T Cell Transcriptional Signatures of Influenza A/H3N2 Antibody Response to High Dose Influenza and Adjuvanted Influenza Vaccine in Older Adults
Abstract
1. Introduction
2. Methods
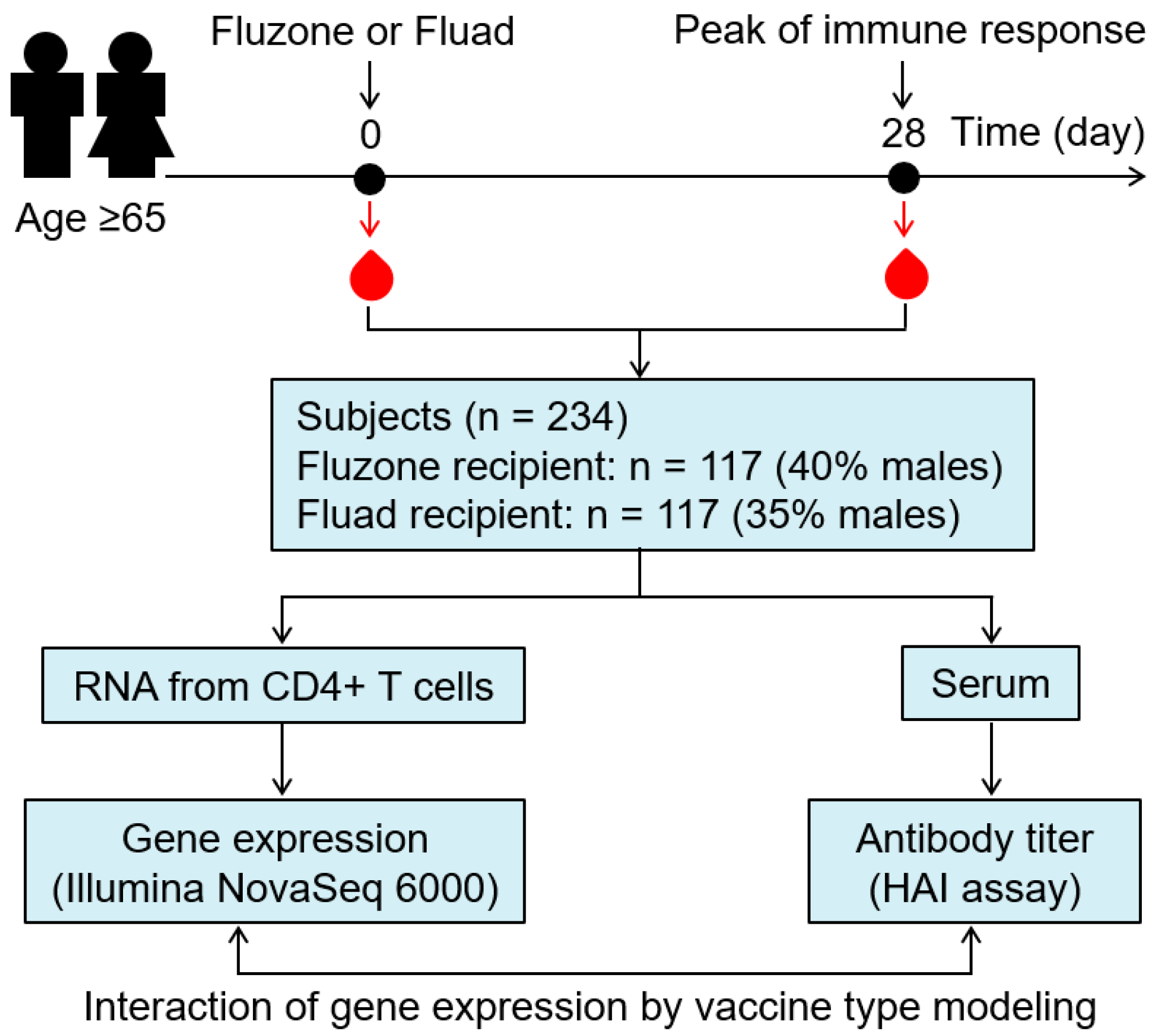
2.1. Study Subjects
2.2. Processing Blood Samples
2.3. Influenza Hemagglutination Inhibition (HAI) Assay
2.4. mRNA Sequencing
2.5. Statistical Analysis
3. Results
3.1. Demographic and Immune Response Characteristics for the Study Cohort
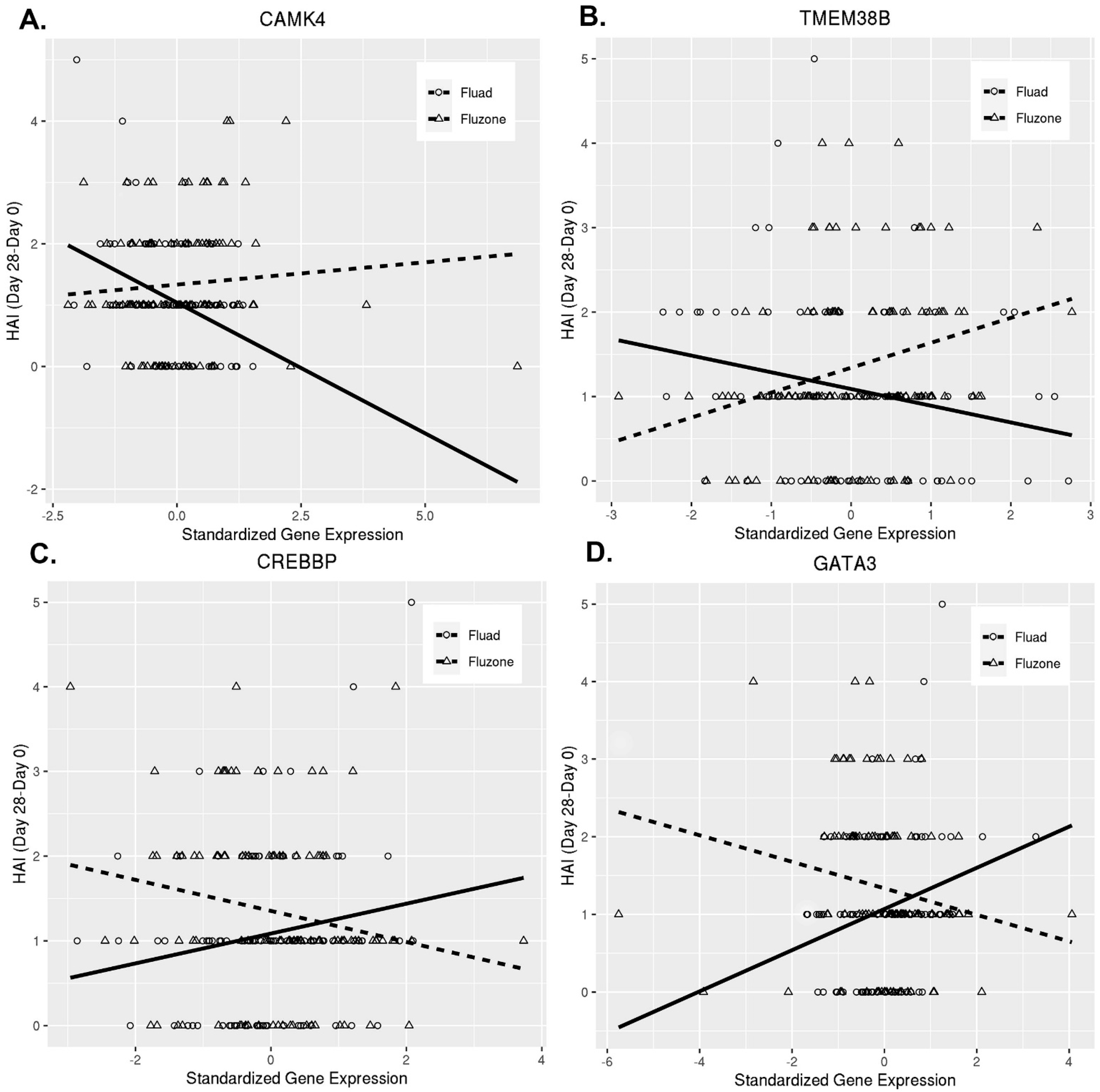
3.2. Humoral Immune Response to Fluad and Fluzone Vaccination Is Shaped by Differential CD4+ T Cell Gene Expression
3.3. Biological Pathways and Immune Functions in T Cells Associated with the Immune Response by Vaccine Type
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Available online: https://www.cdc.gov/flu/about/burden/index.html (accessed on 5 December 2022).
- Estimates of deaths associated with seasonal influenza—United States, 1976–2007. MMWR Morb. Mortal. Wkly. Rep. 2010, 59, 1057–1062.
- Reed, C.; Chaves, S.S.; Daily Kirley, P.; Emerson, R.; Aragon, D.; Hancock, E.B.; Butler, L.; Baumbach, J.; Hollick, G.; Bennett, N.M.; et al. Estimating influenza disease burden from population-based surveillance data in the United States. PLoS ONE 2015, 10, e0118369. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Thompson, W.W.; Viboud, C.G.; Ringholz, C.M.; Cheng, P.Y.; Steiner, C.; Abedi, G.R.; Anderson, L.J.; Brammer, L.; Shay, D.K. Hospitalizations associated with influenza and respiratory syncytial virus in the United States, 1993–2008. Clin. Infect. Dis. 2012, 54, 1427–1436. [Google Scholar] [CrossRef] [PubMed]
- Available online: https://www.cdc.gov/flu/highrisk/65over.htm (accessed on 5 December 2022).
