Activated Chicken Gamma Delta T Cells Are Involved in Protective Immunity against Marek’s Disease
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chicken Housing and Ethics
2.2. Virus Preparation
2.3. Ex Vivo TCRγδ Stimulation
2.4. Experimental Design
2.5. Single-Cell Isolation from Lungs, Spleen, and Blood
2.6. Flow Cytometry
2.7. DNA Extraction
2.8. RNA Extraction and cDNA Synthesis
2.9. Real-Time Quantitative Polymerase Chain Reaction (RT-qPCR)
2.10. Statistical Analysis
3. Results
3.1. TCRγδ Stimulation Induced IFN-γ Production by Chicken γδ T Cells
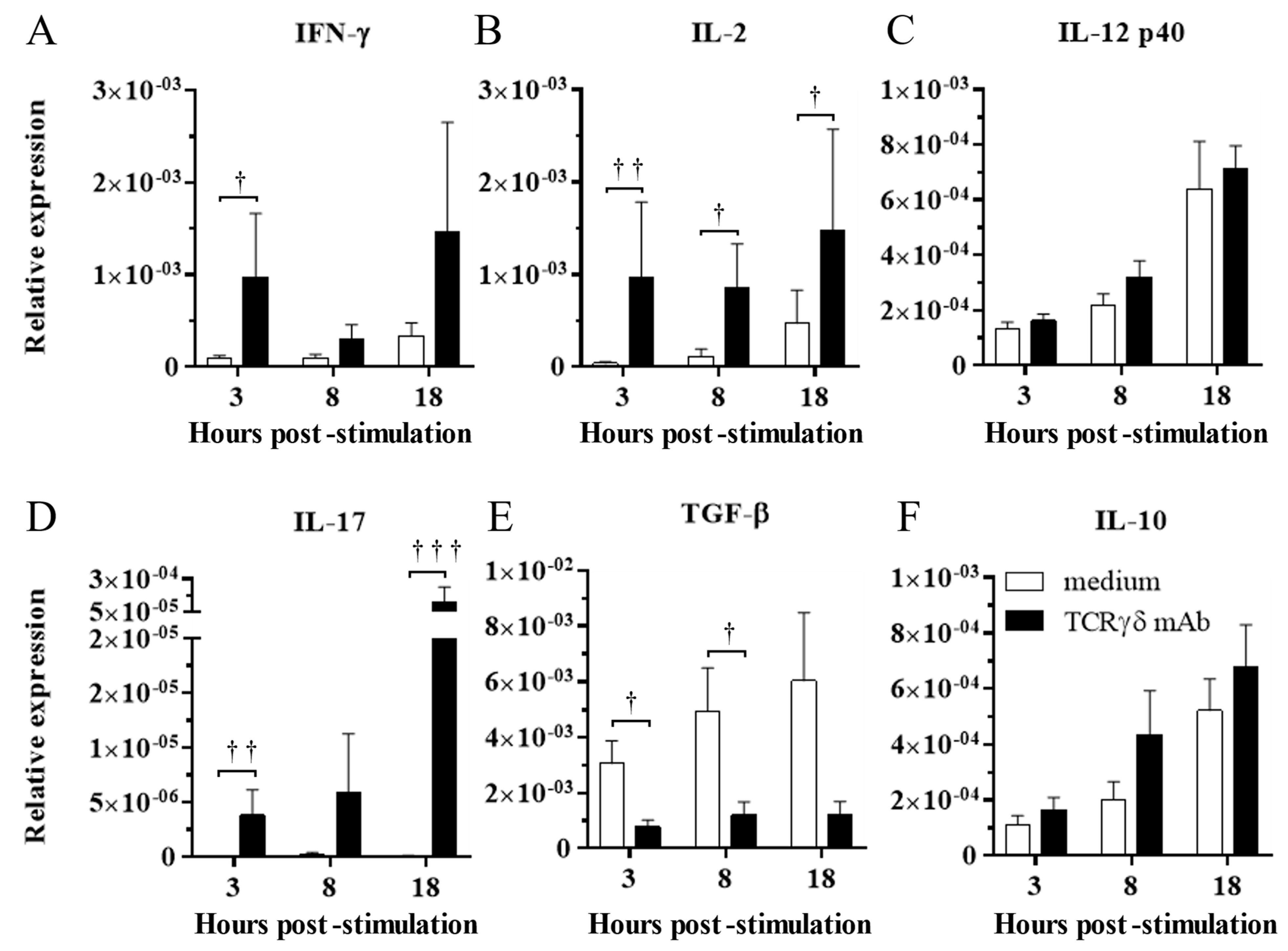
3.2. TCRγδ Stimulation Induced Upregulation of IFN-γ, IL-2, and IL-17 Gene Expression and Downregulation of TGF-β Gene Expression in the PBMCs
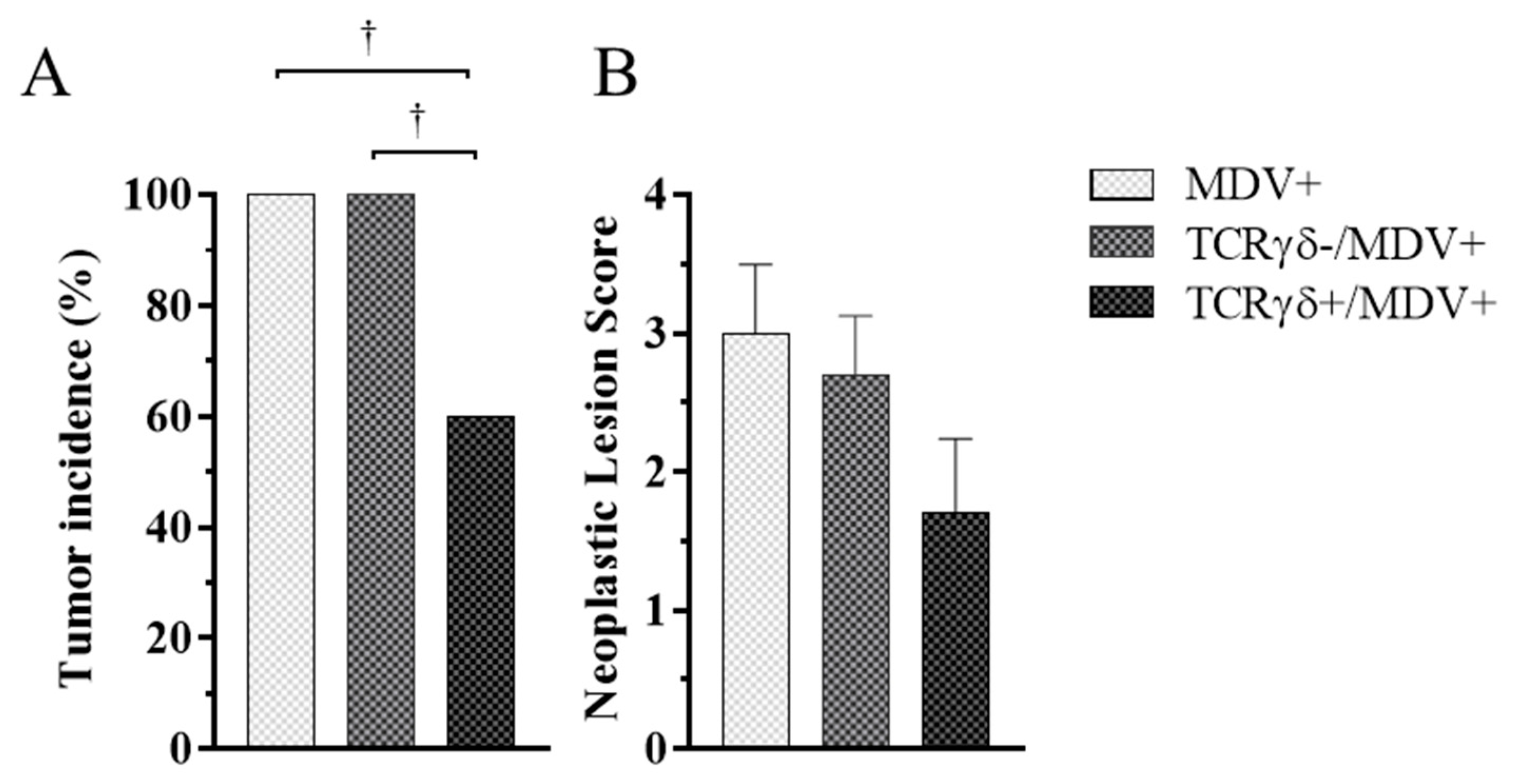
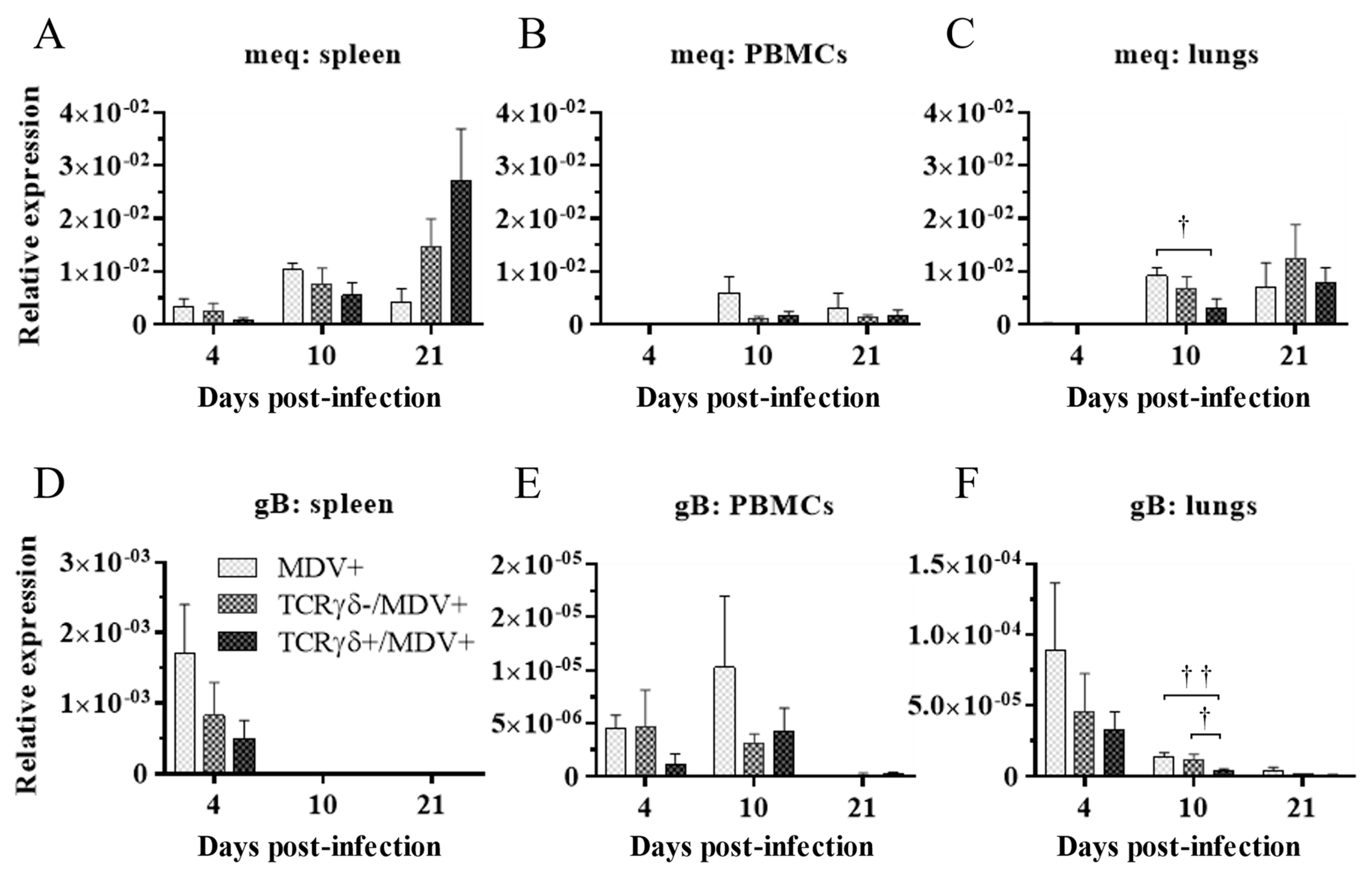
3.3. Adoptive Transfer of TCRγδ-Activated PBMCs Reduced Tumor Incidence and Suppressed Viral Replication in MDV-Challenged Chickens
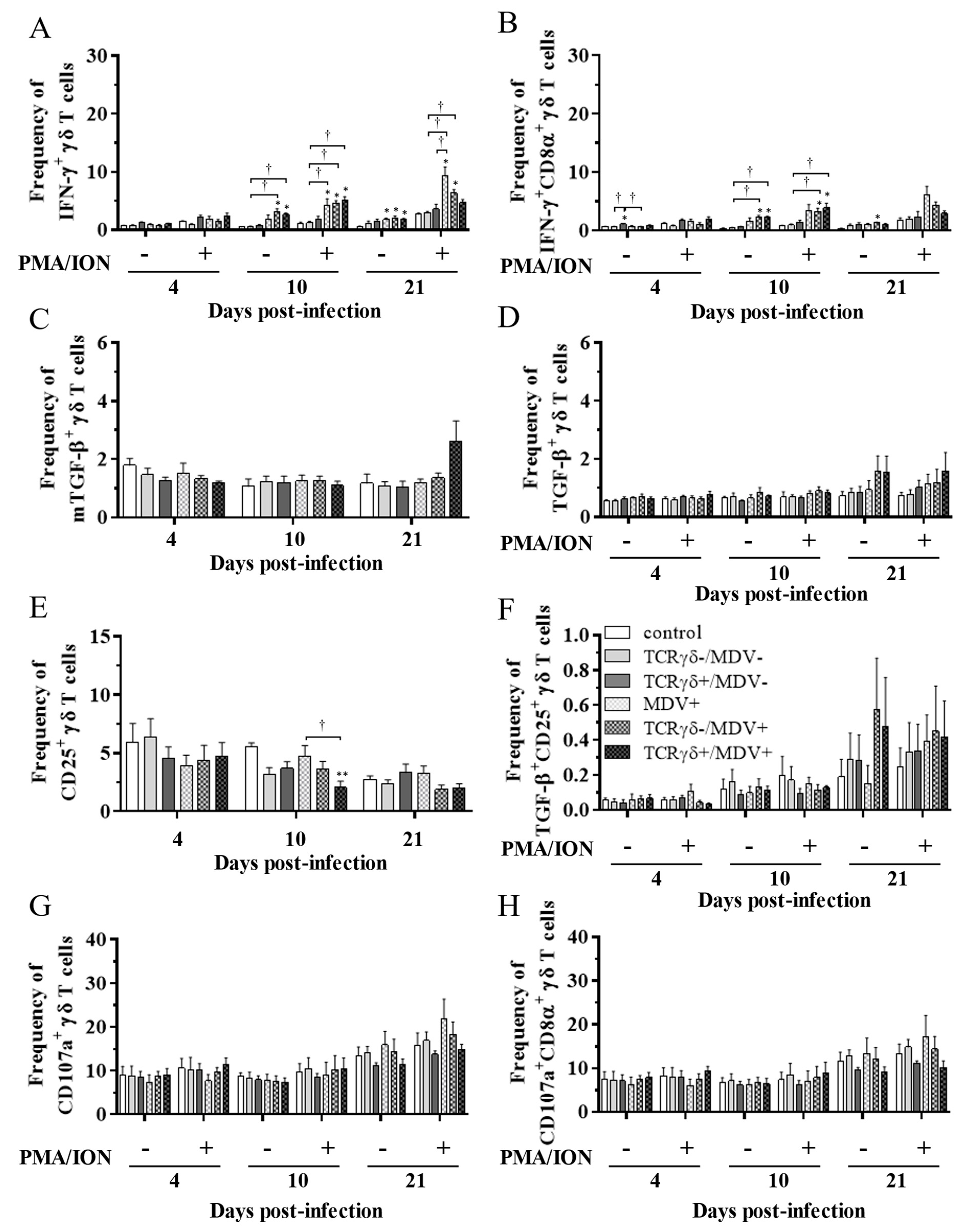
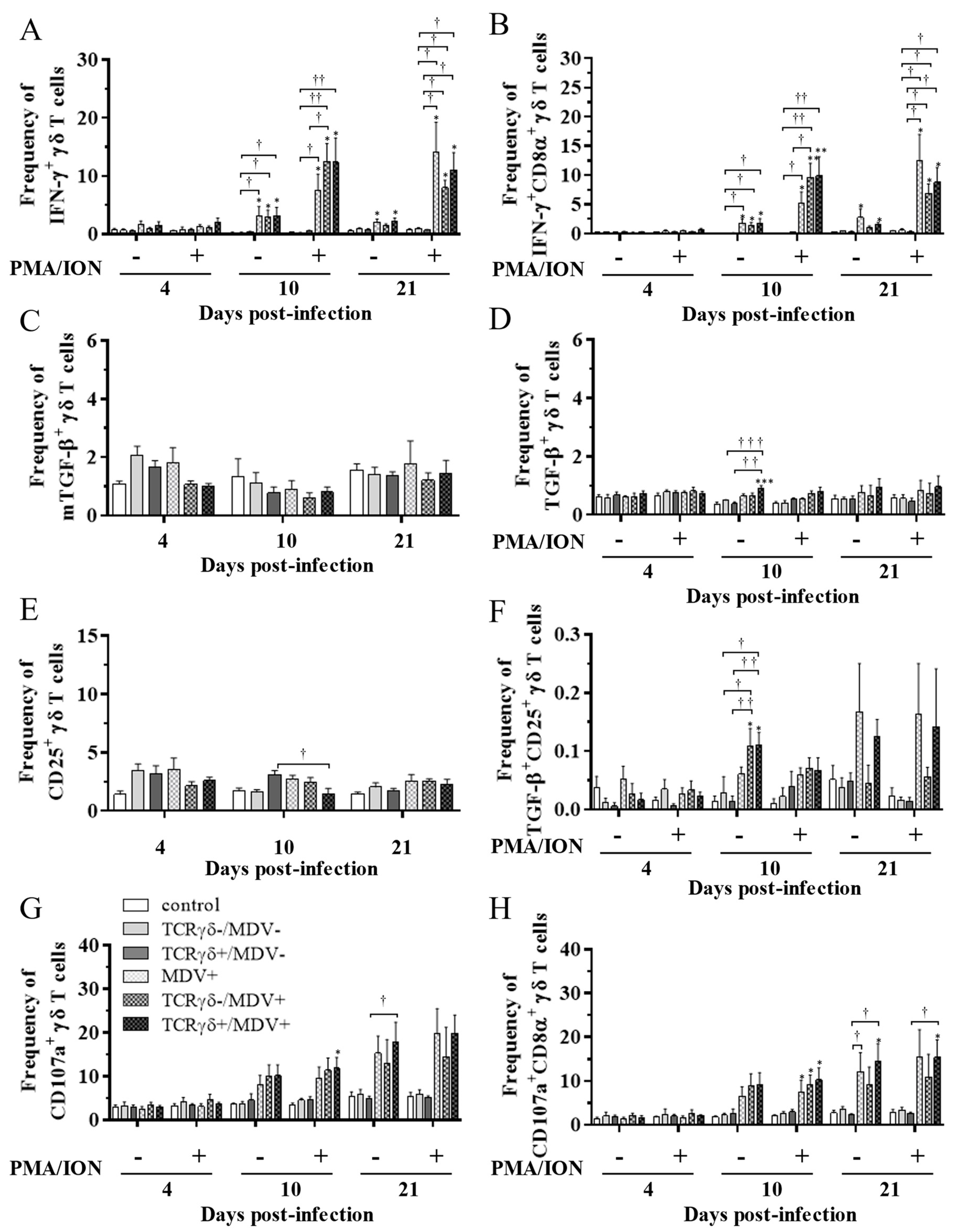
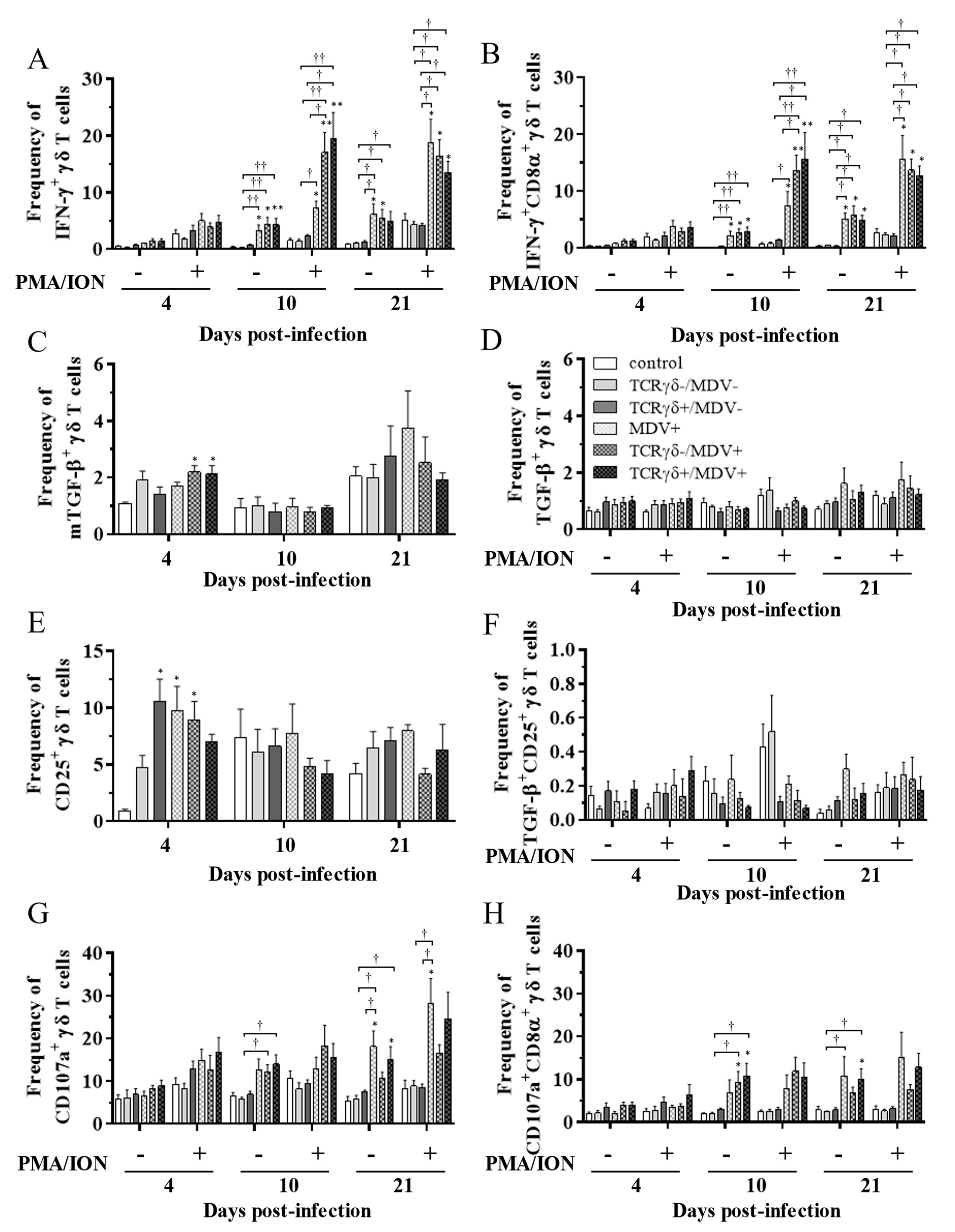
3.4. Lower Frequency of γδ T Cells in the Spleen of the TCRγδ-/MDV+ Group at 10 dpi and Higher Frequency of αβ T Cells in the Spleen and PBMCs of the TCRγδ+/MDV+ Group at 21 dpi
3.5. Circulating γδ T Cells in the TCRγδ+/MDV+ Group Showed a Higher Degranulation Activity at 10 and 21 dpi
3.6. Infusion of TCRγδ-Activated PBMCs Induced IFN-γ Gene Expression in the Spleen at 4, 10, and 21 dpi and Granzyme A Gene Expression at 10 dpi
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chien, Y.; Meyer, C.; Bonneville, M. Γδ T Cells: First Line of Defense and Beyond. Annu. Rev. Immunol. 2014, 32, 121–155. [Google Scholar] [CrossRef] [PubMed]
- Ribot, J.C.; Lopes, N.; Silva-Santos, B. Γδ T Cells in Tissue Physiology and Surveillance. Nat. Rev. Immunol. 2021, 21, 221–232. [Google Scholar] [CrossRef] [PubMed]
- Chan, K.F.; Duarte, J.D.G.; Ostrouska, S.; Behren, A. Γδ T Cells in the Tumor Microenvironment—Interactions With Other Immune Cells. Front. Immunol. 2022, 13, 894315. [Google Scholar] [CrossRef]
- Zhao, Y.; Lin, L.; Xiao, Z.; Li, M.; Wu, X.; Li, W.; Li, X.; Zhao, Q.; Wu, Y.; Zhang, H.; et al. Protective Role of Γδ T Cells in Different Pathogen Infections and Its Potential Clinical Application. J. Immunol. Res. 2018, 2018, 5081634. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matsuyama-Kato, A.; Iseki, H.; Boodhoo, N.; Bavananthasivam, J.; Alqazlan, N.; Abdul-Careem, M.F.; Plattner, B.L.; Behboudi, S.; Sharif, S. Phenotypic Characterization of Gamma Delta (Γδ) T Cells in Chickens Infected with or Vaccinated against Marek’s Disease Virus. Virology 2022, 568, 115–125. [Google Scholar] [CrossRef]
