Oral Antiviral Treatment for COVID-19: A Comprehensive Review on Nirmatrelvir/Ritonavir
Abstract
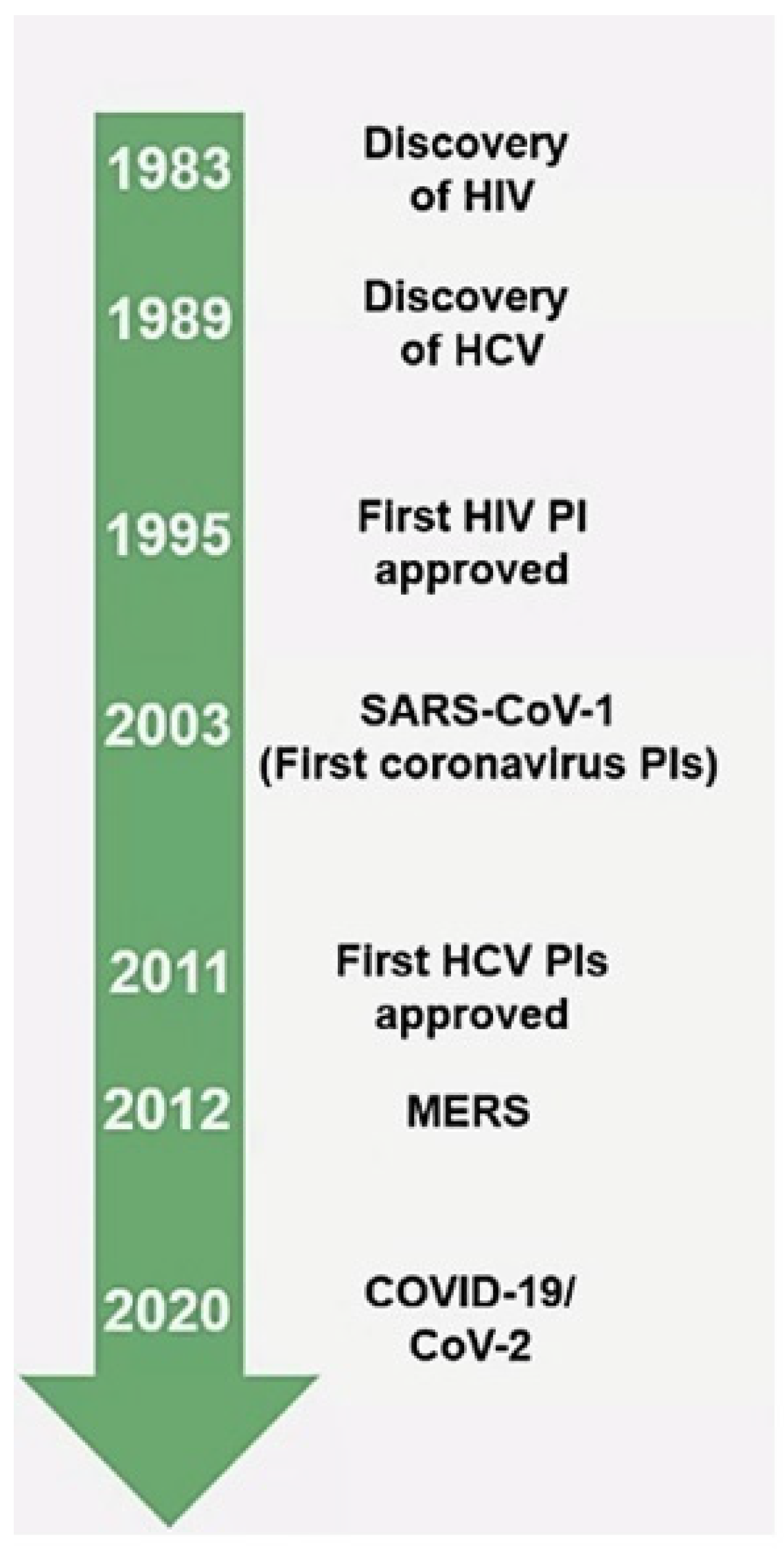
:1. Introduction
2. Methods
3. Opportunities for Early Intervention: Nirmatrelvir/Ritonavir
4. Administration
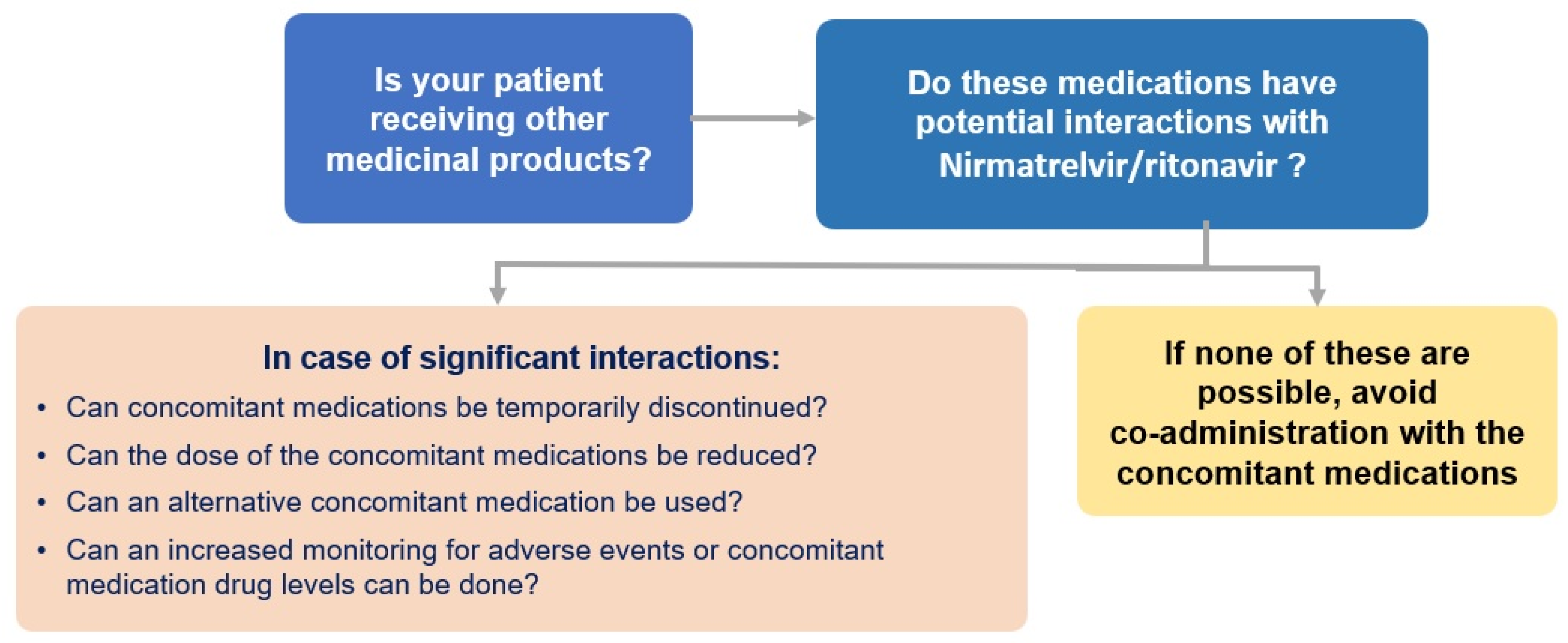
5. A Word on Interactions
6. Guidelines
7. Clinical Trial Data
8. Real-World Evidence
9. Rebound Phenomena
10. Expert Option and Future Directions
11. Limitations of This Study
12. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Parasher, A. COVID-19: Current understanding of its Pathophysiology, Clinical presentation and Treatment. Postgrad Med. J. 2021, 97, 312–320. [Google Scholar] [CrossRef] [PubMed]
- Estiri, H.; Strasser, Z.H.; Murphy, S.N. Individualized prediction of COVID-19 adverse outcomes with MLHO. Sci. Rep. 2021, 11, 5322. [Google Scholar] [CrossRef] [PubMed]
- Logue, J.K.; Franko, N.M.; McCulloch, D.J.; McDonald, D.; Magedson, A.; Wolf, C.R.; Chu, H.Y. Sequelae in Adults at 6 Months After COVID-19 Infection. JAMA Netw. Open 2021, 4, e210830. [Google Scholar] [CrossRef] [PubMed]
- NIH. COVID-19 Treatment Guidelines. Available online: https://www.covid19treatmentguidelines.nih.gov/management/clinical-management-of-adults/nonhospitalized-adults--therapeutic-management/ (accessed on 1 November 2022).
- European Centre for Disease Prevention and Control 2022. High-Risk Groups for COVID-19. Available online: www.ecdc.europa.eu/en/covid-19/high-risk-groups (accessed on 5 November 2022).
