First-Trimester Influenza Infection Increases the Odds of Non-Chromosomal Birth Defects: A Systematic Review and Meta-Analysis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Systematic Search
2.2. Selection and Eligibility Criteria
2.3. Data Extraction
2.4. Data Synthesis and Analysis
2.5. Risk of Bias Assessment and Quality of Evidence
3. Results
3.1. Search and Selection
3.2. Basic Characteristics of Included Studies
3.3. Non-Chromosomal Congenital Malformations
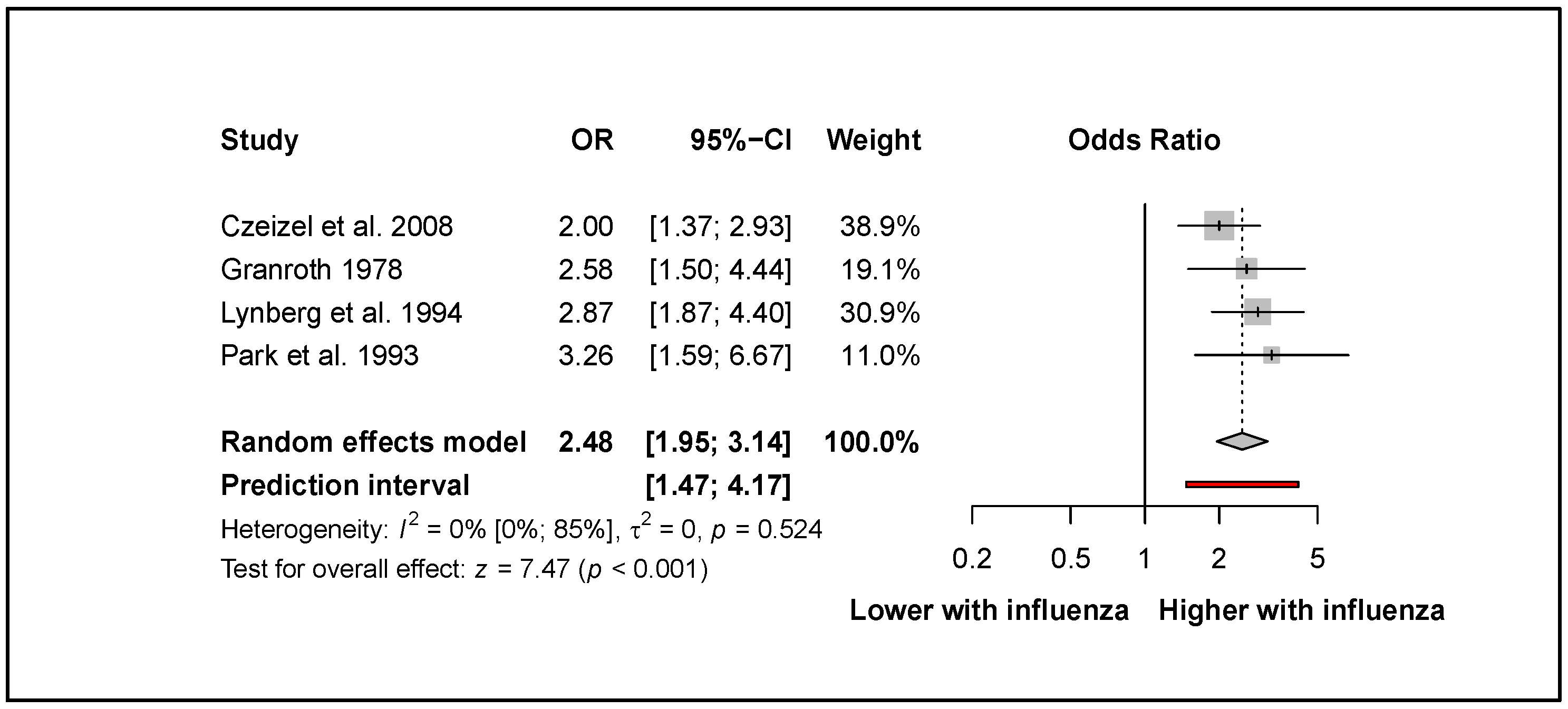
3.4. Influenza Increases the Odds of Neural Tube Defects
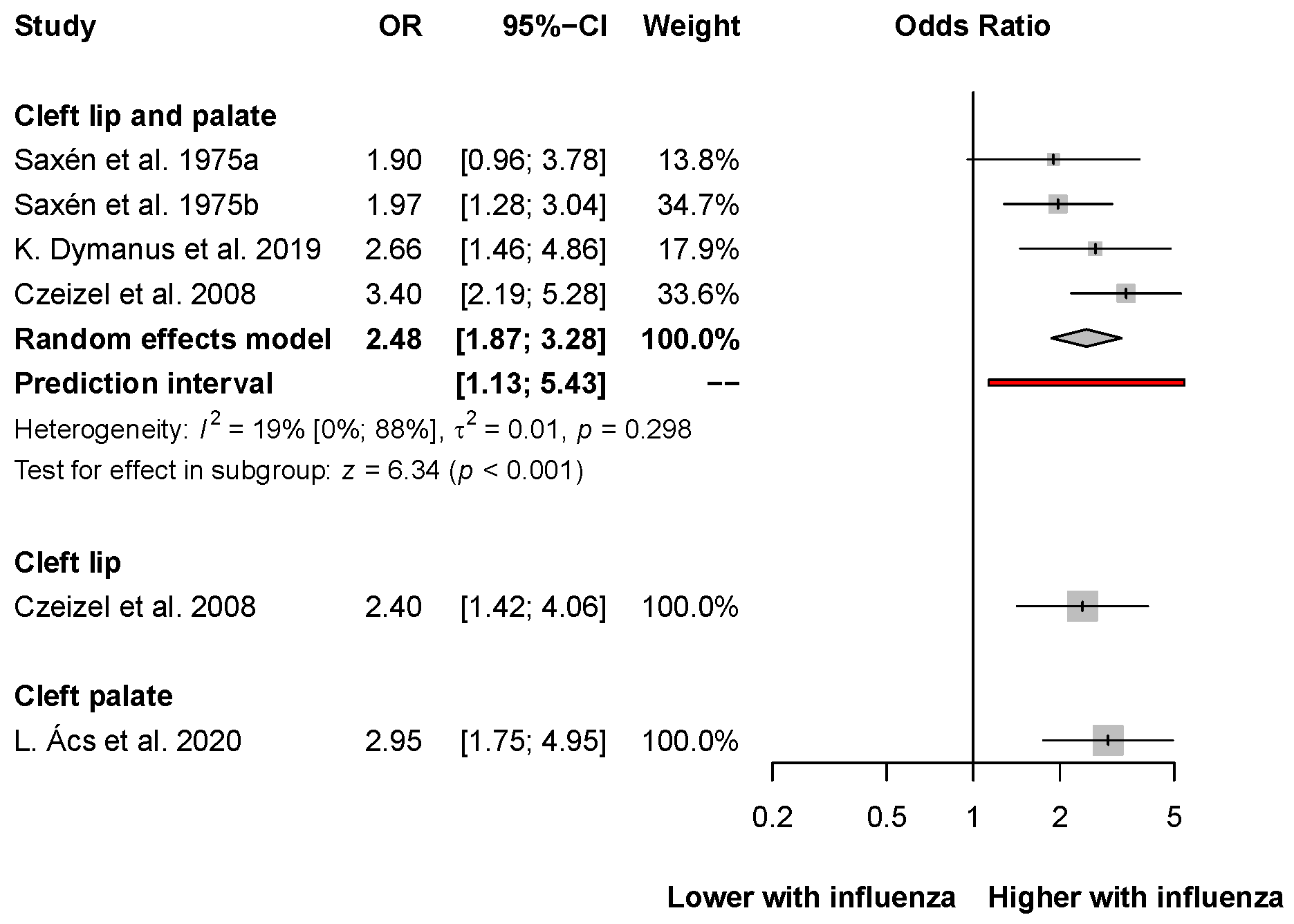
3.5. Oral Clefts
3.6. Congenital Heart Defects
3.7. Other Types of Birth Defects
3.8. Risk of Bias Assessment and Level of Evidence
4. Discussion
4.1. Strengths and Limitation
4.2. Implications for Practice and Research
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Qiao, J. What are the risks of COVID-19 infection in pregnant women? Lancet 2020, 395, 760–762. [Google Scholar] [CrossRef] [Green Version]
- Chen, H.; Guo, J.; Wang, C.; Luo, F.; Yu, X.; Zhang, W.; Li, J.; Zhao, D.; Xu, D.; Gong, Q.; et al. Clinical characteristics and intrauterine vertical transmission potential of COVID-19 infection in nine pregnant women: A retrospective review of medical records. Lancet 2020, 395, 809–815. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Silasi, M.; Cardenas, I.; Kwon, J.Y.; Racicot, K.; Aldo, P.; Mor, G. Viral infections during pregnancy. Am. J. Reprod. Immunol. 2015, 73, 199–213. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Racicot, K.; Mor, G. Risks associated with viral infections during pregnancy. J. Clin. Investig. 2017, 127, 1591–1599. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pleschka, S. Overview of influenza viruses. In Current Topics in Microbiology and Immunology; Springer: Berlin/Heidelberg, Germany, 2013; Volume 370, pp. 1–20. [Google Scholar] [CrossRef]
- Ghebrehewet, S.; MacPherson, P.; Ho, A. Influenza. BMJ 2016, 355, i6258. [Google Scholar] [CrossRef] [Green Version]
- Waller, D.K.; Hashmi, S.S.; Hoyt, A.T.; Duong, H.T.; Tinker, S.C.; Gallaway, M.S.; Olney, R.S.; Finnell, R.H.; Hecht, J.T.; Canfield, M.A. Maternal report of fever from cold or flu during early pregnancy and the risk for noncardiac birth defects, National Birth Defects Prevention Study, 1997–2011. Birth Defects Res. 2018, 110, 342–351. [Google Scholar] [CrossRef]
- Li, M.; Liu, Z.; Lin, Y.; Chen, X.; Li, S.; You, F.; Deng, Y.; Li, N.; Wang, Y.; Zhang, Y.; et al. Maternal influenza-like illness, medication use during pregnancy and risk of congenital heart defects in offspring. J. Matern. Fetal. Neonatal. Med. 2014, 27, 807–811. [Google Scholar] [CrossRef]