- World Health Organization. Global Influenza Strategy 2019–2030; World Health Organization: Geneva, Switzerland, 2019. [Google Scholar]
- Goodwin, K.; Viboud, C.; Simonsen, L. Antibody response to influenza vaccination in the elderly: A quantitative review. Vaccine 2006, 24, 1159–1169. [Google Scholar] [CrossRef] [PubMed]
- Haralambieva, I.H.; Painter, S.D.; Kennedy, R.B.; Ovsyannikova, I.G.; Lambert, N.D.; Goergen, K.M.; Oberg, A.L.; Poland, G.A. The impact of immunosenescence on humoral immune response variation after influenza A/H1N1 vaccination in older subjects. PLoS ONE 2015, 10, e0122282. [Google Scholar] [CrossRef]
- DiazGranados, C.A.; Dunning, A.J.; Kimmel, M.; Kirby, D.; Treanor, J.; Collins, A.; Pollak, R.; Christoff, J.; Earl, J.; Landolfi, V. Efficacy of high-dose versus standard-dose influenza vaccine in older adults. N. Engl. J. Med. 2014, 371, 635–645. [Google Scholar] [CrossRef]
- Domnich, A.; Arata, L.; Amicizia, D.; Puig-Barberà, J.; Gasparini, R.; Panatto, D. Effectiveness of MF59-adjuvanted seasonal influenza vaccine in the elderly: A systematic review and meta-analysis. Vaccine 2017, 35, 513–520. [Google Scholar] [CrossRef]
- Frey, S.E.; Reyes, M.R.; Reynales, H.; Bermal, N.N.; Nicolay, U.; Narasimhan, V.; Forleo-Neto, E.; Arora, A.K. Comparison of the safety and immunogenicity of an MF59®-adjuvanted with a non-adjuvanted seasonal influenza vaccine in elderly subjects. Vaccine 2014, 32, 5027–5034. [Google Scholar] [CrossRef]
- Gravenstein, S.; Davidson, H.E.; Taljaard, M.; Ogarek, J.; Gozalo, P.; Han, L.; Mor, V. Comparative effectiveness of high-dose versus standard-dose influenza vaccination on numbers of US nursing home residents admitted to hospital: A cluster-randomised trial. Lancet Respir. Med. 2017, 5, 738–746. [Google Scholar] [CrossRef]
- Lapi, F.; Marconi, E.; Simonetti, M.; Baldo, V.; Rossi, A.; Sessa, A.; Cricelli, C. Adjuvanted versus nonadjuvanted influenza vaccines and risk of hospitalizations for pneumonia and cerebro/cardiovascular events in the elderly. Expert Rev. Vaccines 2019, 18, 663–670. [Google Scholar] [CrossRef]
- McConeghy, K.W.; Davidson, H.E.; Canaday, D.H.; Han, L.; Saade, E.; Mor, V.; Gravenstein, S. Cluster-randomized Trial of Adjuvanted Versus Nonadjuvanted Trivalent Influenza Vaccine in 823 US Nursing Homes. Clin. Infect. Dis. 2021, 73, e4237–e4243. [Google Scholar] [CrossRef] [PubMed]
- Boikos, C.; Fischer, L.; O’Brien, D.; Vasey, J.; Sylvester, G.C.; Mansi, J.A. Relative Effectiveness of Adjuvanted Trivalent Inactivated Influenza Vaccine Versus Egg-derived Quadrivalent Inactivated Influenza Vaccines and High-dose Trivalent Influenza Vaccine in Preventing Influenza-related Medical Encounters in US Adults ≥ 65 Years During the 2017–2018 and 2018–2019 Influenza Seasons. Clin. Infect. Dis. 2021, 73, 816–823. [Google Scholar] [CrossRef] [PubMed]
- Haralambieva, I.H.; Eberhard, K.G.; Ovsyannikova, I.G.; Grill, D.E.; Schaid, D.J.; Kennedy, R.B.; Poland, G.A. Transcriptional signatures associated with rubella virus-specific humoral immunity after a third dose of MMR vaccine in women of childbearing age. Eur. J. Immunol. 2021, 51, 1824–1838. [Google Scholar] [CrossRef] [PubMed]
- Kaufmann, L.; Syedbasha, M.; Vogt, D.; Hollenstein, Y.; Hartmann, J.; Linnik, J.E.; Egli, A. An optimized hemagglutination inhibition (HI) assay to quantify influenza-specific antibody titers. J. Vis. Exp. 2017, 55833. [Google Scholar] [CrossRef]
- Ovsyannikova, I.G.; Salk, H.M.; Kennedy, R.B.; Haralambieva, I.H.; Zimmermann, M.T.; Grill, D.E.; Oberg, A.L.; Poland, G.A. Gene signatures associated with adaptive humoral immunity following seasonal influenza A/H1N1 vaccination. Genes Immun. 2016, 17, 371–379. [Google Scholar] [CrossRef]
