Unravelling Bile Viromes of Free-Range Laying Chickens Clinically Diagnosed with Spotty Liver Disease: Emergence of Many Novel Chaphamaparvoviruses into Multiple Lineages
Abstract
1. Introduction
2. Materials and Methods
2.1. Sampling and Ethical Approval
2.2. Virus Enrichment and Virus Nucleic Acid Extraction
2.3. Next-Generation Sequencing
2.4. Bioinformatic Analyses
2.5. Comparative Genomics and Phylogenetic Analyses
3. Results
3.1. Genomic Characteristic and Diversity of Sequenced GaChPV
3.2. Comparative Analyses of Coding Genes of GaChPV
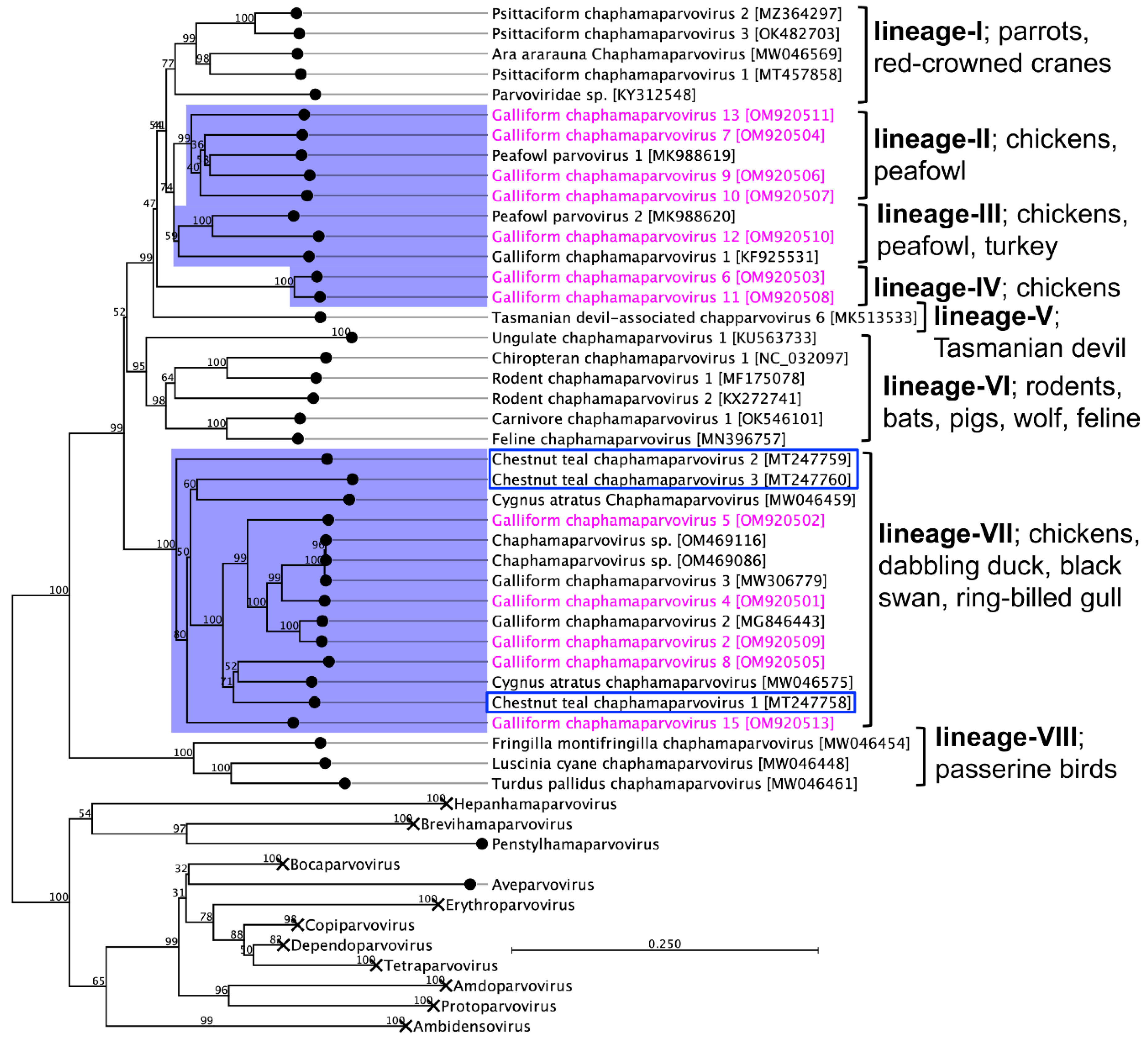
3.3. Emergence of GaChPV into Multiple Lineages
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Roberts, J.R.; Souillard, R.; Bertin, J. Avian diseases which affect egg production and quality. Improv. Saf. Qual. Eggs Egg Prod. 2011, 376–393. [Google Scholar] [CrossRef]
- Phung, C.; Vezina, B.; Anwar, A.; Wilson, T.; Scott, P.C.; Moore, R.J.; Van, T.T.H. Campylobacter hepaticus, the cause of spotty liver disease in chickens: Transmission and routes of infection. Front. Vet. Sci. 2020, 6, 505. [Google Scholar] [CrossRef] [PubMed]
- Quinteros, J.A.; Scott, P.C.; Wilson, T.B.; Anwar, A.M.; Scott, T.; Muralidharan, C.; Van, T.T.H.; Moore, R.J. Isoquinoline alkaloids induce partial protection of laying hens from the impact of Campylobacter hepaticus (spotty liver disease) challenge. Poult. Sci. 2021, 100, 101423. [Google Scholar] [CrossRef] [PubMed]
- Crawshaw, T. A review of the novel thermophilic Campylobacter, Campylobacter hepaticus, a pathogen of poultry. Transbound. Emerg. Dis. 2019, 66, 1481–1492. [Google Scholar]
- Courtice, J.M.; Mahdi, L.K.; Groves, P.J.; Kotiw, M. Spotty liver disease: A review of an ongoing challenge in commercial free-range egg production. Vet. Microbiol. 2018, 227, 112–118. [Google Scholar] [CrossRef]
- Crawshaw, T.R.; Chanter, J.I.; Young, S.C.L.; Cawthraw, S.; Whatmore, A.M.; Koylass, M.S.; Vidal, A.B.; Salguero, F.J.; Irvine, R.M. Isolation of a novel thermophilic Campylobacter from cases of spotty liver disease in laying hens and experimental reproduction of infection and microscopic pathology. Vet. Microbiol. 2015, 179, 315–321. [Google Scholar] [CrossRef]