- Coffey, V.P.; Jessop, W.J. Maternal influenza and congenital deformities: A prospective study. Lancet 1959, 2, 935–938. [Google Scholar] [CrossRef]
- Campbell, W.A. Influenza in early pregnancy; effects on the foetus. Lancet 1953, 1, 173–174. [Google Scholar] [CrossRef]
- Leck, I. Incidence of malformations following influenza epidemics. Br. J. Prev. Soc. Med. 1963, 17, 70–80. [Google Scholar] [CrossRef] [Green Version]
- Doll, R.; Hill, A.B.; Sakula, J. Asian influenza in pregnancy and congenital defects. Br. J. Prev. Soc. Med. 1960, 14, 167–172. [Google Scholar] [CrossRef] [Green Version]
- Luteijn, J.M.; Brown, M.J.; Dolk, H. Influenza and congenital anomalies: A systematic review and meta-analysis. Hum. Reprod. 2014, 29, 809–823. [Google Scholar] [CrossRef] [Green Version]
- Mai, C.T.; Isenburg, J.L.; Canfield, M.A.; Meyer, R.E.; Correa, A.; Alverson, C.J.; Lupo, P.J.; Riehle-Colarusso, T.; Cho, S.J.; Aggarwal, D.; et al. National population-based estimates for major birth defects, 2010–2014. Birth Defects Res. 2019, 111, 1420–1435. [Google Scholar] [CrossRef]
- Canfield, M.A.; Honein, M.A.; Yuskiv, N.; Xing, J.; Mai, C.T.; Collins, J.S.; Devine, O.; Petrini, J.; Ramadhani, T.A.; Hobbs, C.A.; et al. National estimates and race/ethnic-specific variation of selected birth defects in the United States, 1999–2001. Birth Defects Res. Part A Clin. Mol. Teratol. 2006, 76, 747–756. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Brooke, B.S.; Schwartz, T.A.; Pawlik, T.M. MOOSE Reporting Guidelines for Meta-analyses of Observational Studies. JAMA Surg. 2021, 156, 787–788. [Google Scholar] [CrossRef]
- Chandler, J.; Hopewell, S. Cochrane methods--twenty years experience in developing systematic review methods. Syst. Rev. 2013, 2, 76. [Google Scholar] [CrossRef] [Green Version]
- McHugh, M.L. Interrater reliability: The kappa statistic. Biochem. Med. 2012, 22, 276–282. [Google Scholar] [CrossRef]
- Schwarzer, G.; Carpenter, J.R.; Rücker, G. Fixed effect and random effects meta-analysis. In Meta-Analysis with R; Springer: Berlin/Heidelberg, Germany, 2015; pp. 21–53. [Google Scholar]
- Mantel, N.; Haenszel, W. Statistical aspects of the analysis of data from retrospective studies of disease. J. Natl. Cancer Inst. 1959, 22, 719–748. [Google Scholar]
- Thompson, S.G.; Turner, R.M.; Warn, D.E. Multilevel models for meta-analysis, and their application to absolute risk differences. Stat. Methods Med. Res. 2001, 10, 375–392. [Google Scholar] [CrossRef] [PubMed]
- Paule, R.C.; Mandel, J. Consensus Values and Weighting Factors. J. Res. Natl. Bur. Stand. 1982, 87, 377–385. [Google Scholar] [CrossRef] [PubMed]
- Harrer, M.; Cuijpers, P.; Furukawa, T.A.; Ebert, D.D. Doing Meta-Analysis with R: A Hands-On Guide, 1st ed.; Chapman & Hall/CRC Press: Boca Raton, FL, USA; London, UK, 2021. [Google Scholar]