- Quach, H.Q.; Chen, J.; Monroe, J.M.; Ratishvili, T.; Warner, N.D.; Grill, D.E.; Haralambieva, I.H.; Ovsyannikova, I.G.; Poland, G.A.; Kennedy, R.B. The influence of sex, BMI, and age on cellular and humoral immune responses against measles after a 3rd dose of MMR vaccine. J. Infect. Dis. 2022, jiac351. [Google Scholar] [CrossRef]
- Webster, R.; Cox, N.; Stöhr, K. WHO Animal Influenza Manual; WHO/CDS/CSR/NCS/2002.5; World Health Organization: Geneva, Switzerland, 2002. [Google Scholar]
- Zimmermann, M.T.; Kennedy, R.B.; Grill, D.E.; Oberg, A.L.; Goergen, K.M.; Ovsyannikova, I.G.; Haralambieva, I.H.; Poland, G.A. Integration of Immune Cell Populations, mRNA-Seq, and CpG Methylation to Better Predict Humoral Immunity to Influenza Vaccination: Dependence of mRNA-Seq/CpG Methylation on Immune Cell Populations. Front. Immunol. 2017, 8, 445. [Google Scholar] [CrossRef]
- Haralambieva, I.H.; Ovsyannikova, I.G.; Kennedy, R.B.; Zimmermann, M.T.; Grill, D.E.; Oberg, A.L.; Poland, G.A. Transcriptional signatures of influenza A/H1N1-specific IgG memory-like B cell response in older individuals. Vaccine 2016, 34, 3993–4002. [Google Scholar] [CrossRef]
- Kalari, K.R.; Nair, A.A.; Bhavsar, J.D.; O’Brien, D.R.; Davila, J.I.; Bockol, M.A.; Nie, J.; Tang, X.; Baheti, S.; Doughty, J.B. MAP-RSeq: Mayo analysis pipeline for RNA sequencing. BMC Bioinform. 2014, 15, 224. [Google Scholar] [CrossRef]
- Hansen, K.D.; Irizarry, R.A.; Wu, Z. Removing technical variability in RNA-seq data using conditional quantile normalization. Biostatistics 2012, 13, 204–216. [Google Scholar] [CrossRef]
- Storey, J.D. False Discovery Rate. Int. Encycl. Stat. Sci. 2011, 1, 504–508. [Google Scholar]
- Yu, G.; Wang, L.-G.; Han, Y.; He, Q.-Y. clusterProfiler: An R package for comparing biological themes among gene clusters. OMICS J. Integr. Biol. 2012, 16, 284–287. [Google Scholar] [CrossRef] [PubMed]
- Benjamini, Y.; Hochberg, Y. Controlling the false discovery rate: A practical and powerful approach to multiple testing. J. R. Stat. Soc. Ser. B 1995, 57, 289–300. [Google Scholar] [CrossRef]
- Voigt, E.A.; Ovsyannikova, I.G.; Kennedy, R.B.; Grill, D.E.; Goergen, K.M.; Schaid, D.J.; Poland, G.A. Sex differences in older adults’ immune responses to seasonal influenza vaccination. Front. Immunol. 2019, 10, 180. [Google Scholar] [CrossRef]
- Song, W.; Li, D.; Tao, L.; Luo, Q.; Chen, L. Solute carrier transporters: The metabolic gatekeepers of immune cells. Acta Pharm. Sin. B 2020, 10, 61–78. [Google Scholar] [CrossRef]
- Nakaya, H.I.; Wrammert, J.; Lee, E.K.; Racioppi, L.; Marie-Kunze, S.; Haining, W.N.; Means, A.R.; Kasturi, S.P.; Khan, N.; Li, G.-M. Systems biology of vaccination for seasonal influenza in humans. Nat. Immunol. 2011, 12, 786–795. [Google Scholar] [CrossRef]
- Li, S.; Nakaya, H.I.; Kazmin, D.A.; Oh, J.Z.; Pulendran, B. Systems biological approaches to measure and understand vaccine immunity in humans. Semin. Immunol. 2013, 25, 209–218. [Google Scholar] [CrossRef]
- Pulendran, B. Systems vaccinology: Probing humanity’s diverse immune systems with vaccines. Proc. Natl. Acad. Sci. USA 2014, 111, 12300–12306. [Google Scholar] [CrossRef]
- Chawla, S.; Hardingham, G.E.; Quinn, D.R.; Bading, H. CBP: A signal-regulated transcriptional coactivator controlled by nuclear calcium and CaM kinase IV. Science 1998, 281, 1505–1509. [Google Scholar] [CrossRef]
- Filip, A.M.; Klug, J.; Cayli, S.; Fröhlich, S.; Henke, T.; Lacher, P.; Eickhoff, R.; Bulau, P.; Linder, M.; Carlsson-Skwirut, C.; et al. Ribosomal protein S19 interacts with macrophage migration inhibitory factor and attenuates its pro-inflammatory function. J. Biol. Chem. 2009, 284, 7977–7985. [Google Scholar] [CrossRef]