- Van, T.T.H.; Elshagmani, E.; Gor, M.C.; Scott, P.C.; Moore, R.J. Campylobacter hepaticus sp. nov., isolated from chickens with spotty liver disease. Int. J. Syst. Evol. Microbiol. 2016, 66, 4518–4524. [Google Scholar] [CrossRef]
- Van, T.T.H.; Elshagmani, E.; Gor, M.-C.; Anwar, A.; Scott, P.C.; Moore, R.J. Induction of spotty liver disease in layer hens by infection with Campylobacter hepaticus. Vet. Microbiol. 2017, 199, 85–90. [Google Scholar] [CrossRef]
- Shi, M.; Zhang, Y.Z.; Holmes, E.C. Meta-transcriptomics and the evolutionary biology of RNA viruses. Virus Res. 2018, 243, 83–90. [Google Scholar] [CrossRef]
- Vibin, J.; Chamings, A.; Klaassen, M.; Alexandersen, S. Metagenomic characterisation of additional and novel avian viruses from Australian wild ducks. Sci. Rep. 2020, 10, 22284. [Google Scholar] [CrossRef]
- Vibin, J.; Chamings, A.; Collier, F.; Klaassen, M.; Nelson, T.M.; Alexandersen, S. Metagenomics detection and characterisation of viruses in faecal samples from Australian wild birds. Sci. Rep. 2018, 8, 8686. [Google Scholar] [CrossRef]
- Shi, M.; Lin, X.D.; Tian, J.H.; Chen, L.J.; Chen, X.; Li, C.X.; Qin, X.C.; Li, J.; Cao, J.P.; Eden, J.S.; et al. Redefining the invertebrate RNA virosphere. Nature 2016, 540, 539–543. [Google Scholar] [CrossRef] [PubMed]
- Temmam, S.; Monteil-Bouchard, S.; Robert, C.; Baudoin, J.P.; Sambou, M.; Aubadie-Ladrix, M.; Labas, N.; Raoult, D.; Mediannikov, O.; Desnues, C. Characterization of viral communities of biting midges and identification of novel thogotovirus species and Rhabdovirus genus. Viruses 2016, 8, 77. [Google Scholar] [CrossRef] [PubMed]
- Lim, E.S.; Zhou, Y.; Zhao, G.; Bauer, I.K.; Droit, L.; Ndao, I.M.; Warner, B.B.; Tarr, P.I.; Wang, D.; Holtz, L.R. Early life dynamics of the human gut virome and bacterial microbiome in infants. Nat. Med. 2015, 21, 1228–1234. [Google Scholar] [CrossRef] [PubMed]
- Zablocki, O.; van Zyl, L.; Adriaenssens, E.M.; Rubagotti, E.; Tuffin, M.; Cary, S.C.; Cowan, D. High-level diversity of tailed phages, eukaryote-associated viruses, and virophage-like elements in the metaviromes of antarctic soils. Appl. Environ. Microbiol. 2014, 80, 6888–6897. [Google Scholar] [CrossRef]
- Roux, S.; Chan, L.K.; Egan, R.; Malmstrom, R.R.; McMahon, K.D.; Sullivan, M.B. Ecogenomics of virophages and their giant virus hosts assessed through time series metagenomics. Nat. Commun. 2017, 8, 858. [Google Scholar] [CrossRef]