- IntHout, J.; Ioannidis, J.P.; Rovers, M.M.; Goeman, J.J. Plea for routinely presenting prediction intervals in meta-analysis. BMJ Open 2016, 6, e010247. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sterne, J.A.C.; Savović, J.; Page, M.J.; Elbers, R.G.; Blencowe, N.S.; Boutron, I.; Cates, C.J.; Cheng, H.Y.; Corbett, M.S.; Eldridge, S.M.; et al. RoB 2: A revised tool for assessing risk of bias in randomised trials. BMJ 2019, 366, l4898. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McGuinness, L.A.; Higgins, J.P.T. Risk-of-bias VISualization (robvis): An R package and Shiny web app for visualizing risk-of-bias assessments. Res. Synth. Methods 2021, 12, 55–61. [Google Scholar] [CrossRef]
- Tool, G.G.D. GRADEPro Guideline Development Tool. Available online: www.gradepro.org/ (accessed on 19 July 2022).
- Ács, L.; Bányai, D.; Nemes, B.; Nagy, K.; Ács, N.; Bánhidy, F.; Rózsa, N. Maternal-related factors in the origin of isolated cleft palate-A population-based case-control study. Orthod. Craniofac. Res. 2020, 23, 174–180. [Google Scholar] [CrossRef]
- Acs, N.; Bánhidy, F.; Puhó, E.; Czeizel, A.E. Maternal influenza during pregnancy and risk of congenital abnormalities in offspring. Birth Defects Res. Part A Clin. Mol. Teratol. 2005, 73, 989–996. [Google Scholar] [CrossRef]
- Aro, T. Maternal diseases, alcohol consumption and smoking during pregnancy associated with reduction limb defects. Early Hum. Dev. 1983, 9, 49–57. [Google Scholar] [CrossRef]
- Botto, L.D.; Lynberg, M.C.; Erickson, J.D. Congenital heart defects, maternal febrile illness, and multivitamin use: A population-based study. Epidemiology 2001, 12, 485–490. [Google Scholar] [CrossRef] [Green Version]
- Busby, A.; Dolk, H.; Armstrong, B. Eye anomalies: Seasonal variation and maternal viral infections. Epidemiology 2005, 16, 317–322. [Google Scholar] [CrossRef]
- Czeizel, A.E.; Puhó, E.H.; Acs, N.; Bánhidy, F. Use of specified critical periods of different congenital abnormalities instead of the first trimester concept. Birth Defects Res. A Clin. Mol. Teratol. 2008, 82, 139–146. [Google Scholar] [CrossRef]
- Granroth, G. Defects of the central nervous system in Finland: III. Disease and drugs in pregnancy. Early Hum. Dev. 1978, 2, 147–162. [Google Scholar] [CrossRef]
- Lynberg, M.C.; Khoury, M.J.; Lu, X.; Cocian, T. Maternal flu, fever, and the risk of neural tube defects: A population-based case-control study. Am. J. Epidemiol. 1994, 140, 244–255. [Google Scholar] [CrossRef]
- Ou, Y.; Mai, J.; Zhuang, J.; Liu, X.; Wu, Y.; Gao, X.; Nie, Z.; Qu, Y.; Chen, J.; Kielb, C.; et al. Risk factors of different congenital heart defects in Guangdong, China. Pediatr. Res. 2016, 79, 549–558. [Google Scholar] [CrossRef] [Green Version]
