Detection of Zika Virus in Aedes aegypti and Aedes albopictus Mosquitoes Collected in Urban Forest Fragments in the Brazilian Amazon
Abstract
:1. Introduction
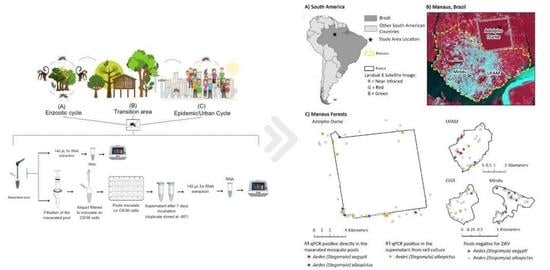
2. Materials and Methods
3. Results and Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Diallo, D.; Sall, A.A.; Diagne, C.T.; Faye, O.; Faye, O.; Ba, Y.; Hanley, K.A.; Buenemann, M.; Weaver, S.C.; Diallo, M. Zika virus emergence in mosquitoes in Southeastern Senegal, 2011. PLoS ONE 2014, 9, e109442. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dick, G.W.A.; Kitchen, S.F.; Haddow, A.J. Zika virus (I). Isolation and serological specificity. Trans. R. Soc. Trop. Med. Hyg. 1952, 46, 509–520. [Google Scholar] [CrossRef] [PubMed]
- Duffy, M.R.; Chen, T.-H.; Hancock, W.T.; Powers, A.M.; Kool, J.L.; Lanciotti, R.S.; Pretrick, M.; Marfel, M.; Holzbauer, S.; Dubray, C.; et al. Zika virus outbreak on Yap Island, Federated States of Micronesia. N. Engl. J. Med. 2009, 360, 2536–2543. [Google Scholar] [CrossRef]
- Zanluca, C.; Melo, V.C.A.D.; Mosimann, A.L.P.; Santos, G.I.V.D.; Santos, C.N.D.D.; Luz, K. First report of autochthonous transmission of Zika virus in Brazil. Mem. Inst. Oswaldo. Cruz. 2015, 110, 569–572. [Google Scholar] [CrossRef]
- Organización Panamericana de la Salud; OPAS/Organización Mundial de la Salud, O. Actualización Epidemiológica: Síndrome Neurológico, Anomalías Congénitas, e Infección Por Virus Zika; Organización Panamericana de la Salud: Washington, DC, USA, 2016. [Google Scholar]
- World Health Organization, W. Zika Strategic Response Framework & Joint Operactions Plan; WHO: Geneva, Switzerland, 2016. [Google Scholar]
- Fernandes, R.S.; O’connor, O.; Bersot, M.I.L.; Girault, D.; Dokunengo, M.R.; Pocquet, N.; Dupont-Rouzeyrol, M.; Lourenço-De-oliveira, R. Vector competence of Aedes aegypti, Aedes albopictus and Culex quinquefasciatus from Brazil and New Caledonia for three Zika virus lineages. Pathogens 2020, 9, 575. [Google Scholar] [CrossRef] [PubMed]
- Leta, S.; Beyene, T.J.; De Clercq, E.M.; Amenu, K.; Kraemer, M.U.G.; Revie, C.W. Global risk mapping for major diseases transmitted by Aedes aegypti and Aedes albopictus. Int. J. Infect. Dis. 2018, 67, 25–35. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boyer, S.; Calvez, E.; Chouin-Carneiro, T.; Diallo, D.; Failloux, A.B. An overview of mosquito vectors of Zika virus. Microbes Infect 2018, 20, 646–660. [Google Scholar] [CrossRef] [PubMed]
- Grard, G.; Caron, M.; Mombo, I.M.; Nkoghe, D.; Mboui Ondo, S.; Jiolle, D.; Fontenille, D.; Paupy, C.; Leroy, E.M. Zika virus in Gabon (Central Africa)—2007: A new threat from Aedes albopictus? PLoS Negl. Trop. Dis. 2014, 8, e2681. [Google Scholar] [CrossRef] [Green Version]
- Pereira dos Santos, T.; Roiz, D.; Santos de Abreu, F.V.; Luz, S.L.B.; Santalucia, M.; Jiolle, D.; Santos Neves, M.S.A.; Simard, F.; Lourenço-de-Oliveira, R.; Paupy, C. Potential of Aedes albopictus as a bridge vector for enzootic pathogens at the urban-forest interface in Brazil. Emerg. Microbes. Infect 2018, 7, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Pereira-dos-santos, T.; Roiz, D.; Lourenço-de-oliveira, R.; Paupy, C. A systematic review: Is Aedes albopictus an efficient bridge vector for zoonotic arboviruses ? Pathogens 2020, 9, 266. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kraemer, M.U.G.; Sinka, M.E.; Duda, K.A.; Mylne, A.Q.N.; Shearer, F.M.; Barker, C.M.; Moore, C.G.; Carvalho, R.G.; Coelho, G.E.; Van Bortel, W.; et al. The global distribution of the arbovirus vectors Aedes aegypti and Ae. albopictus. eLife 2015, 4, e08347. [Google Scholar] [CrossRef]