- Boodhoo, N.; Gurung, A.; Sharif, S.; Behboudi, S. Marek’s Disease in Chickens: A Review with Focus on Immunology. Vet. Res. 2016, 47, 119. [Google Scholar] [CrossRef] [Green Version]
- St.Hill, C.A.; Silva, R.F.; Sharma, J.M. Detection and Localization of Avian Alphaherpesviruses in Embryonic Tissues Following in Ovo Exposure. Virus Res. 2004, 100, 243–248. [Google Scholar] [CrossRef]
- Barrow, A.D.; Burgess, S.C.; Baigent, S.J.; Howes, K.; Nair, V.K. Infection of Macrophages by a Lymphotropic Herpesvirus: A New Tropism for Marek’s Disease Virus. J. Gen. Virol. 2003, 84, 2635–2645. [Google Scholar] [CrossRef]
- Calnek, B.W. Marek’s Disease—A Model for Herpesvirus Oncology. Crit. Rev. Microbiol. 1986, 12, 293–320. [Google Scholar] [CrossRef]
- Bertzbach, L.D.; van Haarlem, D.A.; Härtle, S.; Kaufer, B.B.; Jansen, C.A. Marek’s Disease Virus Infection of Natural Killer Cells. Microorganisms 2019, 7, 588. [Google Scholar] [CrossRef]
- Parvizi, P.; Read, L.; Abdul-Careem, M.F.; Lusty, C.; Sharif, S. Cytokine Gene Expression in Splenic CD4(+) and CD8(+) T-Cell Subsets of Chickens Infected with Marek’s Disease Virus. Viral Immunol. 2009, 22, 31–38. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Sun, S.; Heidari, M. Marek’s Disease Vaccine Activates Chicken Macrophages. J. Vet. Sci. 2018, 19, 375. [Google Scholar] [CrossRef] [PubMed]
- Hao, X.; Li, S.; Li, J.; Yang, Y.; Qin, A.; Shang, S. An Anti-Tumor Vaccine Against Marek’s Disease Virus Induces Differential Activation and Memory Response of Γδ T Cells and CD8 T Cells in Chickens. Front. Immunol. 2021, 12, 645426. [Google Scholar] [CrossRef] [PubMed]
- Laursen, A.M.S.; Kulkarni, R.R.; Taha-Abdelaziz, K.; Plattner, B.L.; Read, L.R.; Sharif, S. Characterizaton of Gamma Delta T Cells in Marek’s Disease Virus (Gallid Herpesvirus 2) Infection of Chickens. Virology 2018, 522, 56–64. [Google Scholar] [CrossRef] [PubMed]
- Yazdanifar, M.; Barbarito, G.; Bertaina, A.; Airoldi, I. Γδ T Cells: The Ideal Tool for Cancer Immunotherapy. Cells 2020, 9, 1305. [Google Scholar] [CrossRef]
- Zhou, J.; Kang, N.; Cui, L.; Ba, D.; He, W. Anti-Γδ TCR Antibody-Expanded Γδ T Cells: A Better Choice for the Adoptive Immunotherapy of Lymphoid Malignancies. Cell. Mol. Immunol. 2012, 9, 34–44. [Google Scholar] [CrossRef] [Green Version]
- Ou, L.; Wang, H.; Liu, Q.; Zhang, J.; Lu, H.; Luo, L.; Shi, C.; Lin, S.; Dong, L.; Guo, Y.; et al. Dichotomous and Stable Gamma Delta T-Cell Number and Function in Healthy Individuals. J. Immunother. Cancer 2021, 9, e002274. [Google Scholar] [CrossRef]