- Wu, Z.; McGoogan, J.M. Characteristics of and Important Lessons From the Coronavirus Disease 2019 (COVID-19) Outbreak in China: Summary of a Report of 72314 Cases From the Chinese Center for Disease Control and Prevention. JAMA 2020, 323, 1239–1242. [Google Scholar] [CrossRef] [PubMed]
- Di Fusco, M.; Shea, K.M.; Lin, J.; Nguyen, J.L.; Angulo, F.J.; Benigno, M.; Malhotra, D.; Emir, B.; Sung, A.H.; Hammond, J.L.; et al. Health outcomes and economic burden of hospitalized COVID-19 patients in the United States. J. Med. Econ. 2021, 24, 308–317. [Google Scholar] [CrossRef]
- Scudellari, M. How the pandemic might play out in 2021 and beyond. Nature 2020, 584, 22–25. [Google Scholar] [CrossRef]
- Sallam, M. COVID-19 Vaccine Hesitancy Worldwide: A Concise Systematic Review of Vaccine Acceptance Rates. Vaccines 2021, 9, 160. [Google Scholar] [CrossRef]
- Bergwerk, M.; Gonen, T.; Lustig, Y.; Amit, S.; Lipsitch, M.; Cohen, C.; Mandelboim, M.; Levin, E.G.; Rubin, C.; Indenbaum, V.; et al. Covid-19 Breakthrough Infections in Vaccinated Health Care Workers. N. Engl. J. Med. 2021, 385, 1474–1484. [Google Scholar] [CrossRef]
- Naaber, P.; Tserel, L.; Kangro, K.; Sepp, E.; Jürjenson, V.; Adamson, A.; Haljasmägi, L.; Rumm, A.P.; Maruste, R.; Kärner, J.; et al. Dynamics of antibody response to BNT162b2 vaccine after six months: A longitudinal prospective study. Lancet Reg. Health Eur. 2021, 10, 100208. [Google Scholar] [CrossRef]
- Harvey, W.T.; Carabelli, A.M.; Jackson, B.; Gupta, R.K.; Thomson, E.C.; Harrison, E.M.; Ludden, C.; Reeve, R.; Rambaut, A.; Consortium, C.-G.U.; et al. SARS-CoV-2 variants, spike mutations and immune escape. Nat. Rev. Microbiol. 2021, 19, 409–424. [Google Scholar] [CrossRef]
- Harris, R.J.; Hall, J.A.; Zaidi, A.; Andrews, N.J.; Dunbar, J.K.; Dabrera, G. Effect of Vaccination on Household Transmission of SARS-CoV-2 in England. N. Engl. J. Med. 2021, 385, 759–760. [Google Scholar] [CrossRef] [PubMed]
- Tan, D.H.S.; Chan, A.K.; Jüni, P.; Tomlinson, G.; Daneman, N.; Walmsley, S.; Muller, M.; Fowler, R.; Murthy, S.; Press, N.; et al. Post-exposure prophylaxis against SARS-CoV-2 in close contacts of confirmed COVID-19 cases (CORIPREV): Study protocol for a cluster-randomized trial. Trials 2021, 22, 224. [Google Scholar] [CrossRef] [PubMed]
- Tanne, J.H. COVID-19: FDA authorises pharmacists to prescribe Paxlovid. BMJ 2022, 378, o1695. [Google Scholar] [CrossRef] [PubMed]
- EMA. COVID-19 Treatments. Available online: https://www.ema.europa.eu/en/human-regulatory/overview/public-health-threats/coronavirus-disease-covid-19/treatments-vaccines/covid-19-treatments (accessed on 5 November 2022).