- Iwamura, T.; Guzman-Holst, A.; Murray, K.A. Accelerating invasion potential of disease vector Aedes aegypti under climate change. Nat. Commun. 2020, 11, 2130. [Google Scholar] [CrossRef]
- Vasilakis, N.; Weaver, S.C. Flavivirus transmission focusing on Zika. Curr. Opin. Virol. 2017, 22, 30–35. [Google Scholar] [CrossRef] [Green Version]
- Terzian, A.C.B.; Zini, N.; Sacchetto, L.; Rocha, R.F.; Parra, M.C.P.; Del Sarto, J.L.; Dias, A.C.F.; Coutinho, F.; Rayra, J.; da Silva, R.A.; et al. Evidence of natural Zika virus infection in neotropical non-human primates in Brazil. Sci. Rep. 2018, 8, 16034. [Google Scholar] [CrossRef] [Green Version]
- Fernandes, R.S.; Bersot, M.I.; Castro, M.G.; Telleria, E.L.; Ferreira-de-Brito, A.; Raphael, L.M.; Bonaldo, M.C.; Lourenço-de-Oliveira, R. Low Vector competence in sylvatic mosquitoes limits Zika virus to initiate an enzootic cycle in South America. Sci. Rep. 2019, 9, 20151. [Google Scholar] [CrossRef] [Green Version]
- Berry, N.; Ferguson, D.; Ham, C.; Hall, J.; Jenkins, A.; Giles, E.; Devshi, D.; Kempster, S.; Rose, N.; Dowall, S.; et al. High Susceptibility, Viral dynamics and persistence of South American Zika virus in New World monkey species. Sci. Rep. 2019, 9, 14495. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rezende, H.R.; Romano, C.M.; Claro, I.M.; Caleiro, G.S.; Sabino, E.C.; Felix, A.C.; Bissoli, J.; Hill, S.; Faria, N.R.; Cardoso da Silva, T.C.; et al. First report of Aedes albopictus infected by dengue and Zika virus in a rural outbreak in Brazil. PLoS ONE 2020, 15, e0229847. [Google Scholar] [CrossRef] [PubMed]
- Alencar, J.; de Mello, C.F.; Marcondes, C.B.; Guimarães, A.É.; Toma, H.K.; Bastos, A.Q.; Silva, S.O.F.; Machado, S.L. Natural infection and vertical transmission of Zika virus in sylvatic mosquitoes Aedes albopictus and Haemagogus leucocelaenus from Rio de Janeiro, Brazil. Trop. Med. Infect. Dis. 2021, 6, 99. [Google Scholar] [CrossRef] [PubMed]
- Parra, M.C.P.; Lorenz, C.; de Aguiar Milhim, B.H.G.; Dibo, M.R.; Guirado, M.M.; Chiaravalloti-Neto, F.; Nogueira, M.L. Detection of Zika RNA virus in Aedes aegypti and Aedes albopictus mosquitoes, São Paulo, Brazil. Infect. Genet. Evol. 2022, 98, 105226. [Google Scholar] [CrossRef]
- da Silva Neves, N.A.; da Silva Ferreira, R.; Morais, D.O.; Pavon, J.A.R.; de Pinho, J.B.; Slhessarenko, R.D. Chikungunya, Zika, mayaro, and equine encephalitis virus detection in adult Culicinae from South Central Mato Grosso, Brazil, during the rainy season of 2018. Braz. J. Microbiol. 2022, 53, 63–70. [Google Scholar] [CrossRef] [PubMed]
- Instituto Brasileiro de Geografia e Estatística, I. Cidades: Amazonas: Manaus. Available online: http://www.cidades.ibge.gov.br/ (accessed on 3 November 2019).