- Roger, T.; Ding, X.; Chanson, A.L.; Renner, P.; Calandra, T. Regulation of constitutive and microbial pathogen-induced human macrophage migration inhibitory factor (MIF) gene expression. Eur. J. Immunol. 2007, 37, 3509–3521. [Google Scholar] [CrossRef] [PubMed]
- Nagai-Singer, M.A.; Morrison, H.A.; Allen, I.C. NLRX1 Is a Multifaceted and Enigmatic Regulator of Immune System Function. Front. Immunol. 2019, 10, 2419. [Google Scholar] [CrossRef] [PubMed]
- Kanehisa, M.; Goto, S. KEGG: Kyoto encyclopedia of genes and genomes. Nucleic Acids Res. 2000, 28, 27–30. [Google Scholar] [CrossRef] [PubMed]
- Katagiri, K.; Hattori, M.; Minato, N.; Kinashi, T. Rap1 functions as a key regulator of T-cell and antigen-presenting cell interactions and modulates T-cell responses. Mol. Cell. Biol. 2002, 22, 1001–1015. [Google Scholar] [CrossRef] [PubMed]
- Voigt, E.A.; Grill, D.E.; Zimmermann, M.T.; Simon, W.L.; Ovsyannikova, I.G.; Kennedy, R.B.; Poland, G.A. Transcriptomic signatures of cellular and humoral immune responses in older adults after seasonal influenza vaccination identified by data-driven clustering. Sci. Rep. 2018, 8, 739. [Google Scholar] [CrossRef]
- Nakaya, H.I.; Clutterbuck, E.; Kazmin, D.; Wang, L.; Cortese, M.; Bosinger, S.E.; Patel, N.B.; Zak, D.E.; Aderem, A.; Dong, T. Systems biology of immunity to MF59-adjuvanted versus nonadjuvanted trivalent seasonal influenza vaccines in early childhood. Proc. Natl. Acad. Sci. USA 2016, 113, 1853–1858. [Google Scholar] [CrossRef]
- Wen, A.Y.; Sakamoto, K.M.; Miller, L.S. The role of the transcription factor CREB in immune function. J. Immunol. 2010, 185, 6413–6419. [Google Scholar] [CrossRef]
- Barton, K.; Muthusamy, N.; Chanyangam, M.; Fischer, C.; Clendenin, C.; Leiden, J.M. Defective thymocyte proliferation and IL-2 production in transgenic mice expressing a dominant-negative form of CREB. Nature 1996, 379, 81–85. [Google Scholar] [CrossRef]
- Zhang, F.; Rincon, M.; Flavell, R.A.; Aune, T.M. Defective Th function induced by a dominant-negative cAMP response element binding protein mutation is reversed by Bcl-2. J. Immunol. 2000, 165, 1762–1770. [Google Scholar] [CrossRef]
- Scherlinger, M.; Li, H.; Pan, W.; Tsokos, M.; Tsokos, G. CaMK4 Controls the Humoral Response via the T Follicular Helper Compartment and Regulates Autoimmunity. Arthritis Rheumatol. 2022, 74 (Suppl. 9), 8524. Available online: https://acrabstracts.org/abstract/camk4-controls-the-humoral-response-via-the-t-follicular-helper-compartment-and-regulates-autoimmunity/ (accessed on 5 December 2022).
- Kaiser, M.; Wiggin, G.R.; Lightfoot, K.; Arthur, J.S.; Macdonald, A. MSK regulate TCR-induced CREB phosphorylation but not immediate early gene transcription. Eur. J. Immunol. 2007, 37, 2583–2595. [Google Scholar] [CrossRef] [PubMed]
- Yu, C.T.; Shih, H.M.; Lai, M.Z. Multiple signals required for cyclic AMP-responsive element binding protein (CREB) binding protein interaction induced by CD3/CD28 costimulation. J. Immunol. 2001, 166, 284–292. [Google Scholar] [CrossRef] [PubMed]
- Hu, S.C.; Chrivia, J.; Ghosh, A. Regulation of CBP-mediated transcription by neuronal calcium signaling. Neuron 1999, 22, 799–808. [Google Scholar] [CrossRef] [PubMed]
- Anderson, K.A.; Means, A.R. Defective signaling in a subpopulation of CD4+ T cells in the absence of Ca2+/calmodulin-dependent protein kinase IV. Mol. Cell. Biol. 2002, 22, 23–29. [Google Scholar] [CrossRef] [PubMed]
- Illario, M.; Giardino-Torchia, M.L.; Sankar, U.; Ribar, T.J.; Galgani, M.; Vitiello, L.; Masci, A.M.; Bertani, F.R.; Ciaglia, E.; Astone, D.; et al. Calmodulin-dependent kinase IV links Toll-like receptor 4 signaling with survival pathway of activated dendritic cells. Blood 2008, 111, 723–731. [Google Scholar] [CrossRef]
- Kanwisher, N.; McDermott, J.; Chun, M.M. The fusiform face area: A module in human extrastriate cortex specialized for face perception. J. Neurosci. 1997, 17, 4302–4311. [Google Scholar] [CrossRef]