- Goyal, A.; Cardozo-Ojeda, E.F.; Schiffer, J.T. Potency and timing of antiviral therapy as determinants of duration of SARS-CoV-2 shedding and intensity of inflammatory response. Sci. Adv. 2020, 6, eabc7112. [Google Scholar] [CrossRef]
- Forrest, J.I.; Rayner, C.R.; Park, J.J.H.; Mills, E.J. Early Treatment of COVID-19 Disease: A Missed Opportunity. Infect Dis. Ther. 2020, 9, 715–720. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, A.; Bertrand, J.; Ke, R.; Comets, E.; de Lamballerie, X.; Malvy, D.; Pizzorno, A.; Terrier, O.; Calatrava, M.R.; Mentré, F.; et al. Timing of Antiviral Treatment Initiation is Critical to Reduce SARS-CoV-2 Viral Load. CPT Pharmacomet. Syst. Pharm. 2020, 9, 509–514. [Google Scholar] [CrossRef] [PubMed]
- Rai, B.; Shukla, A.; Dwivedi, L.K. Incubation period for COVID-19: A systematic review and meta-analysis. Z Gesundh. Wiss. 2022, 30, 2649–2656. [Google Scholar] [CrossRef]
- Eastman, R.T.; Roth, J.S.; Brimacombe, K.R.; Simeonov, A.; Shen, M.; Patnaik, S.; Hall, M.D. Remdesivir: A Review of Its Discovery and Development Leading to Emergency Use Authorization for Treatment of COVID-19. ACS Cent. Sci. 2020, 6, 672–683. [Google Scholar] [CrossRef] [PubMed]
- Rubin, E.J.; Baden, L.R. The Potential of Intentional Drug Development. N. Engl. J. Med. 2022, 386, 1463–1464. [Google Scholar] [CrossRef]
- Yang, H.; Xie, W.; Xue, X.; Yang, K.; Ma, J.; Liang, W.; Zhao, Q.; Zhou, Z.; Pei, D.; Ziebuhr, J.; et al. Design of wide-spectrum inhibitors targeting coronavirus main proteases. PLoS Biol. 2005, 3, e324. [Google Scholar] [CrossRef]
- Owen, D.R.; Allerton, C.M.N.; Anderson, A.S.; Aschenbrenner, L.; Avery, M.; Berritt, S.; Boras, B.; Cardin, R.D.; Carlo, A.; Coffman, K.J.; et al. An oral SARS-CoV-2 M(pro) inhibitor clinical candidate for the treatment of COVID-19. Science 2021, 374, 1586–1593. [Google Scholar] [CrossRef] [PubMed]
- Razali, R.; Asis, H.; Budiman, C. Structure-Function Characteristics of SARS-CoV-2 Proteases and Their Potential Inhibitors from Microbial Sources. Microorganisms 2021, 9, 2481. [Google Scholar] [CrossRef] [PubMed]
- Yang, K.S.; Leeuwon, S.Z.; Xu, S.; Liu, W.R. Evolutionary and Structural Insights about Potential SARS-CoV-2 Evasion of Nirmatrelvir. J. Med. Chem. 2022, 65, 8686–8698. [Google Scholar] [CrossRef] [PubMed]
- Hammond, J.; Leister-Tebbe, H.; Gardner, A.; Abreu, P.; Bao, W.; Wisemandle, W.; Baniecki, M.; Hendrick, V.M.; Damle, B.; Simón-Campos, A.; et al. Oral Nirmatrelvir for High-Risk, Nonhospitalized Adults with COVID-19. N. Engl. J. Med. 2022, 386, 1397–1408. [Google Scholar] [CrossRef]
- Ahmad, B.; Batool, M.; Ain, Q.U.; Kim, M.S.; Choi, S. Exploring the Binding Mechanism of PF-07321332 SARS-CoV-2 Protease Inhibitor through Molecular Dynamics and Binding Free Energy Simulations. Int. J. Mol. Sci. 2021, 22, 9124. [Google Scholar] [CrossRef]
- Vangeel, L.; Chiu, W.; De Jonghe, S.; Maes, P.; Slechten, B.; Raymenants, J.; André, E.; Leyssen, P.; Neyts, J.; Jochmans, D. Remdesivir, Molnupiravir and Nirmatrelvir remain active against SARS-CoV-2 Omicron and other variants of concern. Antivir. Res. 2022, 198, 105252. [Google Scholar] [CrossRef]
- Cooper, C.L.; van Heeswijk, R.P.; Gallicano, K.; Cameron, D.W. A review of low-dose ritonavir in protease inhibitor combination therapy. Clin. Infect. Dis. 2003, 36, 1585–1592. [Google Scholar] [CrossRef]
- PAXLOVID SPC. 2022. Available online: https://www.ema.europa.eu/en/medicines/human/EPAR/paxlovid (accessed on 3 November 2022).