- Park, C.H.; Stewart, W.; Khoury, M.J.; Mulinare, J. Is there etiologic heterogeneity between upper and lower neural tube defects? Am. J. Epidemiol. 1993, 136, 1493–1501. [Google Scholar] [CrossRef]
- Saxén, I. Epidemiology of cleft lip and palate. An attempt to rule out chance correlations. Br. J. Prev. Soc. Med. 1975, 29, 103–110. [Google Scholar] [CrossRef] [Green Version]
- Saxén, I. The association between maternal influenza, drug consumption and oral clefts. Acta Odontol. Scand. 1975, 33, 259–267. [Google Scholar] [CrossRef]
- Dymanus, K.; Yu, J.; Carroll, W.; Lima, M.H.; Linder, D. The association of influenza on the incidence of orofacial clefts. Cleft Palate-Craniofacial. J. 2019, 56, 61–62. [Google Scholar] [CrossRef]
- Morens, D.M.; Daszak, P.; Markel, H.; Taubenberger, J.K. Pandemic COVID-19 Joins History’s Pandemic Legion. mBio 2020, 11, e00812-20. [Google Scholar] [CrossRef]
- Oster, M.E.; Riehle-Colarusso, T.; Alverson, C.J.; Correa, A. Associations between maternal fever and influenza and congenital heart defects. J. Pediatr. 2011, 158, 990–995. [Google Scholar] [CrossRef]
- Kurppa, K.; Holmberg, P.C.; Kuosma, E.; Aro, T.; Saxén, L. Anencephaly and maternal common cold. Teratology 1991, 44, 51–55. [Google Scholar] [CrossRef] [PubMed]
- Medveczky, E.; Puhó, E.; Czeizel, A.E. An evaluation of maternal illnesses in the origin of neural-tube defects. Arch. Gynecol. Obstet. 2004, 270, 244–251. [Google Scholar] [CrossRef] [PubMed]
- Shaw, G.M.; Todoroff, K.; Velie, E.M.; Lammer, E.J. Maternal illness, including fever and medication use as risk factors for neural tube defects. Teratology 1998, 57, 1–7. [Google Scholar] [CrossRef]
- Kennedy, D.; Chitayat, D.; Winsor, E.J.; Silver, M.; Toi, A. Prenatally diagnosed neural tube defects: Ultrasound, chromosome, and autopsy or postnatal findings in 212 cases. Am. J. Med. Genet. 1998, 77, 317–321. [Google Scholar] [CrossRef]
- Avagliano, L.; Massa, V.; George, T.M.; Qureshy, S.; Bulfamante, G.P.; Finnell, R.H. Overview on neural tube defects: From development to physical characteristics. Birth Defects Res. 2019, 111, 1455–1467. [Google Scholar] [CrossRef]
- Chitayat, D.; Matsui, D.; Amitai, Y.; Kennedy, D.; Vohra, S.; Rieder, M.; Koren, G. Folic acid supplementation for pregnant women and those planning pregnancy: 2015 update. J. Clin. Pharmacol. 2016, 56, 170–175. [Google Scholar] [CrossRef]
- Allam, E.; Windsor, L.; Stone, C. Cleft lip and palate: Etiology, epidemiology, preventive and intervention strategies. Anat. Physiol. 2014, 4, 3. [Google Scholar]
- Mitchell, J.C.; Wood, R.J. Management of cleft lip and palate in primary care. J. Pediatr. Health Care 2000, 14, 13–19. [Google Scholar] [CrossRef]
- Jones, M.C. Facial clefting. Etiology and developmental pathogenesis. Clin. Plast. Surg. 1993, 20, 599–606. [Google Scholar] [CrossRef]
