Physical and Chemical Barriers in the Larval Midgut Confer Developmental Resistance to Virus Infection in Drosophila
Abstract
:1. Introduction
2. Materials and Methods
2.1. Virus, Fly Lines, and Husbandry
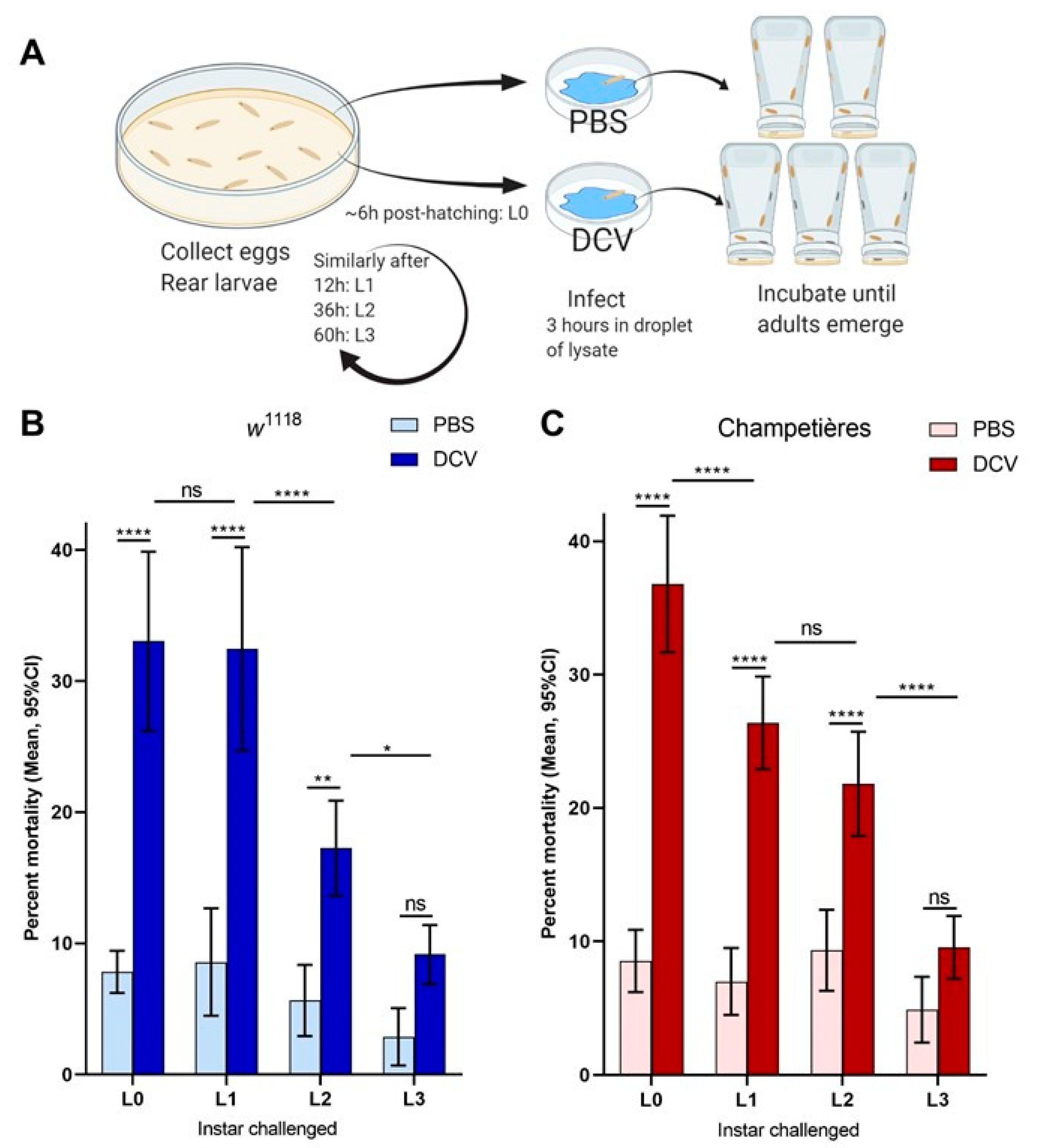