- Gandhi, R.T.; Malani, P.N.; Del Rio, C. COVID-19 Therapeutics for Nonhospitalized Patients. JAMA 2022, 327, 617–618. [Google Scholar] [CrossRef]
- Bhimraj, A.; Morgan, R.L.; Shumaker, A.H.; Baden, L.; Cheng, V.C.C.; Edwards, K.M.; Gallagher, J.C.; Gandhi, R.T.; Muller, W.J.; Nakamura, M.M.; et al. Infectious Diseases Society of America Guidelines on the Treatment and Management of Patients with COVID-19. Clin. Infect. Dis. 2022, ciac724. [Google Scholar] [CrossRef]
- Rochwerg, B.; Siemieniuk, R.A.; Agoritsas, T.; Lamontagne, F.; Askie, L.; Lytvyn, L.; Agarwal, A.; Leo, Y.-S.; Macdonald, H.; Zeng, L.; et al. A living WHO guideline on drugs for COVID-19. BMJ 2020, 370, m3379. [Google Scholar] [CrossRef]
- Arbel, R.; Sagy, Y.W.; Hoshen, M.; Battat, E.; Lavie, G.; Sergienko, R.; Friger, M.; Waxman, J.G.; Dagan, N.; Balicer, R.; et al. Nirmatrelvir Use and Severe Covid-19 Outcomes during the Omicron Surge. N. Engl. J. Med. 2022, 387, 790–798. [Google Scholar] [CrossRef] [PubMed]
- Najjar-Debbiny, R.; Gronich, N.; Weber, G.; Khoury, J.; Amar, M.; Stein, N.; Goldstein, L.H.; Saliba, W. Effectiveness of Paxlovid in Reducing Severe COVID-19 and Mortality in High Risk Patients. Clin. Infect. Dis. 2022, ciac443. [Google Scholar] [CrossRef] [PubMed]
- Wong, C.K.H.; Au, I.C.H.; Lau, K.T.K.; Lau, E.H.Y.; Cowling, B.J.; Leung, G.M. Real-world effectiveness of early molnupiravir or nirmatrelvir-ritonavir in hospitalised patients with COVID-19 without supplemental oxygen requirement on admission during Hong Kong’s omicron BA.2 wave: A retrospective cohort study. Lancet Infect. Dis. 2022. [Google Scholar] [CrossRef]
- Wong, C.K.H.; Au, I.C.H.; Lau, K.T.K.; Lau, E.H.Y.; Cowling, B.J.; Leung, G.M. Real-world effectiveness of molnupiravir and nirmatrelvir plus ritonavir against mortality, hospitalisation, and in-hospital outcomes among community-dwelling, ambulatory patients with confirmed SARS-CoV-2 infection during the omicron wave in Hong Kong: An observational study. Lancet 2022, 400, 1213–1222. [Google Scholar] [CrossRef] [PubMed]
- Yip, T.C.F.; Lui, G.C.Y.; Lai, M.S.M.; Wong, V.W.S.; Tse, Y.K.; Ma, B.H.M.; Hui, E.; Leung, M.K.W.; Chan, H.L.Y.; Hui, D.S.C.; et al. Impact of the use of oral antiviral agents on the risk of hospitalization in community COVID-19 patients. Clin. Infect. Dis. 2022, ciac687. [Google Scholar] [CrossRef]
- Dryden-Peterson, S.; Kim, A.; Kim, A.Y.; Caniglia, E.C.; Lennes, I.; Patel, R.; Gainer, L.; Dutton, L.; Donahue, E.; Gandhi, R.T.; et al. Nirmatrelvir plus ritonavir for early COVID-19 and hospitalization in a large US health system. medRxiv 2022. [Google Scholar] [CrossRef]
- Ganatra, S.; Dani, S.S.; Ahmad, J.; Kumar, A.; Shah, J.; Abraham, G.M.; McQuillen, D.P.; Wachter, R.M.; E Sax, P. Oral Nirmatrelvir and Ritonavir in Non-hospitalized Vaccinated Patients with Covid-19. Clin. Infect. Dis. 2022, ciac673. [Google Scholar] [CrossRef]
- Malden, D.E.; Hong, V.; Lewin, B.J.; Ackerson, B.K.; Lipsitch, M.; Lewnard, J.A.; Tartof, S.Y. Hospitalization and Emergency Department Encounters for COVID-19 After Paxlovid Treatment—California, December 2021-May 2022. MMWR Morb. Mortal. Wkly. Rep. 2022, 71, 830–833. [Google Scholar] [CrossRef]
- Allen, S.; Jonhson, K.; Haddock, J.; Trinkl, J.; Nariani, S.; Joyce, B. Game Changer: Paxlovid Reduces Hospitalizations and Saves Lives. Cosmos Study. 2022. Available online: https://epicresearch.org/articles/game-changer-paxlovid-reduces-hospitalizations-and-saves-lives (accessed on 1 November 2022).