- Voigt, A.; Radlanski, R.J.; Sarioglu, N.; Schmidt, G. [Cleft lip and palate]. Pathologe 2017, 38, 241–247. [Google Scholar] [CrossRef]
- Li, Z.; Ren, A.; Liu, J.; Pei, L.; Zhang, L.; Guo, Z.; Li, Z. Maternal flu or fever, medication use, and neural tube defects: A population-based case-control study in Northern China. Birth Defects Res. Part A Clin. Mol. Teratol. 2007, 79, 295–300. [Google Scholar] [CrossRef]
- Hegyi, P.; Erőss, B.; Izbéki, F.; Párniczky, A.; Szentesi, A. Accelerating the translational medicine cycle: The Academia Europaea pilot. Nat. Med. 2021, 27, 1317–1319. [Google Scholar] [CrossRef]
- Czeizel, A.E.; Acs, N.; Banhidy, F.; Puho, E.H.; Vogt, G. Primary prevention of congenital abnormalities due to high fever related maternal diseases by antifever therapy and folic acid supplementation. Curr. Women’s Health Rev. 2007, 3, 190–201. [Google Scholar] [CrossRef]



| Author, Year | Study Type | Country | Number of the Participant Centers | Examination Period | Number of Patients (Cases-Controls) | Age Groups | Diagnosis of Influenza | Congenital Malformations | Adjusted for |
|---|---|---|---|---|---|---|---|---|---|
| L. Ács et al., 2020 [29] | Case-control | Hungary | No data | 1980–1996, 2007–2009 | 1947 (751–1196) | <23; 23–33; >33 | Prospective, medically recorded data; retrospective questionnaire, supplementary data collection | Cleft palate | |
| Ács et al., 2005 [30] | Case-control | Hungary | No data | 1980–1996 | 3166 (1328–1838) | ≤24; 25–29; >30 | Prospective, medically recorded data; retrospective questionnaire, supplementary data collection | Neural tube defects; Cleft lip/palate; Cleft palate; Esophageal atresia; Pyloric stenosis; Intestinal atresia/stenosis; Rectal/anal stresia/stenosis; Renal a/dysgenesia; Obstructive urinary Cas; Hypospadiasis; Undescended testis; Exomphalos/gastroschisis; Congenital hydrocephaly, Ear CAs, Cardiovascular CAs, Clubfoot, Limb reduction defects, Poly/syndactilia, Diaphragmatic CAs, Other Isolated CAs, Multiple CAs | maternal employment status and use of antipyretic drugs |
| Aro et al., 1983 [31] | Case-control | Finland | No data | 1964–1977 | 906 (453–453) | No data | Personal visits, questionnaire | limb reduction defects | |
| Botto et al., 2001 [32] | Case-control | USA | No data | 1968–1980 | 3934 (905–3029) | 11–19, 20–24, 25–29, 30–34, >35 | Telephone interview | Congenital heart defects, Transposition of great arteries, Tetralogy of Fallot, Atrioventricular septal defect, Ebstein anomaly, Anomalous pulmonary venous return, All right obstructive defects, Tricuspid atresia, All left obstructive defects, Hypoplastic left heart, Aortic stenosis, Aortic coarctation, Ventricular septal defect, Atrial septal defect | maternal race, education, multivitamin use, smoking, alcohol use, chronic illnesses, and period of birth of the child |