- Trebak, M.; Kinet, J.P. Calcium signalling in T cells. Nat. Rev. Immunol. 2019, 19, 154–169. [Google Scholar] [CrossRef]
- Vono, M.; Taccone, M.; Caccin, P.; Gallotta, M.; Donvito, G.; Falzoni, S.; Palmieri, E.; Pallaoro, M.; Rappuoli, R.; Di Virgilio, F.; et al. The adjuvant MF59 induces ATP release from muscle that potentiates response to vaccination. Proc. Natl. Acad. Sci. USA 2013, 110, 21095–21100. [Google Scholar] [CrossRef] [PubMed]
| Variables | Fluad (n = 117) | Fluzone (n = 117) | Total (n = 234) | p-Value |
|---|---|---|---|---|
| Sex | 0.418 * | |||
| Female | 76 (65.0%) | 70 (59.8%) | 146 (62.4%) | |
| Male | 41 (35.0%) | 47 (40.2%) | 88 (37.6%) | |
| Age at enrollment (years) | 0.556 ** | |||
| Median | 71.900 | 71.100 | 71.500 | |
| Q1, Q3 | 68.200, 76.500 | 67.700, 75.600 | 67.900, 76.000 | |
| Ethnicity | 1.000 * | |||
| Non-Hispanic or Latino | 115 (98.3%) | 115 (98.3%) | 230 (98.3%) | |
| Hispanic or Latino | 2 (1.7%) | 2 (1.7%) | 4 (1.7%) | |
| Race | 0.316 * | |||
| Black or African American | 1 (0.9%) | 0 (0.0%) | 1 (0.4%) | |
| White | 116 (99.1%) | 117 (100.0%) | 233 (99.6%) | |
| Weight (kg) | 0.977 ** | |||
| Median | 79.5 | 81.1 | 80.3 | |
| Q1, Q3 | 68.9, 91.1 | 68.1, 90.7 | 68.5, 91.0 | |
| Height (cm) | 0.221 ** | |||
| Median | 164.4 | 165.9 | 164.9 | |
| Q1, Q3 | 159.1, 173.5 | 161.2, 175.2 | 160.1, 174.4 | |
| BMI | 0.333 ** | |||
| Median | 28.7 | 28.0 | 28.3 | |
| Q1, Q3 | 25.3, 31.9 | 24.9, 31.1 | 25.2, 31.6 | |
| HAI titer (Day 28/Day 0) *** | 0.062 ** | |||
| Median | 1.000 | 1.000 | 1.000 | |
| Q1, Q3 | 0.000, 2.000 | 1.000, 2.000 | 0.000, 2.000 | |
| Day 28/Day 0 Gene Expression Change (Interaction Model 1) * | ||||
|---|---|---|---|---|
| Gene Symbol | Entrezgene Description | Estimate | p-Value ** | q-Value *** |
| SLC2A11 | solute carrier family 2 member 11 | 0.6034 | 8.19 × 10−6 | 0.0701 |
| RP11-779O18.2 | pre-mRNA processing factor 31 | −0.5604 | 2.65 × 10−5 | 0.1137 |
| SLC38A5 | solute carrier family 38 member 5 | −0.5324 | 0.0001 | 0.1575 |
| WDR35 | WD repeat domain 35 | 0.5332 | 0.0001 | 0.1575 |
| APOBR | apolipoprotein B receptor | −0.5347 | 0.0001 | 0.1575 |
| CC2D1A | coiled-coil and C2 domain containing 1A | −0.4902 | 0.0002 | 0.1575 |
| MYO9B | myosin IXB | −0.5059 | 0.0002 | 0.1575 |
| PRKD2 | protein kinase D2 | −0.5016 | 0.0002 | 0.1575 |
| SLC25A53 | solute carrier family 25 member 53 | 0.4843 | 0.0003 | 0.1575 |
| TMEM38B | transmembrane protein 38B | 0.4931 | 0.0003 | 0.1575 |
| B4GALT4 | beta-1,4-galactosyltransferase 4 | 0.4931 | 0.0003 | 0.1575 |
| SLC23A3 | solute carrier family 23 member 3 | 0.4830 | 0.0003 | 0.1575 |
| GORASP1 | golgi reassembly stacking protein 1 | 0.4830 | 0.0004 | 0.1575 |
| ERLIN2 | ER lipid raft associated 2 | 0.4828 | 0.0004 | 0.1575 |
| PITPNM1 | phosphatidylinositol transfer protein membrane-associated 1 | −0.4753 | 0.0004 | 0.1575 |
| MAP4 | microtubule associated protein 4 | −0.4993 | 0.0004 | 0.1575 |
| MVB12B | multivesicular body subunit 12B | −0.4904 | 0.0004 | 0.1575 |
| PKIG | cAMP-dependent protein kinase inhibitor gamma | 0.4634 | 0.0005 | 0.1575 |
| POP5 | POP5 homolog, ribonuclease P/MRP subunit | 0.4947 | 0.0005 | 0.1575 |
| C17orf51 | long intergenic non-protein coding RNA 2693 | 0.4690 | 0.0005 | 0.1575 |
| PLEKHG5 | pleckstrin homology and RhoGEF domain containing G5 | −0.4809 | 0.0005 | 0.1575 |
| CAMK4 | calcium-/calmodulin-dependent protein kinase IV | 0.4991 | 0.0008 | 0.1575 |
| PIAS3 | protein inhibitor of activated STAT 3 | −0.4458 | 0.0009 | 0.1598 |
| TRAF7 | TNF receptor-associated factor 7 | −0.4395 | 0.0012 | 0.1684 |
| COX6B1 | cytochrome c oxidase subunit 6B1 | 0.4254 | 0.0017 | 0.1783 |
| GATA3 | GATA-binding protein 3 | −0.4358 | 0.0019 | 0.1861 |