- Lewnard, J.A.; Malden, D.; Hong, V.; Puzniak, L.; Kim, J.S.; Shaw, S.F.; Takhar, H.; Jodar, L.; McLaughlin, J.M.; Tartof, S.Y. Effectiveness of nirmatrelvir-ritonavir against hospital admission: A matched cohort study in a large US healthcare system. medRxiv 2022. [Google Scholar] [CrossRef]
- Wai, A.K.-C.; Chan, C.Y.; Cheung, A.W.-L.; Wang, K.; Chan, S.C.-L.; Lee, T.T.-L.; Luk, L.Y.-F.; Yip, E.T.-F.; Ho, J.W.-K.; Tsui, O.W.-K.; et al. Association of Molnupiravir and Nirmatrelvir-Ritonavir with preventable mortality, hospital admissions and related avoidable healthcare system cost among high-risk patients with mild to moderate COVID-19. Lancet Reg. Health West Pac. 2022, 100602. [Google Scholar] [CrossRef]
- Gentile, I.; Scotto, R.; Moriello, N.S.; Pinchera, B.; Villari, R.; Trucillo, E.; Ametrano, L.; Fusco, L.; Castaldo, G.; Buonomo, A.R.; et al. Nirmatrelvir/Ritonavir and Molnupiravir in the Treatment of Mild/Moderate COVID-19: Results of a Real-Life Study. Vaccines 2022, 10, 1731. [Google Scholar] [CrossRef] [PubMed]
- Loza, A.; Farias, R.; Gavin, N.; Wagner, R.; Hammer, E.; Shields, A. Short-term Pregnancy Outcomes After Nirmatrelvir–Ritonavir Treatment for Mild-to-Moderate Coronavirus Disease 2019 (COVID-19). Obstet. Gynecol. 2022, 140, 447–449. [Google Scholar] [CrossRef] [PubMed]
- Qian, G.; Wang, X.; Patel, N.J.; Kawano, Y.; Fu, X.; Cook, C.E.; Vanni, K.M.M.; Kowalski, E.N.; Banasiak, E.P.; Banasiak, E.P.; et al. Outcomes with and without outpatient SARS-CoV-2 treatment for patients with COVID-19 and systemic autoimmune rheumatic diseases: A retrospective cohort study. medRxiv 2022. [Google Scholar] [CrossRef]
- Ranganath, N.; O’Horo, J.C.; Challener, D.W.; Tulledge-Scheitel, S.M.; Pike, M.L.; O’Brien, R.M.; Razonable, R.R.; Shah, A. Rebound Phenomenon after Nirmatrelvir/Ritonavir Treatment of Coronavirus Disease-2019 in High-Risk Persons. Clin. Infect. Dis. 2022, ciac481. [Google Scholar] [CrossRef]
- Epling, B.P.; Rocco, J.M.; Boswell, K.L.; Laidlaw, E.; Galindo, F.; Kellogg, A.; Das, S.; Roder, A.; Ghedin, E.; Kreitman, A.; et al. Clinical, Virologic, and Immunologic Evaluation of Symptomatic Coronavirus Disease 2019 Rebound Following Nirmatrelvir/Ritonavir Treatment. Clin. Infect. Dis. 2022, ciac663. [Google Scholar] [CrossRef]
- Truong, T.T.; Ryutov, A.; Pandey, U.; Yee, R.; Goldberg, L.; Bhojwani, D.; Aguayo-Hiraldo, P.; Pinsky, B.A.; Pekosz, A.; Shen, L.; et al. Persistent SARS-CoV-2 infection and increasing viral variants in children and young adults with impaired humoral immunity. medRxiv 2021. [Google Scholar] [CrossRef]
- Motyan, J.A.; Mahdi, M.; Hoffka, G.; Tozser, J. Potential Resistance of SARS-CoV-2 Main Protease (Mpro) against Protease Inhibitors: Lessons Learned from HIV-1 Protease. Int. J. Mol. Sci. 2022, 23, 3507. [Google Scholar] [CrossRef]
- Bajema, K.; Wang, X.Q.; Hynes, D.M.; Rowneki, M.; Hickok, A.; Cunningham, F.; Bohnert, A.; Boyko, E.J.; Iwashyna, T.J.; Maciejewski, M.L.; et al. Early Adoption of Anti–SARS-CoV-2 Pharmacotherapies Among US Veterans With Mild to Moderate COVID-19, January and February 2022. JAMA Netw. Open 2022, 5, e2241434. [Google Scholar] [CrossRef]
- Helleberg, M.; Niemann, C.U.; Moestrup, K.S.; Kirk, O.; Lebech, A.M.; Lane, C.; Lundgren, J. Persistent COVID-19 in an Immunocompromised Patient Temporarily Responsive to Two Courses of Remdesivir Therapy. J. Infect. Dis. 2020, 222, 1103–1107. [Google Scholar] [CrossRef]
- ClinicalTrials.gov. (2022). NCT. Available online: www.clinicaltrials.gov/ct2/show/NCT05047601 (accessed on 1 November 2022).