| Busby et al., 2005 [33] | England | No data | 1987–1994 | 275 | General practitioner data, laboratory data | Anophtalmia, Micophtalmia | |||
| Czeizel et al., 2008 [34] | Case-control | Hungary | No data | 1980–1996 | 3754 (1349–2405) | No data | Prospective, medically recorded data; retrospective questionnaire, supplementray data collection | Neural-tube defects, Anencephalus+-spina bifida, Spina bifida aperta/cystica, Encephalocele, occipital, Microcephaly, primary, Congenital hydrocephalus, CAs of eye, Anophthalmia–microphthalmia, Primary congenital glaucoma, Congenital cataract, Ocular coloboma, CAs of ear, Auditory canal+ear Cas, An/microtia, Others, unspecified, Cardiovascular CAs, Transposition of great vessels, Ventricular septal defect, Atrial septal defect, type II, Hypoplastic left heart, Patent ductus arteriosus, CAs of aorta, CAs of pulmonary valve, Others or unspecified, Brachial cyst, cleft, fistula, preauricular sinus, CAs of respiratory system, Cleft palate, Robin sequence, Cleft lip+-cleft palate, Cleft lip, Cleft lip with palate, Esophageal atresia/stenosis with or without tracheoesophageal fistula, Cong hypertrophic pyloric stenosis, Atresia/stenosis of small intestine, Atresia/stenosis of rectum/anal canal, Other CAs of digestive system, Hirschprung’s disease, Other CAs of intestine, Other CAs of digestive system, Undescended testis (diagnosed after 3rd postnatal month), Hypospadias (without coronal type), Other CAs of genital organs, Renal a/dysgenesis, Obstructive CAs of urinary tract, Cystic kidney (diseases), Obstructive CAs of renal pelvis and ureter (hydronephrosis, constricture of ureteropelvic junction and ureterovesical orifice), Other CAs of urinary tract, Other CAs of kidney, Other CAs of bladder and urethrea, Clubfoot, Poly/syndactyly, Polydactyly, Syndactyly (without minor), Limb deficiencies, Other CAs of limbs, CAs of diaphragm, CAs of abdominal wall (exomphalos and gastroschisis are not differentiated), Multiple CAs (major gene mutations and chromosomal aberrations are excluded) | |
| Dymanus et al., 2019 [41] | Retrospective observational population study | USA | No data | 2004–2013 | 58,270 | No data | Cleft lip | ||
| Granroth 1978 [35] | Case-control | Finland | No data | 1965–1973 | 1420 (710–710) | No data | Questionnaire | Anencephalia, Spina bifida, Congenital hydrocephaly, Microcephaly, Hydrancephaly, Polydactylia | |
| Li et al., 2014 [8] | Case-control | China | No data | 2010–2011 | 710 (294–416) | <20, 20–24, 25–29, 30–34, ≥35 | Questionnaire | All congenital heart defects, Septal defects, Conotruncal defects, Right-sided obstructive defects, Left-sided obstructive defects, Anomalous pulmonary venous return, Other isolated CAs | maternal age, maternal education, maternal BMI, supplementation, and history of pregnancy with any defect |
