2011, 50, 65–68. [Google Scholar] [CrossRef] [PubMed]
- Rhodes, S.G.; Palmer, N.; Graham, S.P.; Bianco, A.E.; Hewinson, R.G.; Vordermeier, H.M. Distinct response kinetics of gamma interferon and interleukin-4 in bovine tuberculosis. Infect. Immun. 2000, 68, 5393–5400. [Google Scholar] [CrossRef]
- Ronca, S.E.; Ruff, J.C.; Murray, K.O. A 20-year historical review of West Nile virus since its initial emergence in North America: Has West Nile virus become a neglected tropical disease? PLoS Negl. Trop. Dis. 2021, 15, e0009190. [Google Scholar] [CrossRef]
- ClinicalTrials.gov. Search for: West Nile. Card Results. Available online: https://clinicaltrials.gov/search?cond=west%20nile&page=1 (accessed on 19 October 2023).
- Gottlieb, R.L.; Vaca, C.E.; Paredes, R.; Mera, J.; Webb, B.J.; Perez, G.; Oguchi, G.; Ryan, P.; Nielsen, B.U.; Brown, M.; et al. Early Remdesivir to Prevent Progression to Severe COVID-19 in Outpatients. N. Engl. J. Med. 2022, 386, 305–315. [Google Scholar] [CrossRef]
| Articles In Vitro | |||||||
| Author | Ref. N | Cellular Line or Technique | WNV Strain | Increased Cytokines | Decreased Cytokines | Unchanged Cytokines | |
| Kumar M | [26] | Transformed human neuroblastoma (SK-N-SH) | WNV NY99 | TNF-α, IL-1β, IL-6, IL-8 | NA | IL-18 | |
| Constant O | [27] | Human brain-like endothelial cells (hBLEC) | WNV 3125/France/2018 | TNF-α, IL-1β, IL-4, IL-6, IL-8, IL-17, CCL2, CCL3, CCL4, CCL5, CXCL10, IFN-α, IFN-β, GM-CSF | NA | NA | |
| Verma S | [28] | Human brain cortical astrocytes (HBCA) | WNV NY99 | IL-1β, IL-6, IL-8 | NA | NA | |
| Huang B | [29] | Transformed human neuroblastoma (SK-N-SH) | WNVKUN (MRM16) + NSW2012 | IL-2, CCL2, CCL5, CXCL10, IFN-β | NA | NA | |
| Bhide K | [30] | Human brain microvascular endothelial cells (HBMECs) | WNV Goshawk | IL-1β, IL-6, CCL2, CCL5, CXCL10 | NA | NA | |
| Cheeran MC | [31] | Human glial cell cultures (microglia/astrocytes) | WNV NY99 | TNF-α, IL-6, IL-8, CCL2, CCL3, CCL4, CCL5, CXCL10 | NA | IL-1β, IL-10, IFN-α, IFN-γ | |
| Nelson J | [32] | Human neural stem cell (hNSC)-derived neuron/astrocyte co-cultures | WNV NY99 | TNF-α, IL-1β, IL-1ra, IL-2, IL-4, IL-5, IL-6, IL-7, IL-8, IL-9, IL-12, IL-15, IL-17, IL-18, CCL3, CCL4, CCL5, CXCL9, CXCL10, IFN-γ, GM-CSF, VEGF, FGF, PDGF-BB, Eotaxin | NA | NA | |
| Zhang H | [33] | Human glial cell line (U251) | WNV NY99 | TNF-α, IL-1β, IL-6 | NA | NA | |
| Durrant DM | [34] | Mice CNS tissue | WNV 3000.0259 | IL-1α, IL-1β | NA | NA | |
| Getts DR | [35] | Mice CNS tissue | WNV Sarafend | TNF-α, IL-6 | NA | NA | |