- ClinicalTrials.gov. (2022). NCT. Available online: https://clinicaltrials.gov/ct2/show/NCT05011513 (accessed on 1 November 2022).
- ClinicalTrials.gov. (2022). NCT. Available online: https://clinicaltrials.gov/ct2/show/NCT05438602 (accessed on 1 November 2022).
- ClinicalTrials.gov. (2022). NCT. Available online: https://clinicaltrials.gov/ct2/show/NCT05261139 (accessed on 1 November 2022).
| Absolute Risk Reduction | Relative Risk Reduction | Drug Interactions | Administration | Pregnancy | Adverse Events | |
|---|---|---|---|---|---|---|
| Nirmatrelvir/ritonavir | 6.3→0.8% | 88% | Anticipated drug interactions of concern | Oral | Safe in pregnancy | Minor |
| Remdesivir | 5.3→0.7% | 87% | Few/no anticipated drug interactions | Intravenous | Safe in pregnancy | Minor |
| Molnupiravir | 9.7→6.8% | 30% | Few/no anticipated drug interactions | Oral | Not recommended in pregnancy | Mutagenicity |
| Sotrovimab | 7→1% | 85% | Few/no anticipated drug interactions | Intravenous | Safe in pregnancy | Minor |
| Reference | Country of Origin | Population Characteristics | Outcomes |
|---|---|---|---|
| Arbel et al., 2022; Oral Nirmatrelvir and Severe COVID-19 Outcomes During the Omicron Surge [35] https://doi.org/10.21203/rs.3.rs-1705061/v1 | Israel |
| Primary endpoint: COVID-19 hospitalization Secondary endpoint: Death 68% RRR for COVID-19 hospitalization > 65 years with prior immunity and 85% without prior immunity; 79% RRR for death in people aged >65 years No impact on younger patients (confounders noted) |
| Najjar-Debbiny et al., 2022; Effectiveness of Paxlovid in Reducing Severe COVID-19 and Mortality in High-Risk Patients [36] https://doi.org/10.1093/cid/ciac443 | Israel |
| Composite Endpoint: Severe COVID-19 or COVID-19 specific mortality 46% RRR for Pax, 80% RRR for vaccine Effectiveness similar in vaccinated and unvaccinated |
| Wong et al., 2022; Real-world effectiveness of molnupiravir and nirmatrelvir; ritonavir among COVID-19 inpatients during Hong Kong’s Omicron BA.2 wave: an observational study [37] https://doi.org/10.1016/S1473-3099(22)00507-2 | Hong Kong |
| Composite Endpoint: death, IMV, or ICU admission 43% reduction in composite endpoint for Pax Shorter length of hospital stays in survivors 40% reduction in composite endpoint for molnupiravir |
| Wong et al., 2022; Real-world effectiveness of molnupiravir and nirmatrelvir; ritonavir against mortality, hospitalization, and in-hospital outcomes among community-dwelling, ambulatory COVID-19 patients during the BA.2.2 wave in Hong Kong: an observational study [38] https://doi.org/10.1108/2022.05.26.22275631 | Hong Kong |
| Endpoints: Death, COVID-19-related hospitalization, and composite of in-patient death, IMV, or ICU admission 31% reduction in hospitalization; 75% reduction in death; protection of Pax > molnupiravir |
| Yip et al., 2022; Impact of the use of oral antiviral agents on the risk of hospitalization in community COVID-19 patients [39] https://doi.org/10.1093/cid/ciac687 | Hong Kong |
| Primary endpoint: Hospitalization Secondary endpoint: Death, IMV, or ICU admission 21% reduction in hospitalization with Pax, no impact with molnupiravir No difference for secondary endpoint |
| Ganatra S et al., 2022; Oral Nirmatrelvir and Ritonavir in Non-hospitalized Vaccinated Patients with COVID-19 [41] https://doi.org/10.1093/cid/ciac673 | USA |
| Primary outcome: Composite ER visits, hospitalization, or death Secondary endpoints: Individual components and symptoms 45% RRR in primary composite outcome Ten deaths noted, all in the non-Pax cohort |
| Dryden-Petersonet, S et al., 2022; Nirmatrelvir plus ritonavir for early COVID-19 and hospitalization in a large US health system [40] https://doi.org/10.1108/2022.06.14.22276393 | USA |