| Lynberg et al., 1994 [36] | Case-control | USA | No data | 1968–1980 | 329 (31–298) | No data | Questionnaire | Anencephalia, Spina bifida, Encephalocele | maternal age, education, smoking, alcohol consumption, and periconceptional multivitamin use |
| Ou et al., 2015 [37] | Case-control | China | 39 | 2004–2013 | 8068 (4034–4034) | <30, 30–34, 35–40, >40 | Questionnaire | Cardiovascular CAs, Ventricular septal defect, Atrial septal defect, Pulmonary stenosis, Dextro-transposition of great arteries, Tetralogy of Fallot | |
| Park et al., 1993 [38] | Case-control | USA | No data | 1968–1980 | 1490 (304–1186) | 11–19, 20–34, >35 | Questionnaire | Anencephalia, Spina bifida | |
| Saxen et al., 1975 [39] | Case-control | Finland | No data | 1972–1973 | 388 (194–194) | ≥30 (23%) | Maternity records, interview records, death certificates | Cleft lip and palate | |
| Saxen et al., 1975 [40] | Case-control | Finland | No data | 1967–1971 | 1198 (599–599) | ≥30 (30.7%) | Maternity records, interview records, death certificates | Cleft lip and palate |
| Study | Congenital Heart Defects | Odds Ratios |
|---|---|---|
| Li et al., 2014 [8] | Anomalous pulmonary venous return | 0.80 (CI: 0.36−1.79) |
| Czeizel et al., 2008 [34] | Atrial septal defect | 1.70 (CI: 0.42−6.96) |
| Czeizel et al., 2008 [34] | CAs of the aorta | 4.60 (CI: 1.41−15.01) |
| Czeizel et al., 2008 [34] | CAs of the pulmonary valve | 1.20 (CI: 0.38−3.75) |
| Li et al., 2014 [8] | Conotruncal defects | 1.71 (CI: 1.13−2.58) |
| Czeizel et al., 2008 [34] | Hypoplastic left heart | 1.60 (CI: 0.31−8.26) |
| Li et al., 2014 [8] | Left−sided obstructive heart defects | 1.50 (CI: 0.84−2.68) |
| Czeizel et al., 2008 [34] | Patent ductus arteriosus | 0.80 (CI: 0.08−7.55) |
| Li et al., 2014 [8] | Right−sided obstructive heart defects | 1.40 (CI: 0.80−2.45) |
| Li et al., 2014 [8] | Septal defects | 1.92 (CI: 1.31−2.82) |
| Czeizel et al., 2008 [34] | Transposition of great vessels | 2.90 (CI: 0.90−9.32) |
| Czeizel et al., 2008 [34] | Ventricular septal defect | 2.70 (CI: 1.59−4.58) |
| Study | Congenital Heart Defects | Adjusted Odds Ratio | Adjusted for | Type of Logistic Regression |
| Botto et al., 2001 [32] | Anomalous pulmonary venous return | 2.20 (CI: 0.29−16.51) | maternal race, education, multivitamin use, smoking, alcohol use, chronic illnesses, and period of birth of the child | Multiple |
| Botto et al., 2001 [32] | Aortic coarctation | 3.80 (CI: 1.62−8.91) | ||
| Botto et al., 2001 [32] | Aortic stenosis | 4.00 (CI: 0.90−17.84) | ||
| Botto et al., 2001 [32] | Atrial septal defect | 1.00 (CI: 0.12−8.60) | ||