- Chang, W.S.; Eden, J.S.; Hall, J.; Shi, M.; Rose, K.; Holmes, E.C. Metatranscriptomic analysis of virus diversity in urban wild birds with paretic disease. J. Virol. 2020, 94, e00606-20. [Google Scholar] [CrossRef]
- Pénzes, J.J.; Söderlund-Venermo, M.; Canuti, M.; Eis-Hübinger, A.M.; Hughes, J.; Cotmore, S.F.; Harrach, B. Reorganizing the family Parvoviridae: A revised taxonomy independent of the canonical approach based on host association. Arch. Virol. 2020, 165, 2133–2146. [Google Scholar] [CrossRef]
- Reuter, G.; Boros, Á.; Delwart, E.; Pankovics, P. Novel circular single-stranded DNA virus from turkey faeces. Arch. Virol. 2014, 159, 2161–2164. [Google Scholar] [CrossRef]
- Palinski, R.M.; Mitra, N.; Hause, B.M. Discovery of a novel Parvovirinae virus, porcine parvovirus 7, by metagenomic sequencing of porcine rectal swabs. Virus Genes 2016, 52, 564–567. [Google Scholar] [CrossRef]
- Duarte, M.A.; Silva, J.M.F.; Brito, C.R.; Teixeira, D.S.; Melo, F.L.; Ribeiro, B.M.; Nagata, T.; Campos, F.S. Faecal virome analysis of wild animals from Brazil. Viruses 2019, 11, 803. [Google Scholar] [CrossRef] [PubMed]
- Sarker, S. Molecular and phylogenetic characterisation of a highly divergent novel Parvovirus (Psittaciform chaphamaparvovirus 2) in Australian neophema parrots. Pathogens 2021, 10, 1559. [Google Scholar] [CrossRef] [PubMed]
- Van, T.T.H.; Gor, M.-C.; Anwar, A.; Scott, P.C.; Moore, R.J. Campylobacter hepaticus, the cause of spotty liver disease in chickens, is present throughout the small intestine and caeca of infected birds. Vet. Microbiol. 2017, 207, 226–230. [Google Scholar] [CrossRef] [PubMed]
- Sarker, S. Metagenomic detection and characterisation of multiple viruses in apparently healthy Australian Neophema birds. Sci. Rep. 2021, 11, 20915. [Google Scholar] [CrossRef] [PubMed]
- Sarker, S.; Das, S.; Lavers, J.L.; Hutton, I.; Helbig, K.; Imbery, J.; Upton, C.; Raidal, S.R. Genomic characterization of two novel pathogenic avipoxviruses isolated from pacific shearwaters (Ardenna spp.). BMC Genom. 2017, 18, 298. [Google Scholar] [CrossRef]