2.2. Larval Oral Infection
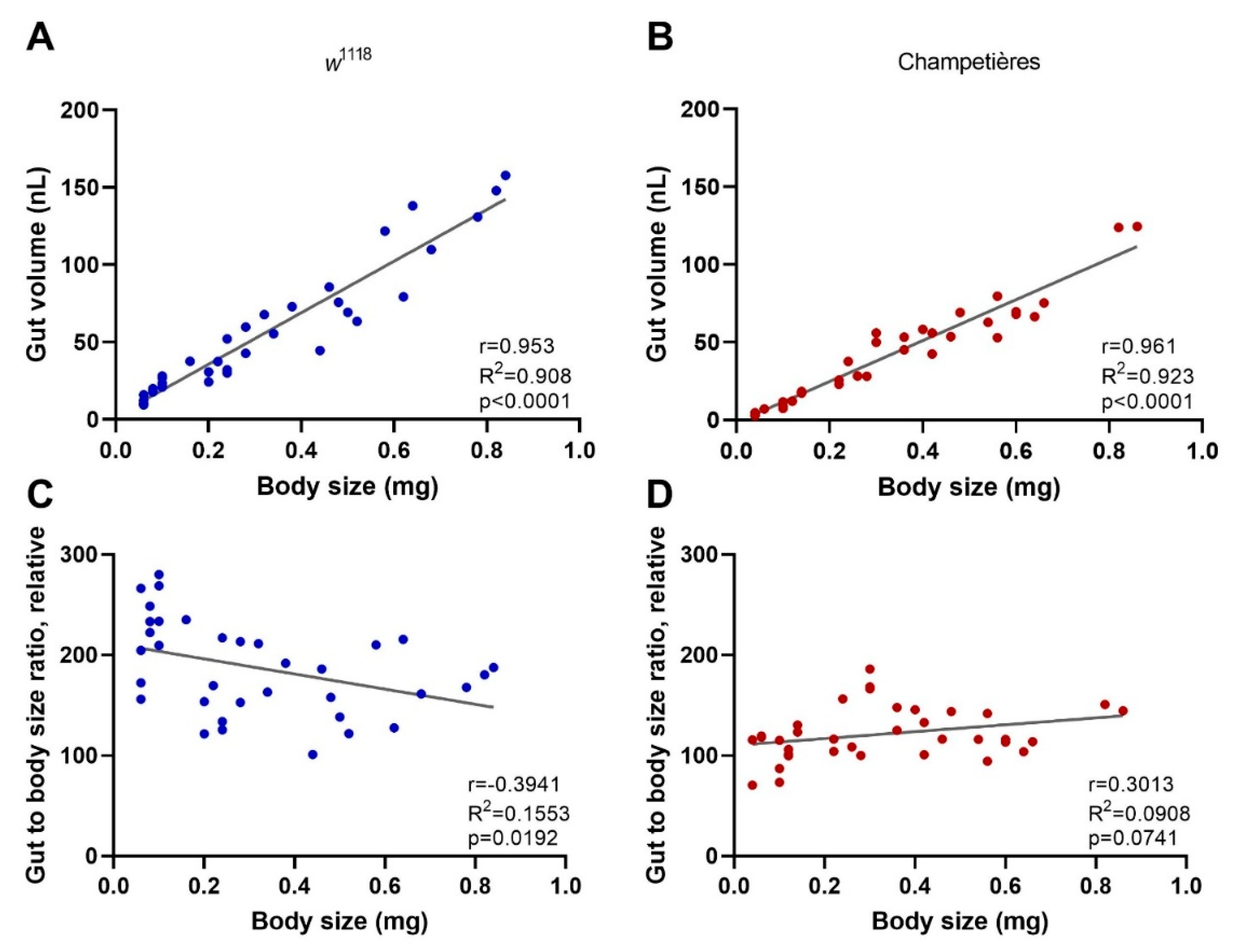
2.3. Gut Volume Measurements
2.4. Adult Oral Infection
2.5. PCR and RT-qPCR
2.6. Microscopy
2.7. Cell Culture
2.8. Statistical Analysis
3. Results
3.1. Mortality Decreases When Oral DCV