- Schat, K.A.; Calnek, B.W.; Fabricant, J. Characterisation of Two Highly Oncogenic Strains of Marek’s Disease Virus 1 2. Avian Pathol. 1982, 11, 593–605. [Google Scholar] [CrossRef] [Green Version]
- Abdul-Careem, M.F.; Haq, K.; Shanmuganathan, S.; Read, L.R.; Schat, K.A.; Heidari, M.; Sharif, S. Induction of Innate Host Responses in the Lungs of Chickens Following Infection with a Very Virulent Strain of Marek’s Disease Virus. Virology 2009, 393, 250–257. [Google Scholar] [CrossRef]
- Yssel, H.; De Vries, J.E.; Koken, M.; Van Blitterswijk, W.; Spits, H. Serum-Free Medium for Generation and Propagation of Functional Human Cytotoxic and Helper T Cell Clones. J. Immunol. Methods 1984, 72, 219–227. [Google Scholar] [CrossRef]
- Bavananthasivam, J.; Alizadeh, M.; Astill, J.; Alqazlan, N.; Matsuyama-Kato, A.; Shojadoost, B.; Taha-Abdelaziz, K.; Sharif, S. Effects of Administration of Probiotic Lactobacilli on Immunity Conferred by the Herpesvirus of Turkeys Vaccine against Challenge with a Very Virulent Marek’s Disease Virus in Chickens. Vaccine 2021, 39, 2424–2433. [Google Scholar] [CrossRef] [PubMed]
- Abdul-Careem, M.F.; Hunter, B.D.; Nagy, É.; Read, L.R.; Sanei, B.; Spencer, J.L.; Sharif, S. Development of a Real-Time PCR Assay Using SYBR Green Chemistry for Monitoring Marek’s Disease Virus Genome Load in Feather Tips. J. Virol. Methods 2006, 133, 34–40. [Google Scholar] [CrossRef]
- Brisbin, J.T.; Zhou, H.; Gong, J.; Sabour, P.; Akbari, M.R.; Haghighi, H.R.; Yu, H.; Clarke, A.; Sarson, A.J.; Sharif, S. Gene Expression Profiling of Chicken Lymphoid Cells after Treatment with Lactobacillus Acidophilus Cellular Components. Dev. Comp. Immunol. 2008, 32, 563–574. [Google Scholar] [CrossRef] [PubMed]
- Abdul-Careem, M.F.; Hunter, B.D.; Sarson, A.J.; Parvizi, P.; Haghighi, H.R.; Read, L.; Heidari, M.; Sharif, S. Host Responses Are Induced in Feathers of Chickens Infected with Marek’s Disease Virus. Virology 2008, 370, 323–332. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brisbin, J.T.; Gong, J.; Parvizi, P.; Sharif, S. Effects of Lactobacilli on Cytokine Expression by Chicken Spleen and Cecal Tonsil Cells. Clin. Vaccine Immunol. 2010, 17, 1337–1343. [Google Scholar] [CrossRef] [Green Version]
- Yitbarek, A.; Rodriguez-Lecompte, J.C.; Echeverry, H.M.; Munyaka, P.; Barjesteh, N.; Sharif, S.; Camelo-Jaimes, G. Performance, Histomorphology, and Toll-like Receptor, Chemokine, and Cytokine Profile Locally and Systemically in Broiler Chickens Fed Diets Supplemented with Yeast-Derived Macromolecules. Poult. Sci. 2013, 92, 2299–2310. [Google Scholar] [CrossRef]