- Athukorala, A.; Phalen, D.N.; Das, A.; Helbig, K.J.; Forwood, J.K.; Sarker, S. Genomic characterisation of a highly divergent Siadenovirus (Psittacine siadenovirus F) from the critically endangered orange-bellied parrot (Neophema chrysogaster). Viruses 2021, 13, 1714. [Google Scholar] [CrossRef] [PubMed]
- Sutherland, M.; Sarker, S.; Vaz, P.K.; Legione, A.R.; Devlin, J.M.; Macwhirter, P.L.; Whiteley, P.L.; Raidal, S.R. Disease surveillance in wild Victorian cacatuids reveals co-infection with multiple agents and detection of novel avian viruses. Vet. Microbiol. 2019, 235, 257–264. [Google Scholar] [CrossRef] [PubMed]
- Sarker, S.; Isberg, R.S.; Moran, L.J.; Araujo, D.R.; Elliott, N.; Melville, L.; Beddoe, T.; Helbig, J.K. Crocodilepox virus evolutionary genomics supports observed poxvirus infection dynamics on saltwater crocodile (Crocodylus porosus). Viruses 2019, 11, 1116. [Google Scholar] [CrossRef]
- Bankevich, A.; Nurk, S.; Antipov, D.; Gurevich, A.A.; Dvorkin, M.; Kulikov, A.S.; Lesin, V.M.; Nikolenko, S.I.; Pham, S.; Prjibelski, A.D.; et al. SPAdes: A new genome assembly algorithm and its applications to single-cell sequencing. J. Comput. Biol. A J. Comput. Mol. Cell Biol. 2012, 19, 455–477. [Google Scholar] [CrossRef] [PubMed]
- Benson, D.A.; Cavanaugh, M.; Clark, K.; Karsch-Mizrachi, I.; Lipman, D.J.; Ostell, J.; Sayers, E.W. GenBank. Nucleic Acids Res. 2013, 41, D36–D42. [Google Scholar] [CrossRef]
- Katoh, K.; Standley, D.M. MAFFT multiple sequence alignment software version 7: Improvements in performance and usability. Mol. Biol. Evol. 2013, 30, 772–780. [Google Scholar] [CrossRef] [PubMed]
- Vibin, J.; Chamings, A.; Klaassen, M.; Bhatta, T.R.; Alexandersen, S. Metagenomic characterisation of avian parvoviruses and picornaviruses from Australian wild ducks. Sci. Rep. 2020, 10, 12800. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Wang, H.; Liu, X.; Li, Y.; Chen, J.; Zhang, J.; Wang, X.; Shen, S.; Wang, H.; Deng, F.; et al. Genomic and transcriptional analyses of novel parvoviruses identified from dead peafowl. Virology 2020, 539, 80–91. [Google Scholar] [CrossRef] [PubMed]
- Canuti, M.; Verhoeven, J.T.P.; Munro, H.J.; Roul, S.; Ojkic, D.; Robertson, G.J.; Whitney, H.G.; Dufour, S.C.; Lang, A.S. Investigating the diversity and host range of novel parvoviruses from North American ducks using epidemiology, phylogenetics, genome structure, and codon usage analysis. Viruses 2021, 13, 193. [Google Scholar] [CrossRef] [PubMed]