| IL36A | interleukin 36 alpha | 0.4159 | 0.0024 | 0.1967 |
| MAP3K13 | mitogen-activated protein kinase 13 | 0.4208 | 0.0025 | 0.1995 |
| CREBBP | CREB-binding protein | −0.3594 | 0.0086 | 0.2562 |
| Baseline (Day 0) Gene Expression (Interaction Model 2) * | ||||
| Gene Symbol | Entrezgene Description | Estimate | p-Value ** | q-Value *** |
| FAM136A | family with sequence similarity 136 member A | 0.5098 | 0.0002 | 0.4912 |
| POLA1 | DNA polymerase alpha 1, catalytic subunit | −0.4990 | 0.0002 | 0.4912 |
| CCER2 | coiled-coil glutamate rich protein 2 | 0.4806 | 0.0004 | 0.4912 |
| MLLT4/AFDN | afadin, adherens junction formation factor | 0.4812 | 0.0004 | 0.4912 |
| AMY2B | amylase alpha 2B | 0.4763 | 0.0004 | 0.4912 |
| HECTD3 | HECT domain E3 ubiquitin protein ligase 3 | 0.4935 | 0.0005 | 0.4912 |
| C2orf66 | chromosome 2 open reading frame 66 | 0.4616 | 0.0006 | 0.4912 |
| PIGW | phosphatidylinositol glycan anchor biosynthesis class W | 0.4605 | 0.0006 | 0.4912 |
| SLC23A3 | solute carrier family 23 member 3 | −0.4616 | 0.0006 | 0.4912 |
| BAIAP3 | BAI1-associated protein 3 | 0.4622 | 0.0007 | 0.4912 |
| ITIH4 | inter-alpha-trypsin inhibitor heavy chain 4 | 0.4559 | 0.0007 | 0.4912 |
| MUSTN1 | musculoskeletal, embryonic nuclear protein 1 | 0.4587 | 0.0007 | 0.4912 |
| MBTPS2 | membrane bound transcription factor peptidase, site 2 | −0.4660 | 0.0008 | 0.4912 |
| MYO19 | myosin XIX | 0.4509 | 0.0008 | 0.4912 |
| NLRX1 | NLR family member X1 | −0.4588 | 0.0009 | 0.4912 |
| RP5-966M1.6 | SREBF2 antisense RNA 1 | 0.4501 | 0.0009 | 0.4912 |
| PIAS3 | protein inhibitor of activated STAT 3 | 0.4545 | 0.0009 | 0.4912 |
| C14orf119 | chromosome 14 open reading frame 119 | −0.4441 | 0.0010 | 0.4912 |
| ALDH8A1 | aldehyde dehydrogenase 8 family member A1 | 0.4435 | 0.0011 | 0.4912 |
| MRPS28 | mitochondrial ribosomal protein S28 | −0.4512 | 0.0012 | 0.4912 |
| Day 28 Gene Expression (Interaction Model 3) * | ||||
| Gene Symbol | Entrezgene Description | Estimate | p-Value ** | q-Value *** |
| COQ4 | coenzyme Q4 | 0.5698 | 2.15 × 10−5 | 0.2293 |
| EEFSEC | eukaryotic elongation factor, selenocysteine tRNA-specific | −0.5304 | 0.0001 | 0.3727 |
| NEIL1 | nei-like DNA glycosylase 1 | 0.5243 | 0.0001 | 0.3727 |
| SLC25A14 | solute carrier family 25 member 14 | 0.5000 | 0.0002 | 0.4405 |
| CNTD2/CCNP | cyclin P | 0.4896 | 0.0003 | 0.4405 |
| PSMC3IP | PSMC3 interacting protein | 0.4946 | 0.0003 | 0.4405 |
| PPM1N | protein phosphatase, Mg2+/Mn2+-dependent 1N | 0.4857 | 0.0003 | 0.4405 |
| MYO19 | myosin XIX | 0.4800 | 0.0003 | 0.4405 |
| BAIAP3 | BAI1-associated protein 3 | 0.4668 | 0.0007 | 0.7302 |
| BIVM | basic, immunoglobulin-like variable motif containing | 0.4614 | 0.0008 | 0.7302 |
| ECHDC2 | enoyl-CoA hydratase domain containing 2 | 0.4586 | 0.0008 | 0.7302 |
| TTC8 | tetratricopeptide repeat domain 8 | 0.4493 | 0.0008 | 0.7364 |
| POGLUT1 | protein O-glucosyltransferase 1 | 0.4451 | 0.0009 | 0.7598 |
| TRIP6 | thyroid hormone receptor interactor 6 | 0.4316 | 0.0011 | 0.7658 |
| SAPCD1 | suppressor APC domain containing 1 | 0.4472 | 0.0012 | 0.7658 |
| CYP4F12 | cytochrome P450 family 4 subfamily F member 12 | 0.4541 | 0.0014 | 0.7658 |
| MFI2/MELTF | melanotransferrin | 0.4405 | 0.0014 | 0.7658 |
| LPPR2/PLPPR2 | phospholipid phosphatase-related 2 | 0.4282 | 0.0014 | 0.7658 |
| FN3KRP | fructosamine 3 kinase-related protein | 0.4198 | 0.0016 | 0.7658 |
| RABL2A | RAB, member of RAS oncogene family-like 2A | 0.4191 | 0.0016 | 0.7658 |
| KEGG Pathway * | Set Size | p-Value | FDR *** |
|---|---|---|---|
| Herpes simplex virus 1 infection | 388 | 1.48 × 10−6 | 0.001 |
| MAPK signaling pathway ** | 150 | 3.11 × 10−6 | 0.001 |
| Endocytosis | 142 | 2.60 × 10−5 | 0.003 |