| Botto et al., 2001 [32] | Ebstein anomaly | 3.00 (CI: 0.39−23.19) | ||
| Botto et al., 2001 [32] | Hypoplastic left heart | 1.60 (CI: 0.39−6.55) | ||
| Botto et al., 2001 [32] | Left−sided obstructive heart defects | 2.90 (CI: 1.49−5.65) | ||
| Botto et al., 2001 [32] | Right−sided obstructive heart defects | 2.50 (CI: 1.14−5.49) | ||
| Botto et al., 2001 [32] | Septal defects | 2.00 (CI: 0.38−14.28) | ||
| Botto et al., 2001 [32] | Tetralogy of Fallot | 0.50 (CI: 0.08−3.00) | ||
| Botto et al., 2001 [32] | Transposition of great vessels | 2.10 (CI: 0.80−5.51) | ||
| Botto et al., 2001 [32] | Tricuspid atresia | 7.90 (CI: 0.80−78.47) | ||
| Botto et al., 2001 [32] | Ventricular septal defect | 2.00 (CI: 1.11−3.62) | ||
| Li et al., 2014 [8] | Anomalous pulmonary venous return | 0.68 (CI: 0.28−1.65) | maternal age, maternal education, maternal body mass index (BMI), supplementation, and history of pregnancy with any defect | Multivariate |
| Li et al., 2014 [8] | Conotruncal defects | 1.60 (CI: 1.01−2.52) | ||
| Li et al., 2014 [8] | Left−sided obstructive heart defects | 1.55 (CI: 0.82−2.93) | ||
| Li et al., 2014 [8] | Septal defects | 2.12 (CI: 1.38−3.26) | ||
| Li et al., 2014 [8] | Right−sided obstructive heart defects | 1.26 (CI: 0.68−2.34) | ||
| Ou et al., 2015 [37] | Atrial septal defect | 2.71 (CI: 1.17−6.33) | maternal age, maternal education, maternal pregnancy history, maternal environmental risk exposures, maternal perinatal diseases, and medication use in the first trimester | Multivariate |
| Ou et al., 2015 [37] | Pulmonary stenosis | 0.62 (CI: 0.07−5.58) | ||
| Ou et al., 2015 [37] | Transposition of great vessels | 1.15 (CI: 0.07−19.22) | ||
| Ou et al., 2015 [37] | Ventricular septal defect | 1.19 (CI: 0.71−1.99) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mátrai, Á.; Teutsch, B.; Váradi, A.; Hegyi, P.; Pethő, B.; Fujisawa, A.; Váncsa, S.; Lintner, B.; Melczer, Z.; Ács, N. First-Trimester Influenza Infection Increases the Odds of Non-Chromosomal Birth Defects: A Systematic Review and Meta-Analysis. Viruses 2022, 14, 2708. https://doi.org/10.3390/v14122708
Mátrai Á, Teutsch B, Váradi A, Hegyi P, Pethő B, Fujisawa A, Váncsa S, Lintner B, Melczer Z, Ács N. First-Trimester Influenza Infection Increases the Odds of Non-Chromosomal Birth Defects: A Systematic Review and Meta-Analysis. Viruses. 2022; 14(12):2708. https://doi.org/10.3390/v14122708
Chicago/Turabian StyleMátrai, Ákos, Brigitta Teutsch, Alex Váradi, Péter Hegyi, Boglárka Pethő, Akari Fujisawa, Szilárd Váncsa, Balázs Lintner, Zsolt Melczer, and Nándor Ács. 2022. "First-Trimester Influenza Infection Increases the Odds of Non-Chromosomal Birth Defects: A Systematic Review and Meta-Analysis" Viruses 14, no. 12: 2708. https://doi.org/10.3390/v14122708