- Matos, M.; Bilic, I.; Viloux, N.; Palmieri, N.; Albaric, O.; Chatenet, X.; Tvarogová, J.; Dinhopl, N.; Heidl, S.; Liebhart, D.; et al. A novel Chaphamaparvovirus is the etiological agent of hepatitis outbreaks in pheasants (Phasianus colchicus) characterized by high mortality. Transbound. Emerg. Dis. 2022, 69, e2093–e2104. [Google Scholar] [CrossRef] [PubMed]
- Palya, V.J. Parvovirus Infections of Waterfowl. In Diseases of Poultry, 14th ed.; Swayne, D.E., Boulianne, M., Logue, C.M., McDougald, L.R., Nair, V., Suarez, D.L., Eds.; Wiley-Blackwell: Hoboken, NJ, USA, 2020; pp. 474–497. [Google Scholar]
- Jenner, R. Spotty Liver Syndrome—An Emerging Disease? In Proceedings of the Australian Veterinary Poultry Association Scientific Meeting, Sydney, Australia, 6–7 February 2001. [Google Scholar]
- Petrovska, L.; Tang, Y.; Jansen van Rensburg, M.J.; Cawthraw, S.; Nunez, J.; Sheppard, S.K.; Ellis, R.J.; Whatmore, A.M.; Crawshaw, T.R.; Irvine, R.M. Genome reduction for niche association in Campylobacter hepaticus, a cause of spotty liver disease in poultry. Front. Cell. Infect. Microbiol. 2017, 7, 354. [Google Scholar] [CrossRef]
- Yang, S.; Peng, P.; Zhaoyuan, Q.; Wang, X.; Wang, C. Genetic relationship of the 1780–1760 Ma Dykes and the coeval volcanics in the Lvliang area, North China. Acta Geol. Sin. Engl. Ed. 2016, 90, 133–134. [Google Scholar] [CrossRef]
- Sarker, S. Characterization of a novel complete-genome sequence of a galliform Chaphamaparvovirus from a free-range laying chicken clinically diagnosed with spotty liver disease. Microbiol. Resour. Announc. 2022, e0101722. [Google Scholar] [CrossRef]
- de Souza, W.M.; Romeiro, M.F.; Fumagalli, M.J.; Modha, S.; de Araujo, J.; Queiroz, L.H.; Durigon, E.L.; Figueiredo, L.T.M.; Murcia, P.R.; Gifford, R.J. Chapparvoviruses occur in at least three vertebrate classes and have a broad biogeographic distribution. J. Gen. Virol. 2017, 98, 225–229. [Google Scholar] [CrossRef]
- Sykes, J.E. Chapter 14—Canine parvovirus Infections and Other Viral Enteritides. In Canine and Feline Infectious Diseases; Sykes, J.E., Ed.; W.B. Saunders: Saint Louis, MO, USA, 2014; pp. 141–151. [Google Scholar]