- BRASIL. Ministério da Saúde Monitoramento Dos Casos de Arboviroses Até a Semana Epidemiológica 9 de 2022. Ministério Saúde—Bol. Epidemiológico 2022, 53, 1–14. [Google Scholar]
- Hendy, A.; Hernandez-Acosta, E.; Chaves, B.A.; Fé, N.F.; Valério, D.; Mendonça, C.; de Lacerda, M.V.G.; Buenemann, M.; Vasilakis, N.; Hanley, K.A. Into the woods: Changes in mosquito community composition and presence of key vectors at increasing distances from the urban edge in urban forest parks in Manaus, Brazil. Acta Trop. 2020, 206, 105441. [Google Scholar] [CrossRef]
- Hendy, A.; Valério, D.; Fé, N.F.; Hernandez-Acosta, E.; Mendonça, C.; Andrade, E.; Pedrosa, I.; Costa, E.R.; Júnior, J.T.A.; Assunção, F.P.; et al. Microclimate and the vertical stratification of potential bridge vectors of mosquito-borne viruses captured by nets and ovitraps in a Central Amazonian forest bordering Manaus, Brazil. Sci. Rep. 2021, 11, 21129. [Google Scholar] [CrossRef] [PubMed]
- Hendy, A.; Hernandez-Acosta, E.; Valério, D.; Mendonça, C.; Costa, E.R.; Júnior, J.T.A.; Assunção, F.P.; Scarpassa, V.M.; Gordo, M.; Fé, N.F.; et al. The vertical stratification of potential bridge vectors of mosquito-borne viruses in a Central Amazonian forest bordering Manaus, Brazil. Sci. Rep. 2020, 10, 18254. [Google Scholar] [CrossRef]
- Hendy, A.; Hernandez-Acosta, E.; Valério, D.; Fé, N.F.; Mendonça, C.R.; Costa, E.R.; de Andrade, E.S.; Júnior, J.T.A.; Assunção, F.P.; Scarpassa, V.M.; et al. Where boundaries become bridges: Mosquito community composition, key vectors, and environmental associations at forest edges in the Central Brazilian Amazon. PLoS Negl. Trop. Dis. 2023, 17, e0011296. [Google Scholar] [CrossRef]
- Consoli, R.A.G.; Lourenço-de-Oliveira, R. Principais Mosquitos de Importância Sanitária No Brasil, 1st ed.; Fiocruz, Ed.; Fiocruz: Rio de Janeiro, Brazil, 1994; ISBN 8585676035. [Google Scholar]
- Patel, P.; Landt, O.; Kaiser, M.; Faye, O.; Koppe, T.; Lass, U.; Sall, A.A.; Niedrig, M. Development of One-Step quantitative reverse transcription PCR for the rapid detection of flaviviruses. J. Virol. 2013, 10, 58. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Johnson, B.W.; Russell, B.J.; Lanciotti, R.S. Serotype-specific detection of dengue viruses in a fourplex real-time reverse transcriptase PCR assay. J. Clin. Microbiol. 2005, 43, 4977–4983. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lanciotti, R.S.; Kosoy, O.L.; Laven, J.J.; Velez, J.O.; Lambert, A.J.; Johnson, A.J.; Stanfield, S.M.; Duffy, M.R. Genetic and serologic properties of Zika virus associated with an epidemic, Yap State, Micronesia, 2007. Emerg. Infect. Dis. 2008, 14, 1232–1239. [Google Scholar] [CrossRef] [PubMed]
- Domingo, C.; Patel, P.; Yillah, J.; Weidmann, M.; Méndez, J.A.; Nakouné, E.R.; Niedrig, M. Advanced yellow fever virus genome detection in point-of-care facilities and reference laboratories. J. Clin. Microbiol. 2012, 50, 4054–4060. [Google Scholar] [CrossRef] [Green Version]
- Contreras, D.; Arumugaswami, V. Zika virus infectious cell culture system and the in vitro prophylactic effect of interferons. J. Vis. Exp 2016, 114, 54767. [Google Scholar] [CrossRef]
- Ayllón, T.; Campos, R.D.M.; Brasil, P.; Morone, F.C.; Cardoso, D.; Câmara, P.; Louzada, G.; Meira, S.; Tannich, E.; Pedro, R.S.; et al. Early evidence for Zika virus circulation among Aedes aegypti mosquitoes, Rio de Janeiro, Brazil. Emerg. Infect. Dis. 2017, 23, 1411–1412. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gordo, M.; Calleia, F.O.; Vasconcelos, S.A.; Leite, J.J.F.; Stephen, F.F. The Challenges of survival in a concrete jungle: Conservation of the pied tamarin (Saguinus bicolor) in the urban landscape of Manaus, Brazil. In Primates in Fragments; Marsh, L., Chapman, C., Eds.; Springer: New York, NY, USA, 2013; pp. 357–370. ISBN 9781461488392. [Google Scholar]