- Crhanova, M.; Hradecka, H.; Faldynova, M.; Matulova, M.; Havlickova, H.; Sisak, F.; Rychlik, I. Immune Response of Chicken Gut to Natural Colonization by Gut Microflora and to Salmonella Enterica Serovar Enteritidis Infection. Infect. Immun. 2011, 79, 2755–2763. [Google Scholar] [CrossRef] [Green Version]
- Abdul-Careem, M.F.; Hunter, B.D.; Parvizi, P.; Haghighi, H.R.; Thanthrige-Don, N.; Sharif, S. Cytokine Gene Expression Patterns Associated with Immunization against Marek’s Disease in Chickens. Vaccine 2007, 25, 424–432. [Google Scholar] [CrossRef]
- Sarson, A.J.; Abdul-Careem, M.F.; Read, L.R.; Brisbin, J.T.; Sharif, S. Expression of Cytotoxicity-Associated Genes in Marek’s Disease Virus—Infected Chickens. Viral Immunol. 2008, 21, 267–272. [Google Scholar] [CrossRef]
- Dunne, M.R.; Madrigal-Estebas, L.; Tobin, L.M.; Doherty, D.G. (E)-4-Hydroxy-3-Methyl-but-2 Enyl Pyrophosphate-Stimulated Vγ9Vδ2 T Cells Possess T Helper Type 1-Promoting Adjuvant Activity for Human Monocyte-Derived Dendritic Cells. Cancer Immunol. Immunother. 2010, 59, 1109–1120. [Google Scholar] [CrossRef]
- Ismaili, J.; Olislagers, V.; Poupot, R.; Fournié, J.-J.; Goldman, M. Human Γδ T Cells Induce Dendritic Cell Maturation. Clin. Immunol. 2002, 103, 296–302. [Google Scholar] [CrossRef]
- Eberl, M.; Roberts, G.W.; Meuter, S.; Williams, J.D.; Topley, N.; Moser, B. A Rapid Crosstalk of Human Γδ T Cells and Monocytes Drives the Acute Inflammation in Bacterial Infections. PLoS Pathog. 2009, 5, e1000308. [Google Scholar] [CrossRef]
- Boodhoo, N.; Behboudi, S. Marek’s Disease Virus-Specific T Cells Proliferate, Express Antiviral Cytokines but Have Impaired Degranulation Response. Front. Immunol. 2022, 13, 973762. [Google Scholar] [CrossRef] [PubMed]
- Boodhoo, N.; Behboudi, S. Differential Virus-Specific IFN-Gamma Producing T Cell Responses to Marek’s Disease Virus in Chickens With B19 and B21 MHC Haplotypes. Front. Immunol. 2022, 12, 784359. [Google Scholar] [CrossRef] [PubMed]
- Song, K.D.; Lillehoj, H.S.; Choi, K.D.; Zarlenga, D.; Han, J.Y. Expression and Functional Characterization of Recombinant Chicken Interferon-Gamma. Vet. Immunol. Immunopathol. 1997, 58, 321–333. [Google Scholar] [CrossRef] [PubMed]
- Kano, R.; Konnai, S.; Onuma, M.; Ohashi, K. Cytokine Profiles in Chickens Infected with Virulent and Avirulent Marek’s Disease Viruses: Interferon-Gamma Is a Key Factor in the Protection of Marek’s Disease by Vaccination. Microbiol. Immunol. 2009, 53, 224–232. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Haq, K.; Elawadli, I.; Parvizi, P.; Mallick, A.I.; Behboudi, S.; Sharif, S. Interferon-γ Influences Immunity Elicited by Vaccines against Very Virulent Marek’s Disease Virus. Antiviral Res. 2011, 90, 218–226. [Google Scholar] [CrossRef]