| Stonedahl S | [36] | Mice brain slice cultures | WNV TX02 | Il-4, IL-6, IL-10, CCL2, CCL5, IFN-β, IFN-γ | NA | NA | |
| Daniels BP | [37] | Brain microvascular endothelial cells (BMECs) | WNV 3000.0259 | TNF-α, IL-1β, IFN-β | NA | NA | |
| Articles in vivo | |||||||
| Author | Ref. N | Species | WNV Strain | Increased Cytokines | Decreased Cytokines | Unchanged Cytokines | |
| Patel S | [38] | Mouse | WNV ITA09 | TNF-α, IFN-γ | NA | NA | |
| Luo H | [39] | Mouse | WNV NY99 | TNF-α, IL-12, CCL2, CCL4, CXCL10 | NA | NA | |
| Jasim Uddin M | [40] | Horse | WNV NSW2011 | IFN-α, CXCL10, ISG15, IRF7, IL-22 | IFN-γ | NA | |
| Natekar J P | [41] | Mouse | WNV NY99, WNV Eg101 | IFN-α | NA | NA | |
| Constant O | [27] | Mouse | WNV-3125/France/2018 | TNF-α, CCL5 | NA | IL-1β | |
| Krause K | [42] | Mouse | WNV NY99 | TNF-α, IFN-α, IFN-γ, IFN-β, IL-12p40, IL-6, CCL2, CCL4, CXCL10, IL-10 (blood sample), IL-1β, IL-1α, IL-5, GM-CSF, CCL5, CXCL2, CXCL9, IL-13, G-CSF, M-CSF, CXCL1, CCL3, IL-12p70, Eotaxin | NA | IL-10 (CNS sample) | |
| Saxena V | [43] | Mouse | WNV H8912 | IFN-β, IL-10, IL-1β | NA | TNF-α, IFN-α, IL-6 | |
| Rothan H A | [44] | Mouse | WNV NY99, WNV Eg101 | TNF-α, IFN-α, IFN-γ, IL-6, CCL2, CXCL10, IL-1α, CXCL9 | NA | IL-5, G-CSF | |
| Wang P | [45] | Mouse | WNV-2741 | TNF-α, IFN-β, IL-6, CXCL1, CXCL5, IL-22 | NA | NA | |
| Welte T | [46] | Mouse | WNV NY99 | IL-10, IL-17 | TGF-β | NA | |
| Michlmayr D | [47] | Mouse | WNV NY99 | TNF-α, IFN-α, IFN-β, IL-6, CCL2, CCL4, CCL10, TGF-β, IRF7, IL-1β, CCL5, CXCL2, CXCL9, CCL11, CXCL1, CCL7, CCL8, CCL3, RIG-I | NA | CXCL12 | |
| Durrant DM | [48] | Mouse | WNV NY99 | CCL2, CCL4, CXCL10, IL-1β, CCL5, CXCL9, CXCL12, CCL7, CCL3 | NA | NA | |
| Peña J | [49] | Mouse | WNV NY99 | IL-12p40, IL-12, IL-6, CCL2, IL-10, IL-1α, GM-CSF, CCL5, IL-13, G-CSF, CCL11, CXCL1 | IFN-γ, IL-2 | NA | |
| Durrant DM | [34] | Mouse | WNV NY99 | IFN-α, IFN-β, IL-1β, IL-1α | NA | NA | |
| Seitz S | [50] | Mouse | WNV NY99 | IFN-γ, CCL2, CXCL10, CCL5, CXCL9, CCL7, CCL3 | NA | NA | |
| Maximova OA | [51] | Non-human primates | WNV NY99 | TNF-α, IFN-γ, CCL2, CXCL10, CXCL11, CXCL8, CCL5, CXCL1, CXCL13, CCL8, CCL3 | NA | NA | |
| Clarke P | [52] | Mouse | WNV NY99 | TNF-α, CCL2, CXCL10, CXCL11, CCL5, CXCL9, CXCL13, CCL7, CCL3, CCL12 | NA | NA | |
| Rosen SF | [53] | Mouse | WNV-NS5-E218A | CXCL16 | NA | NA | |
| Getts DR | [35] | Mouse | NA | TNF-α, IL-6 | NA | NA | |
| Clarke P | [54] | Mouse | WNV NY99 | TNF-α, IFN-β, IL-6, CCL2, CXCL10, IL-4, IL-10, IFIT1, IRF1, CCL5 | NA | NA | |