- de Oliveira, E.X.; de Souza, L.L.; de Lima, D.F.; da Silva, M. Comportamento alimentar e interações intergrupais de Saguinus bicolor (Primates: Callitrichidae) em um fragmento florestal urbano na cidade de Manaus, Amazonas. Neotrop. Primates 2020, 26, 25–32. [Google Scholar]
- Pollett, S.; Fauver, J.R.; Maljkovic Berry, I.; Melendrez, M.; Morrison, A.; Gillis, L.D.; Johansson, M.A.; Jarman, R.G.; Grubaugh, N.D. Genomic epidemiology as a public health tool to combat mosquito-borne virus outbreaks. J. Infect. Dis. 2020, 221, S308–S318. [Google Scholar] [CrossRef] [PubMed]

| Location | Collection Method | Date Collected (M/D/Y) * | Distance (m) | ** NDVI | Species | Number of Mosquitoes per Pool | Ct of ZIKV-Positive Samples in C6/36 | Ct of ZIKV-Positive Samples in Mosquito Macerate |
|---|---|---|---|---|---|---|---|---|
| 1 | BG-Sentinel | 05/10/2018 | 0 | Low | Aedes albopictus | 9 | 33.41 | *** N |
| 1 | Aspirator | 07/16/2018 | 50 | Low | Aedes albopictus | 2 | 35.23 | N |
| 3 | BG-Sentinel | 04/26/2018 | 0 | Medium | Aedes albopictus | 1 | 33.96 | N |
| 3 | BG-Sentinel | 06/12/2018 | 0 | Low | Aedes albopictus | 10 | 36.3 | N |
| 3 | BG-Sentinel | 08/22/2018 | 500 | low | Aedes albopictus | 1 | 33.75 | N |
| 3 | BG-Sentinel | 04/12/2018 | 50 | Low | Aedes aegypti | 1 | N | 35.6 |
| 3 | BG-Sentinel | 05/23/2018 | 0 | High | Aedes aegypti | 1 | N | 35.4 |
| 4 | BG-Sentinel | 01/15/2019 | 0 | Medium | Aedes albopictus | 2 | N | 35.1 |
| 4 | Aspirator | 02/01/2019 | 0 | Medium | Aedes albopictus | 3 | 34.42 | N |
| 4 | BG-Sentinel | 02/01/2019 | 0 | Low | Aedes aegypti | 1 | N | 37.5 |
| 4 | BG-Sentinel | 02/01/2019 | 0 | Medium | Aedes albopictus | 3 | 33.21 | N |
| 4 | BG-Sentinel | 02/05/2019 | 0 | Medium | Aedes albopictus | 1 | 33.39 | N |
| 4 | Net | 02/07/2019 | 0 | High | Aedes albopictus | 2 | 37.2 | N |
| 4 | Net | 04/09/2019 | 500 | Low | Aedes albopictus | 1 | 34.78 | N |
| 4 | Net | 05/06/2019 | 0 | Low | Aedes albopictus | 1 | N | 33.8 |
| 4 | BG-Sentinel | 05/28/2019 | 0 | Low | Aedes albopictus | 2 | N | 34.8 |
| 4 | Net | 06/10/2019 | 0 | Low | Aedes albopictus | 1 | N | 35.1 |
| 4 | BG-Sentinel | 12/06/2019 | 0 | Medium | Aedes albopictus | 1 | N | 35.9 |
| 4 | BG-Sentinel | 01/24/2020 | 0 | Low | Aedes albopictus | 1 | 33.85 | N |
| 4 | Aspirator | 02/28/2020 | 0 | Low | Aedes albopictus | 1 | 36 | N |
| 4 | Net | 05/18/2021 | 0 | High | Aedes albopictus | 1 | 37.5 | N |
| 4 | Net | 06/02/2021 | 0 | High | Aedes albopictus | 1 | 36.9 | N |
| 4 | Net | 06/09/2021 | 0 | Low | Aedes albopictus | 3 | 36.4 | N |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gomes, E.O.; Sacchetto, L.; Teixeira, M.; Chaves, B.A.; Hendy, A.; Mendonça, C.; Guimarães, I.; Linhares, R.; Brito, D.; Valério, D.; et al. Detection of Zika Virus in Aedes aegypti and Aedes albopictus Mosquitoes Collected in Urban Forest Fragments in the Brazilian Amazon. Viruses 2023, 15, 1356. https://doi.org/10.3390/v15061356
Gomes EO, Sacchetto L, Teixeira M, Chaves BA, Hendy A, Mendonça C, Guimarães I, Linhares R, Brito D, Valério D, et al. Detection of Zika Virus in Aedes aegypti and Aedes albopictus Mosquitoes Collected in Urban Forest Fragments in the Brazilian Amazon. Viruses. 2023; 15(6):1356. https://doi.org/10.3390/v15061356
Chicago/Turabian StyleGomes, Erika Oliveira, Lívia Sacchetto, Maurício Teixeira, Bárbara Aparecida Chaves, Adam Hendy, Claudia Mendonça, Izabele Guimarães, Ramon Linhares, Daniela Brito, Danielle Valério, and et al. 2023. "Detection of Zika Virus in Aedes aegypti and Aedes albopictus Mosquitoes Collected in Urban Forest Fragments in the Brazilian Amazon" Viruses 15, no. 6: 1356. https://doi.org/10.3390/v15061356