- Bertzbach, L.D.; Harlin, O.; Härtle, S.; Fehler, F.; Vychodil, T.; Kaufer, B.B.; Kaspers, B. IFNα and IFNγ Impede Marek’s Disease Progression. Viruses 2019, 11, 1103. [Google Scholar] [CrossRef] [Green Version]
- Gao, Y.; Yang, W.; Pan, M.; Scully, E.; Girardi, M.; Augenlicht, L.H.; Craft, J.; Yin, Z. Γδ T Cells Provide an Early Source of Interferon γ in Tumor Immunity. J. Exp. Med. 2003, 198, 433–442. [Google Scholar] [CrossRef] [Green Version]
- Toka, F.N.; Kenney, M.A.; Golde, W.T. Rapid and Transient Activation of Γδ T Cells to IFN-γ Production, NK Cell-Like Killing, and Antigen Processing during Acute Virus Infection. J. Immunol. 2011, 186, 4853–4861. [Google Scholar] [CrossRef]
- Walliser, I.; Göbel, T.W. Chicken IL-17A Is Expressed in Aβ and Γδ T Cell Subsets and Binds to a Receptor Present on Macrophages, and T Cells. Dev. Comp. Immunol. 2018, 81, 44–53. [Google Scholar] [CrossRef]
- Papotto, P.H.; Ribot, J.C.; Silva-Santos, B. IL-17+ Γδ T Cells as Kick-Starters of Inflammation. Nat. Immunol. 2017, 18, 604–611. [Google Scholar] [CrossRef]
- Omar, A.R.; Schat, K.A. Syngeneic Marek’s Disease Virus (MDV)-Specific Cell-Mediated Immune Responses against Immediate Early, Late, and Unique MDV Proteins. Virology 1996, 222, 87–99. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Markowski-Grimsrud, C.J.; Schat, K.A. Cytotoxic T Lymphocyte Responses to Marek’s Disease Herpesvirus-Encoded Glycoproteins. Vet. Immunol. Immunopathol. 2002, 90, 133–144. [Google Scholar] [CrossRef]
- Chen, C.-Y.; Xie, Q.-M.; Xue, Y.; Ji, J.; Chang, S.; Ma, J.-Y.; Bi, Y.-Z. Characterization of Cytotoxicity-Related Gene Expression in Response to Virulent Marek’s Disease Virus Infection in the Bursa of Fabricius. Res. Vet. Sci. 2013, 94, 496–503. [Google Scholar] [CrossRef] [PubMed]
- St.Leger, A.J.; Koelle, D.M.; Kinchington, P.R.; Verjans, G.M.G.M. Local Immune Control of Latent Herpes Simplex Virus Type 1 in Ganglia of Mice and Man. Front. Immunol. 2021, 12, 723809. [Google Scholar] [CrossRef]
- Pereira, R.A.; Simon, M.M.; Simmons, A. Granzyme A, a Noncytolytic Component of CD8 + Cell Granules, Restricts the Spread of Herpes Simplex Virus in the Peripheral Nervous Systems of Experimentally Infected Mice. J. Virol. 2000, 74, 1029–1032. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Knickelbein, J.E.; Khanna, K.M.; Yee, M.B.; Baty, C.J.; Kinchington, P.R.; Hendricks, R.L. Noncytotoxic Lytic Granule–Mediated CD8 + T Cell Inhibition of HSV-1 Reactivation from Neuronal Latency. Science 2008, 322, 268–271. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bavananthasivam, J.; Alqazlan, N.; Alizadeh, M.; Matsuyama-Kato, A.; Astill, J.; Kulkarni, R.R.; Sharif, S. The Regulatory Microenvironment in Feathers of Chickens Infected with Very Virulent Marek’s Disease Virus. Viruses 2022, 14, 112. [Google Scholar] [CrossRef]