| Quick ED | [55] | Mouse | WNV NY99 | TNF-α, IFN-α, IL-6, CCL2, CXCL10, IL-4, IL-10, IRF1, IL-1β, IL-7, CCL5, IL-13, CCL7, CCL3 | NA | NA | |
| Quick ED | [56] | Mouse | WNV NY99 | TNF-α, IL-6, CCL2, CXCL10, CCL5, CXCL1, CCL3, TRAIL | NA | NA | |
| Garber C | [57] | Mouse | WNV NY99, WNV-NS5-E218A | IL-1β | NA | NA | |
| Wang T | [58] | Mouse | WNV-2741 | TNF-α, IFN-α, IFN-β, IL-12, IL-6 | NA | NA | |
| Arjona A | [59] | Mouse | WNV-2741 | TNF-α, IFN-α, IL-12, IL-6, IL-1β, MIF | NA | NA | |
| Lim SM | [60] | Mouse | WNV NY99 | TNF-α, IFN-γ, IL-12b, IL-12, IL-6, CCL2, CCL3, CCL4, CCL5, CCL8, CXCL10, IL-10, IL-1β, CXCL2, CXCL9, CCL11, CXCL1, CXCL13, CCL25 | CXCL12 | IL-18, IL-23, IL-17, CCL1, CCL20, CCL24 | |
| Daniels BP | [37] | Mouse | NY-2000 | TNF-α, IFN-β | NA | NA | |
| Wang P | [61] | Mouse | NA | TNF-α, IFN-α, IL-6 | NA | NA | |
| Town T | [62] | Mouse | WNV-2741 | IL-12b, IL-12p40, IL-23 | NA | NA | |
| Bai F | [63] | Mouse | WNV-2741 | IFN-γ, IL-10 | NA | NA | |
| Kumar M | [64] | Mouse | Eg101, WNV NY99 | TNF-α, IFN-γ, IL-6, CCL2, CCL4, CXCL10, IL-10, IL-1β, IL-1α, IL-5, IL-7, GM-CSF, CCL5, CXCL2, CXCL9, IL-15, IL-13, IL-17, G-CSF, M-CSF, CCL11, CXCL1, CXCL5, CCL3 | NA | NA | |
| Ramos HJ | [65] | Mouse | TX 2002-HC | IFN-β, IL-6, CCL2, IL-1β | NA | NA | |
| Kumar M | [66] | Mouse | Eg101, WNV NY99 | CCL4, CXCL10, IL-1α, IL-1β, IL-7, CCL5, CXCL1, CXCL12, CXCL13, CCL7, CCL8, CCL3, CCL19 | NA | NA | |
| Kumar M | [67] | Mouse | WNV NY99 | TNF-α, IFN-γ, CCL2, CXCL10, IL-10, IL-1β, CCL5, CXCL1, CCL3 | NA | NA | |
| Kumar M | [68] | Mouse | WNV NY99 | TNF-α, IFN-γ, CCL2, CXCL10, IL-1β, CCL5, CXCL9, G-CSF, CXCL1 | NA | NA | |
| Kumar M | [69] | Mouse | WNV NY99 | TNF-α, IFN-γ, IL-6, CCL2, IL-1β, CCL5, CXCL1, CCL3 | NA | NA | |
| Sabouri AH | [70] | Mouse | Eg101 | TNF-α, IFN-α, IL-6, CCL2, CCL3 | CCL5 | CCL4, CXCL10, CXCL9 | |
| Daffis S | [71] | Mouse | WNV 3004.19.00 | IFN-α, IFN-β | NA | NA | |
| Stonedahl S | [36] | Mouse | TX02 | IFN-γ, IFN-β, IL-6, CCL2, IL-4, IL-10, MX1, IFIT1, IRF1, CCL5 | NA | NA | |
| Paul AM | [72] | Mouse | WNV-2741 | IFN-α, IFN-β, IRF7 | NA | NA | |
| Xie G | [73] | Mouse | NS4B-P38G | TNF-α, IL-12p40, IL-10, IL-1β, IL-17, IFN-γ, IL-6 | NA | NA | |
| Bourgeois MA | [74] | Horse | WNV NY99 | IL-15, IL-9, IL-22 | NA | NA | |
| Suen WW | [75] | Rabbit | NSW2011, TX8667 | TNF-α, IFN-γ, IFN-β, IL-6, CXCL10, IL-4, IL-10 | NA | NA | |
| Acharya D | [76] | Mouse | WNV-2741 | TNF-α, IFN-γ, IFN-α, IFN-β, IL-12p40, IL-6, IL-10 | NA | NA | |