- Scott, A.B.; Singh, M.; Groves, P.; Hernandez-Jover, M.; Barnes, B.; Glass, K.; Moloney, B.; Black, A.; Toribio, J.-A. Biosecurity practices on Australian commercial layer and meat chicken farms: Performance and perceptions of farmers. PLoS ONE 2018, 13, e0195582. [Google Scholar] [CrossRef]


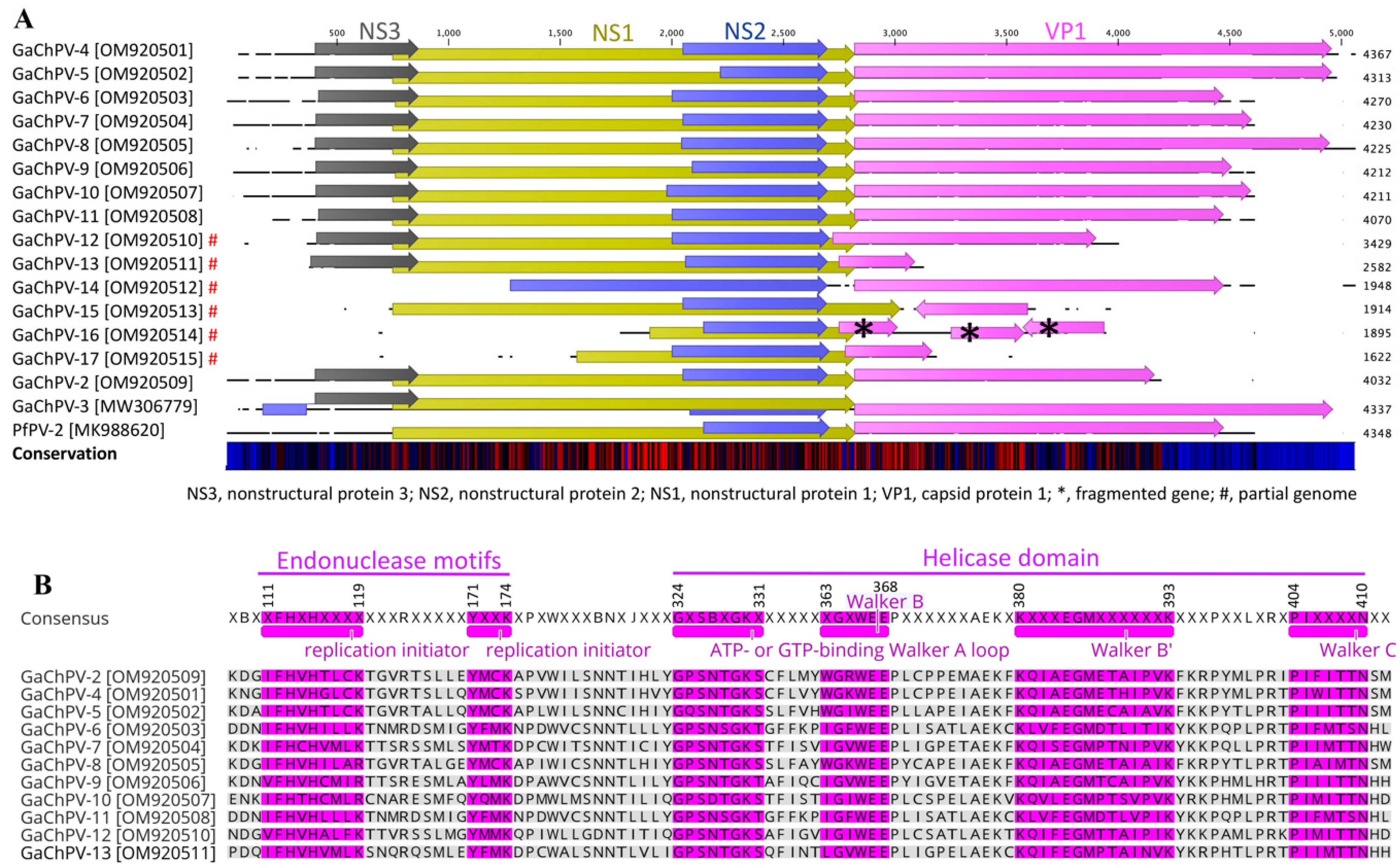
| SL | GaChPV (GenBank Accession No.) | Length (nt) | Best BlastN Match (Organism/Query Coverage (%)/E-Value/GenBank Accession No.) | nt Similarity (%) |
|---|---|---|---|---|
| 1 | GaChPV-4 (OM920501) | 4367 | Galliform chaphamaparvovirus 2/97/0.0/MG846442.1 | 79.90 |
| 2 | GaChPV-5 (OM920502) | 4311 | Galliform chaphamaparvovirus 3/63/0.0/MW306779.1 | 74.90 |
| 3 | GaChPV-6 (OM920503) | 4270 | Peafowl parvovirus 2/33/3.00 × 10−44/MK988620.1 | 64.11 |
| 4 | GaChPV-7 (OM920504) | 4230 | Peafowl parvovirus 1/29/1.00 × 10−125/MK988619.1 | 73.95 |
| 5 | GaChPV-8 (OM920505) | 4225 | Chestnut teal chaphamaparvovirus 1/87/0/MT247758.1 | 73.73 |
| 6 | GaChPV-9 (OM920506) | 4212 | Pavo cristatus parvoviridae sp./30/0/MW046349.1 | 76.56 |
| 7 | GaChPV-10 (OM920507) | 4211 | Peafowl parvovirus 1/36/6.00 × 10−79/MK988619.1 | 71.12 |
| 8 | GaChPV-11 (OM920508) | 4070 | Cygnus columbianus Chaphamaparvovirus/19/4.00 × 10−30/MW046623.1 | 76.22 |
| 9 | GaChPV-2 (OM920509) | 4032 | Galliform chaphamaparvovirus 2/97/0.0/MG846443.1 | 87.39 |
| 10 | GaChPV-12 (OM920510) | 3429 | Peafowl parvovirus 2/67/2.00 × 10−127/MK988620.1 | 71.15 |
| 11 | GaChPV-13 (OM920511) | 2582 | Peafowl parvovirus 2/30/6.00 × 10−62/MK988620.1 | 73.30 |
| 12 | GaChPV-14 (OM920512) | 1948 | Parvoviridae sp./19/6.00 × 10−17/MT138323.1 | 67.62 |
| 13 | GaChPV-15 (OM920513) | 1914 | Ara ararauna Chaphamaparvovirus/52/4.00 × 10−52/MW046364.1 | 71.59 |
| 14 | GaChPV-16 (OM920514) | 1894 | Pavo cristatus parvoviridae sp./77/0.0/MW046349.1 | 78.66 |
| 15 | GaChPV-17 (OM920515) | 1622 | Peafowl parvovirus 2/92/0.0/ MK988620.1 | 71.29 |
| NS3 | NS2 | NS1 | VP1 | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| SL No | Genome (GenBank Accession No) | Gene Coordinate (nt Length) | AA Similarity (%) | Best BLAST Match | Gene Coordinate (nt Length) | AA Similarity (%) | Best BLAST Match | Gene Coordinate (nt Length) | AA Similarity (%) | Best BLAST Match | Gene Coordinate (nt Length) | AA Similarity (%) | Best BLAST Match |