| Human papillomavirus infection | 158 | 3.77 × 10−5 | 0.003 |
| Thyroid hormone signaling pathway | 69 | 4.57 × 10−5 | 0.003 |
| Kaposi sarcoma-associated herpesvirus infection | 101 | 3.19 × 10−4 | 0.011 |
| Tight junction ** | 81 | 3.38 × 10−4 | 0.011 |
| C-type lectin receptor signaling pathway | 56 | 3.54 × 10−4 | 0.011 |
| Folate biosynthesis | 14 | 4.15 × 10−4 | 0.011 |
| PD-L1 expression and PD-1 checkpoint pathway in cancer | 45 | 4.18 × 10−4 | 0.011 |
| Glucagon signaling pathway | 51 | 4.18 × 10−4 | 0.011 |
| Rap1 signaling pathway ** | 100 | 4.56 × 10−4 | 0.011 |
| Relaxin signaling pathway | 55 | 4.59 × 10−4 | 0.011 |
| TNF signaling pathway ** | 62 | 6.22 × 10−4 | 0.013 |
| GnRH signaling pathway | 44 | 6.59 × 10−4 | 0.013 |
| NOD-like receptor signaling pathway ** | 95 | 9.54 × 10−4 | 0.017 |
| Hepatitis B | 84 | 1.08 × 10−3 | 0.018 |
| Glycosaminoglycan biosynthesis—chondroitin/dermatan sulfate | 16 | 1.24 × 10−3 | 0.019 |
| Notch signaling pathway ** | 40 | 1.34 × 10−3 | 0.020 |
| Human T-cell leukemia virus 1 infection | 128 | 1.45 × 10−3 | 0.020 |
| Glutamatergic synapse | 44 | 1.67 × 10−3 | 0.022 |
| IL-17 signaling pathway ** | 40 | 1.78 × 10−3 | 0.023 |
| Transcriptional misregulation in cancer | 103 | 2.01 × 10−3 | 0.025 |
| Human cytomegalovirus infection | 112 | 2.52 × 10−3 | 0.028 |
| Epstein-Barr virus infection | 114 | 2.66 × 10−3 | 0.028 |
| Phospholipase D signaling pathway | 69 | 2.72 × 10−3 | 0.028 |
| Viral carcinogenesis | 110 | 2.96 × 10−3 | 0.029 |
| Calcium signaling pathway ** | 90 | 2.96 × 10−3 | 0.029 |
| Neutrophil extracellular trap formation | 87 | 3.05 × 10−3 | 0.029 |
| Estrogen signaling pathway | 56 | 3.14 × 10−3 | 0.029 |
| AGE-RAGE signaling pathway in diabetic complications | 52 | 3.50 × 10−3 | 0.030 |
| Growth hormone synthesis, secretion, and action | 55 | 4.56 × 10−3 | 0.036 |
| Circadian entrainment | 39 | 4.63 × 10−3 | 0.036 |
| Serotonergic synapse | 41 | 4.94 × 10−3 | 0.038 |
| Insulin signaling pathway | 72 | 5.22 × 10−3 | 0.039 |
| RNA polymerase | 29 | 6.12 × 10−3 | 0.044 |
| Inflammatory mediator regulation of TRP channels ** | 49 | 6.28 × 10−3 | 0.044 |
| Fatty acid elongation | 16 | 6.55 × 10−3 | 0.045 |
| Th1 and Th2 cell differentiation ** | 50 | 7.04 × 10−3 | 0.046 |
| Necroptosis | 76 | 7.04 × 10−3 | 0.046 |
| Dopaminergic synapse | 55 | 7.21 × 10−3 | 0.047 |
| Apoptosis ** | 90 | 7.35 × 10−3 | 0.047 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Haralambieva, I.H.; Quach, H.Q.; Ovsyannikova, I.G.; Goergen, K.M.; Grill, D.E.; Poland, G.A.; Kennedy, R.B. T Cell Transcriptional Signatures of Influenza A/H3N2 Antibody Response to High Dose Influenza and Adjuvanted Influenza Vaccine in Older Adults. Viruses 2022, 14, 2763. https://doi.org/10.3390/v14122763
Haralambieva IH, Quach HQ, Ovsyannikova IG, Goergen KM, Grill DE, Poland GA, Kennedy RB. T Cell Transcriptional Signatures of Influenza A/H3N2 Antibody Response to High Dose Influenza and Adjuvanted Influenza Vaccine in Older Adults. Viruses. 2022; 14(12):2763. https://doi.org/10.3390/v14122763
Chicago/Turabian StyleHaralambieva, Iana H., Huy Quang Quach, Inna G. Ovsyannikova, Krista M. Goergen, Diane E. Grill, Gregory A. Poland, and Richard B. Kennedy. 2022. "T Cell Transcriptional Signatures of Influenza A/H3N2 Antibody Response to High Dose Influenza and Adjuvanted Influenza Vaccine in Older Adults" Viruses 14, no. 12: 2763. https://doi.org/10.3390/v14122763
APA StyleHaralambieva, I. H., Quach, H. Q., Ovsyannikova, I. G., Goergen, K. M., Grill, D. E., Poland, G. A., & Kennedy, R. B. (2022). T Cell Transcriptional Signatures of Influenza A/H3N2 Antibody Response to High Dose Influenza and Adjuvanted Influenza Vaccine in Older Adults. Viruses, 14(12), 2763. https://doi.org/10.3390/v14122763