- Heidari, M.; Delekta, P.C. Transcriptomic Analysis of Host Immune Response in the Skin of Chickens Infected with Marek’s Disease Virus. Viral Immunol. 2017, 30, 377–387. [Google Scholar] [CrossRef]
- Heidari, M.; Wang, D.; Delekta, P.; Sun, S. Marek’s Disease Virus Immunosuppression Alters Host Cellular Responses and Immune Gene Expression in the Skin of Infected Chickens. Vet. Immunol. Immunopathol. 2016, 180, 21–28. [Google Scholar] [CrossRef] [PubMed]
- Minami, Y.; Kono, T.; Miyazaki, T.; Taniguchi, T. The IL-2 Receptor Complex: Its Structure, Function, and Target Genes. Annu. Rev. Immunol. 1993, 11, 245–268. [Google Scholar] [CrossRef] [PubMed]
- Braukmann, M.; Methner, U.; Berndt, A. Avian CD25(+) Gamma/Delta (Γδ) T Cells after Salmonella Exposure. Vet. Immunol. Immunopathol. 2015, 168, 14–18. [Google Scholar] [CrossRef] [PubMed]
- Gurung, A.; Kamble, N.; Kaufer, B.B.; Pathan, A.; Behboudi, S. Association of Marek’s Disease Induced Immunosuppression with Activation of a Novel Regulatory T Cells in Chickens. PLoS Pathog. 2017, 13, e1006745. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Migalovich Sheikhet, H.; Villacorta Hidalgo, J.; Fisch, P.; Balbir-Gurman, A.; Braun-Moscovici, Y.; Bank, I. Dysregulated CD25 and Cytokine Expression by Γδ T Cells of Systemic Sclerosis Patients Stimulated With Cardiolipin and Zoledronate. Front. Immunol. 2018, 9, 753. [Google Scholar] [CrossRef] [PubMed]
- Placek, K.; Schultze, J.L.; Aschenbrenner, A.C. Epigenetic Reprogramming of Immune Cells in Injury, Repair, and Resolution. J. Clin. Investig. 2019, 129, 2994–3005. [Google Scholar] [CrossRef] [Green Version]
- Beatson, R.E.; Parente-Pereira, A.C.; Halim, L.; Cozzetto, D.; Hull, C.; Whilding, L.M.; Martinez, O.; Taylor, C.A.; Obajdin, J.; Luu Hoang, K.N.; et al. TGF-Β1 Potentiates Vγ9Vδ2 T Cell Adoptive Immunotherapy of Cancer. Cell Rep. Med. 2021, 2, 100473. [Google Scholar] [CrossRef]
- Parvizi, P.; Read, L.R.; Abdul-Careem, M.F.; Sarson, A.J.; Lusty, C.; Lambourne, M.; Thanthrige-Don, N.; Burgess, S.C.; Sharif, S. Cytokine Gene Expression in Splenic CD4+ and CD8+ T Cell Subsets of Genetically Resistant and Susceptible Chickens Infected with Marek’s Disease Virus. Vet. Immunol. Immunopathol. 2009, 132, 209–217. [Google Scholar] [CrossRef]
- Mumm, J.B.; Emmerich, J.; Zhang, X.; Chan, I.; Wu, L.; Mauze, S.; Blaisdell, S.; Basham, B.; Dai, J.; Grein, J.; et al. IL-10 Elicits IFNγ-Dependent Tumor Immune Surveillance. Cancer Cell 2011, 20, 781–796. [Google Scholar] [CrossRef] [Green Version]