| 1 | GaChPV-4 (OM920501) | 297–740 (444) | 74.83 | NS3 (GaChV-3, QRK03700.1) | 1917–2525 (609) | 81.14 | GaChV-3, QRK03699.1 | 623–2647 (2025) | 77.63 | GaChV-3, QRK03698.1 | 2644–4317 (1674) | 70.02 | GaChV-3, QRK03701.1 |
| 2 | GaChPV-5 (OM920502) | 285–728 (444) | 69.39 | NS3 (GaChV-3, QRK03700.1) | 2067–2519 (453) | 72.67 | WDChPV, QMI57952.1 | 611–2629 (2019) | 64.45 | GaChV-3, QRK03698.1 | 2626–4290 (1665) | 59.33 | CTChPV-1, QMI57831.1 |
| 3 | GaChPV-6 (OM920503) | 343–780 (438) | 44.76 | ORF1 (ChFV, QSH48278.1) | 1906–2580 (675) | 53.63 | PfPV-2, QGJ83205.1 | 684–2684 (2001) | 45.94 | PfPV-2, QGJ83204.1 | 2659–4173 (1515) | 44.04 | PfPV-2, QGJ83206.1 |
| 4 | GaChPV-7 (OM920504) | 335–778 (444) | 51.35 | ORF1 (ChFV, QSH48278.1) | 1946–2587 (642) | 65.71 | PfPV-1, QGJ83202.1 | 670–2673 (2004) | 58.15 | PfPV-2, QGJ83201.1 | 2666–4216 (1551) | 56.18 | PfPV-1, QGJ83203.1 |
| 5 | GaChPV-8 (OM920505) | 81–524 (444) | 61.22 | NS3 (GaChV-3, QRK03700.1) | 1695–2312 (618) | 68.91 | CTChPV-1, QMI57830.1 | 407–2434 (2028) | 61.63 | GaChPV-2, AXL64657.1 | 2431–4110 (1680) | 60.34 | DAChPV-1, QRK03681.1 |
| 6 | GaChPV-9 (OM920506) | 335–778 (444) | 42.86 | HP (PsChPV-1, QZW33714.1) | 1991–2590 (600) | 68.97 | PfPV-1, QGJ83202.1 | 685–2673 (1989) | 59.21 | PfPV-1, QGJ83201.1 | 2666–4135 (1500) | 65.00 | PCPV, QTE03716.1 |
| 7 | GaChPV-10 (OM920507) | 307–759 (453) | 49.65 | HP (PsChPV-1, QZW33714.1) | 1858–2571 (714) | 64.29 | PfPV-1, QGJ83202.1 | 651–2657 (2007) | 56.29 | PfPV-1, QGJ83201.1 | 2650–4194 (1545) | 53.76 | PfPV-2, QGJ83206.1 |
| 8 | GaChPV-11 (OM920508) | 145–582 (438) | 45.14 | ORF1 (ChFV, QSH48278.1) | 1708–2382 (675) | 52.75 | AAPV, QTE04008.1 | 474–2486 (2013) | 45.29 | PfPV-2, QGJ83204.1 | 2461–3978 (1518) | 46.18 | PfPV-2, QGJ83206.1 |
| 9 | GaChPV-2 (OM920509) | 346–789 (444) | 74.15 | NS3 (GaChV-3, QRK03700.1) | 1966–2577 (612) | 78.86 | GaChV-3, QRK03699.1 | 672–2693 (2022) | 88.26 | GaChPV-2, AXL64657.1 | 2690–3997 (1308) | 85.20 | GaChPV-2, AXL64658.1 |
| 10 | GaChPV-12 (OM920510) | 61–477 (417) | 48.53 | ORF1 (ChFV, QSH48278.1) | 1600–2280 (681) | 67.63 | PfPV-2, QGJ83205.1 | 369–2357 (1989) | 59.16 | PfPV-2, QGJ83204.1 | 2287–3327 (1041) | 61.41 | PfPV-2, QGJ83206.1 |