| Primary endpoint: Hospitalization D14 Hospitalization in 40 (0.66%) with Pax and 232 (0.96%) control (adjusted RR: 0.55 (95% CI: 0.38 to 0.80, p = 0.002)) 39 deaths control vs. no deaths Pax Low baseline rate with greatest impact in unvaccinated and obese patients |
| Malden DE et al., 2022; Hospitalization and Emergency Department Encounters for COVID-19 After Paxlovid Treatment—California, December 2021–May 2022 MMWR (cdc.gov) [42] | USA |
| Endpoints: Hospitalization or ER COVID-19-related illness during the 5–15 days after Pax < 1% of all patients 6 patients hospitalized and 39 ER were either unvaccinated or vaccinated with 1 dose (17.8%) vs. all treated patients (9.7%), 2 deaths (multiple comorbidities and age) |
| Allen S et al., 2022; Game Changer: Paxlovid Reduces Hospitalization and Saves Lives (epicresearch.org) [43] | USA |
| Endpoints: Hospital admission, death, or discharge Hospitalization: Pax 1.86% vs. control 9.6% Death: Pax 0.12% vs. control 1.23% Patients prescribed Pax ~5× less likely to be hospitalized and 10× less likely to die after COVID-19 Patients > 45 years much more likely to be prescribed Nirmatrelvir/r |
| Lewnard et al., 2022; Effectiveness of nirmatrelvir-ritonavir against hospital admission: a matched cohort study in a large US healthcare system [44] https://doi.org/10.1101/2022.10.02.22280623 | USA |
| Primary endpoints: Effectiveness against hospital admission and acute respiratory infection (ARI)-associated hospital admission over 15 and 30 days Pax dispensed within 0–5 days of symptom onset; 88.1% (95% CI: 49.0–97.5%) over 15 days and 71.9% (25.3–90.0%) over 30 days effectiveness in preventing any admission 88.3% (12.9–98.8%) over 15 days and 87.3% (18.3–98.5%) over 30 days effectiveness in preventing ARI-associated hospital admissions Pax initiation at any point of disease; 86.6% (54.9–96.3%) and 78.0% (46.2–91.4%) effectiveness in preventing all hospital admissions over 15 and 30 days, respectively 93.7% (52.5–99.4%) and 92.8% (53.9–99.1%) in preventing ARI-associated hospital admissions over 15 and 30 days, respectively Similar effectiveness regardless of total vaccine doses (≥2 doses) |
| Gentile et al., 2022; Nirmatrelvir/Ritonavir and Molnupiravir in the Treatment of Mild/Moderate COVID-19: Results of a Real-Life Study [46] https://doi.org/10.3390/vaccines10101731 | Italy |
| Endpoints: 1.6% overall hospitalization rate over 14 days 2.1% hospitalization in molnupiravir group vs. 0.9% in Pax 12.1% reported at least one ADR. No statistically significant difference in ADRs reported between groups (8.9% molnupiravir vs. 16.2% Pax, p = 0.075) |
| Qian et al., 2022; Outcomes with and without outpatient SARS-CoV-2 treatment for patients with COVID-19 and systemic autoimmune rheumatic diseases: A retrospective cohort study [48] https://doi.org/10.1101/2022.10.27.22281629 | USA |
| Endpoint: Outpatient treatment reduced the odds of severe COVID-19 by 88% 8% of those receiving oral treatment experienced COVID-19 rebound |
| Loza et al., 2022; Short-term Pregnancy Outcomes After Nirmatrelvir–Ritonavir Treatment for Mild-to-Moderate Coronavirus Disease 2019 (COVID-19) [47] https://doi.org/10.1097/AOG.0000000000004900 | USA |
| Endpoint: No adverse perinatal effects were observed |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Akinosoglou, K.; Schinas, G.; Gogos, C. Oral Antiviral Treatment for COVID-19: A Comprehensive Review on Nirmatrelvir/Ritonavir. Viruses 2022, 14, 2540. https://doi.org/10.3390/v14112540
Akinosoglou K, Schinas G, Gogos C. Oral Antiviral Treatment for COVID-19: A Comprehensive Review on Nirmatrelvir/Ritonavir. Viruses. 2022; 14(11):2540. https://doi.org/10.3390/v14112540
Chicago/Turabian StyleAkinosoglou, Karolina, Georgios Schinas, and Charalambos Gogos. 2022. "Oral Antiviral Treatment for COVID-19: A Comprehensive Review on Nirmatrelvir/Ritonavir" Viruses 14, no. 11: 2540. https://doi.org/10.3390/v14112540