- Ouyang, W.; O’Garra, A. IL-10 Family Cytokines IL-10 and IL-22: From Basic Science to Clinical Translation. Immunity 2019, 50, 871–891. [Google Scholar] [CrossRef]
- Murugaiyan, G.; Saha, B. Protumor vs Antitumor Functions of IL-17. J. Immunol. 2009, 183, 4169–4175. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maniati, E.; Soper, R.; Hagemann, T. Up for Mischief? IL-17/Th17 in the Tumour Microenvironment. Oncogene 2010, 29, 5653–5662. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Silva-Santos, B.; Serre, K.; Norell, H. Γδ T Cells in Cancer. Nat. Rev. Immunol. 2015, 15, 683–691. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.-H.; Tung-Chieh Chang, J.; Liao, C.-T.; Chen, Y.-S.; Kuo, M.-L.; Shen, C.-R. Interleukin 17 and Peripheral IL-17-Expressing T Cells Are Negatively Correlated with the Overall Survival of Head and Neck Cancer Patients. Oncotarget 2018, 9, 9825–9837. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bertzbach, L.D.; Laparidou, M.; Härtle, S.; Etches, R.J.; Kaspers, B.; Schusser, B.; Kaufer, B.B. Unraveling the Role of B Cells in the Pathogenesis of an Oncogenic Avian Herpesvirus. Proc. Natl. Acad. Sci. USA 2018, 115, 11603–11607. [Google Scholar] [CrossRef] [PubMed]






| Target | Fluorochrome | Clone | Host Species | Source |
|---|---|---|---|---|
| CD3ε | Pacific Blue | CT-3 | mouse | Southern Biotech |
| CD4 | PE-Cyanine 7 or allophycocyanin | CT-4 | mouse | Southern Biotech |
| CD8α | fluorescein-5-isothiocyanate | CT-8 | mouse | Southern Biotech |
| TCRγδ | phycoerythrin | TCR-1 | mouse | Southern Biotech |
| CD25 | fluorescein-5-isothiocyanate | AbD13504 | human | Bio-Rad Laboratories Inc. Hercules, CA, USA |
| IFN-γ | biotin | 5C.123.08 | mouse | Invitrogen |
| Streptavidin | allophycocyanin | Invitrogen | ||
| TGF-β | allophycocyanin | 1D11 | mouse | Bio-Techne, Inc. Minneapolis, MN, USA |
| CD107a | PE-Cyanine 7 | LEP100 hybridoma cells | mouse | Developmental Studies Hybridoma Bank, Iowa City, IA, USA |
| Target Gene | Primer Sequence | Accession Number | Reference | |
|---|---|---|---|---|
| β-actin | Forward | 5′-CAACACAGTGCTGTCTGGTGGTA-3′ | X00182 | [23] |
| Reverse | 5′-ATCGTACTCCTGCTTGCTGATCC-3′ | |||
| meq | Forward | 5′-GTCCCCCCTCGATCTTTCTC-3′ | AY571783 | [22] |
| Reverse | 5′-CGTCTGCTTCCTGCGTCTTC-3′ | |||
| gB | Forward | 5′-GTCTGTTCAATTCGCCATGCTCC-3′ | AY129966 | [24] |
| Reverse | 5′-CCTTCCTAATGTTGCACTCGCTG-3′ | |||
| IFN-γ | Forward | 5′-ACACTGACAAGTCAAAGCCGCACA-3′ | X99774 | [25] |
| Reverse | 5′-AGTCGTTCATCGGGAGCTTGGC-3′ | |||
| IL-2 | Forward | 5′-TGCAGTGTTACCTGGGAGAAGTGGT-3′ | NM_204153.2 | [26] |