| 11 | GaChPV-13 (OM920511) | 8–403 (396) | 54.00 | ORF1 (ChFV, QSH48278.1) | 1583–2209 (627) | 62.43 | PfPV-1, QGJ83202.1 | 292–2292 (2001) | 56.59 | PfPV-1, QGJ83201.1 | 2250–2543 (294) | 63.95 | PCPV, QTE03716.1 |
| 12 | GaChPV-14 (OM920512) | 2–202 (201) | 36.92 | PfPV-1, QGJ83202.1 | 281–1795 (1515) | 48.04 | RcPV, QKE54986.1 | ||||||
| 13 | GaChPV-15 (OM920513) | 908–1525 (618) | 63.90 | CTChPV-1, QMI57830.1 | 25–1659 (1635) | 54.73 | DAChPV-2, QRK03694.1 | 1830–1681 (150) | 51.02 | WDChPV, QMI57935.1 | |||
| 14 | GaChPV-16 (OM920514) | 388–936 (549) | 72.13 | PfPV-1, QGJ83202.1 | 147–1022 (876) | 55.67 | PfPV-1, QGJ83201.1 | 980–1195 (216) | 63.33 | PfPV-2, QGJ83206.1 | |||
| 1430–1729 (300) | 45.83 | PfPV-1, QGJ83203.1 | |||||||||||
| 1882–1721 (162) | 56.60 | PfPV-1, QGJ83203.1 | |||||||||||
| 15 | GaChPV-17 (OM920515) | 498–1181 (684) | 64.16 | PfPV-2, QGJ83205.1 | 74–1258 (1185) | 56.93 | PfPV-2, QGJ83204.1 | 1227–1586 (360) | 71.17 | PfPV-2, QGJ83206.1 | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sarker, S.; Talukder, S.; Anwar, A.; Van, T.T.H.; Petrovski, S. Unravelling Bile Viromes of Free-Range Laying Chickens Clinically Diagnosed with Spotty Liver Disease: Emergence of Many Novel Chaphamaparvoviruses into Multiple Lineages. Viruses 2022, 14, 2543. https://doi.org/10.3390/v14112543
Sarker S, Talukder S, Anwar A, Van TTH, Petrovski S. Unravelling Bile Viromes of Free-Range Laying Chickens Clinically Diagnosed with Spotty Liver Disease: Emergence of Many Novel Chaphamaparvoviruses into Multiple Lineages. Viruses. 2022; 14(11):2543. https://doi.org/10.3390/v14112543
Chicago/Turabian StyleSarker, Subir, Saranika Talukder, Arif Anwar, Thi Thu Hao Van, and Steve Petrovski. 2022. "Unravelling Bile Viromes of Free-Range Laying Chickens Clinically Diagnosed with Spotty Liver Disease: Emergence of Many Novel Chaphamaparvoviruses into Multiple Lineages" Viruses 14, no. 11: 2543. https://doi.org/10.3390/v14112543
APA StyleSarker, S., Talukder, S., Anwar, A., Van, T. T. H., & Petrovski, S. (2022). Unravelling Bile Viromes of Free-Range Laying Chickens Clinically Diagnosed with Spotty Liver Disease: Emergence of Many Novel Chaphamaparvoviruses into Multiple Lineages. Viruses, 14(11), 2543. https://doi.org/10.3390/v14112543