| Articles in humans | |||||||
| Author | Ref. N | Number of Patients | Sample and Timing of Cytokine Analysis | Increased Cytokines | Decreased Cytokines | Unchanged Cytokines | Reference Group |
| Lino A | [77] | 29 | Serum, late phase | G-CSF | NA | NA | Subjects with a history of asymptomatic WNV infection |
| Constant O | [78] | 58 | Serum, acute phase CSF, acute phase | Serum: IL-1α, IL-1β, IL-4, IL-6, IL-8, IL-10, IL-13, IL-17A, IFNα, IFNγ, CCL2, CXCL10 CSF: IFNγ | NA | Serum: IL-12p70, TNFα, GM-CSF, CCL3, CCL4 CSF: IL-1α, IL-1β, IL-4, IL-6, IL-8, IL-10, IL-12p70, IL-13, IL-17A, IFNα, TNFα, GM-CSF, CCL2, CCL3, CCL4, CXCL10 | Serum: healthy individuals CSF: meningitis vs. encephalitis |
| Zidovec-Lepej S | [23] | 60 | Serum, acute phase CSF, acute phase | Serum: IL-2, IL-5, IL-6, IL-9, IL-10, IL-13, IL-17A, IL-17F, IL-21, IL-22, IFNγ, TNFα CSF: IL-6 | Serum: IL-4 CSF: IL-2, IL-4, IL-5, IL-17A, IL-17F, IL-21, TNFα | CSF: IL-9, IL-10, IL-13, IL-22, IFNγ | Serum: healthy individuals CSF: paired serum |
| Qian F | [79] | 59 | Serum, late phase | NA | IL-4 | NA | Subjects with a history of asymptomatic WNV infection |
| Qian F | [80] | 49 | Serum, late phase | NA | IL-1β, CXCL10 | NA | Subjects with a history of asymptomatic WNV infection |
| Normandin E | [81] | 4 | CSF, acute phase | IL-6, IL-16 | CCL4 | IL-7, IL-8, IL-15, CCL2, CCL22 | Healthy individuals |
| Leis AA | [82] | 1 | Serum, acute phase | TNFα | NA | IL-1β, IL-2, IL-4, IL-5, IL-6, IL-8, IL-10, IL-12p70, IL-13, IL-17A, IL-17F, IFNγ | Cytokine reference levels |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pavesi, A.; Tiecco, G.; Rossi, L.; Sforza, A.; Ciccarone, A.; Compostella, F.; Lovatti, S.; Tomasoni, L.R.; Castelli, F.; Quiros-Roldan, E. Inflammatory Response Associated with West Nile Neuroinvasive Disease: A Systematic Review. Viruses 2024, 16, 383. https://doi.org/10.3390/v16030383
Pavesi A, Tiecco G, Rossi L, Sforza A, Ciccarone A, Compostella F, Lovatti S, Tomasoni LR, Castelli F, Quiros-Roldan E. Inflammatory Response Associated with West Nile Neuroinvasive Disease: A Systematic Review. Viruses. 2024; 16(3):383. https://doi.org/10.3390/v16030383
Chicago/Turabian StylePavesi, Alessandro, Giorgio Tiecco, Luca Rossi, Anita Sforza, Andrea Ciccarone, Federico Compostella, Sofia Lovatti, Lina Rachele Tomasoni, Francesco Castelli, and Eugenia Quiros-Roldan. 2024. "Inflammatory Response Associated with West Nile Neuroinvasive Disease: A Systematic Review" Viruses 16, no. 3: 383. https://doi.org/10.3390/v16030383