| Reverse | 5′-ACTTCCGGTGTGATTTAGACCCGT-3′ | |||
| IL-12p40 | Forward | 5′-TTGCCGAAGAGCACCAGCCG-3′ | AY262752.1 | [25] |
| Reverse | 5′-CGGTGTGCTCCAGGTCTTGGG-3′ | |||
| IL-17A | Forward | 5′-TATCAGCAAACGCTCACTGG-3′ | NM_204460.2 | [27] |
| Reverse | 5′-AGTTCACGCACCTGGAATG-3′ | |||
| IL-10 | Forward | 5′-AGCAGATCAAGGAGACGTTC-3′ | AJ621614 | [28] |
| Reverse | 5′-ATCAGCAGGTACTCCTCGAT-3′ | |||
| TGF-β | Forward | 5′-CGGCCGACGATGAGTGGCTC-3′ | NM_001318456.1 | [25] |
| Reverse | 5′-CGGGGCCCATCTCACAGGGA-3′ | |||
| granzyme A | Forward | 5′-TGGGTGTTAACAGCTGCTCATTGC-3′ | NM_204457.2 | [29] |
| Reverse | 5′-CACCTGAATCCCCTCGACATGAGT-3′ | |||
| perforin | Forward | 5′-ATGGCGCAGGTGACAGTGA-3′ | XM_046929135.1 | [29] |
| Reverse | 5′-TGGCCTGCACCGGTAATTC-3′ | |||
| Target Gene | Cycling Parameters | Efficiency | ||||
|---|---|---|---|---|---|---|
| Amplification | Melting Temp (°C) | |||||
| Denaturation | Annealing | Extension | Number of Cycles | |||
| β-actin | 95 °C 10 s | 58 °C 5 s | 72 °C 10 s | 40 | 97 | 2 |
| meq | 95 °C 10 s | 64 °C 5 s | 72 °C 8 s | 45 | 97 | 1.8 |
| gB | 95 °C 10 s | 64 °C 5 s | 72 °C 8 s | 45 | 97 | 1.93 |
| IFN-γ | 95 °C 10 s | 60 °C 5 s | 72 °C 10 s | 50 | 97 | 1.98 |
| IL-2 | 95 °C 10 s | 58 °C 5 s | 72 °C 10 s | 50 | 97 | 2 |
| IL-12p40 | 95 °C 10 s | 64 °C 5 s | 72 °C 10 s | 45 | 95 | 2 |
| IL-17A | 95 °C 10 s | 60 °C 5 s | 72 °C 10 s | 55 | 97 | 1.9 |
| IL-10 | 95 °C 1 s | 55 °C 5 s | 72 °C 5 s | 55 | 97 | 1.78 |
| TGF-β | 95 °C 10 s | 60 °C 5 s | 72 °C 10 s | 40 | 97 | 2 |
| granzyme A | 95 °C 10 s | 55 °C 5 s | 72 °C 10 s | 50 | 97 | 2.02 |
| perforin | 95 °C 10 s | 64 °C 5 s | 72 °C 10 s | 50 | 97 | 1.91 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Matsuyama-Kato, A.; Shojadoost, B.; Boodhoo, N.; Raj, S.; Alizadeh, M.; Fazel, F.; Fletcher, C.; Zheng, J.; Gupta, B.; Abdul-Careem, M.F.; et al. Activated Chicken Gamma Delta T Cells Are Involved in Protective Immunity against Marek’s Disease. Viruses 2023, 15, 285. https://doi.org/10.3390/v15020285
Matsuyama-Kato A, Shojadoost B, Boodhoo N, Raj S, Alizadeh M, Fazel F, Fletcher C, Zheng J, Gupta B, Abdul-Careem MF, et al. Activated Chicken Gamma Delta T Cells Are Involved in Protective Immunity against Marek’s Disease. Viruses. 2023; 15(2):285. https://doi.org/10.3390/v15020285
Chicago/Turabian StyleMatsuyama-Kato, Ayumi, Bahram Shojadoost, Nitish Boodhoo, Sugandha Raj, Mohammadali Alizadeh, Fatemeh Fazel, Charlotte Fletcher, Jiayu Zheng, Bhavya Gupta, Mohamed Faizal Abdul-Careem, and et al. 2023. "Activated Chicken Gamma Delta T Cells Are Involved in Protective Immunity against Marek’s Disease" Viruses 15, no. 2: 285. https://doi.org/10.3390/v15020285