Infection Occurs Later in Larval Development
3.2. Body Size Does Not Explain the Increased Resistance Phenotype in Drosophila Larvae
3.3. In Adult Flies a Compromised PM Leads to Higher DCV-Induced Mortality
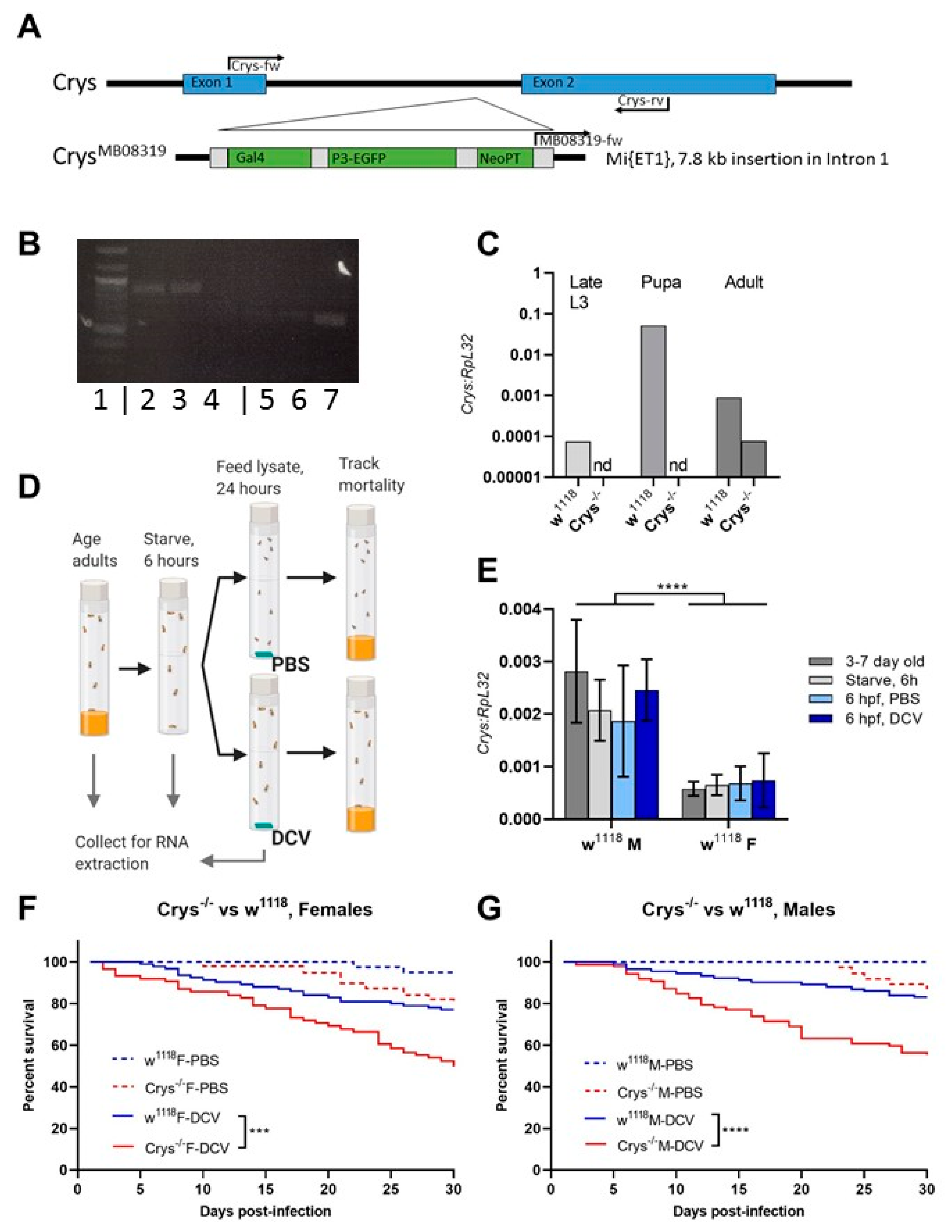
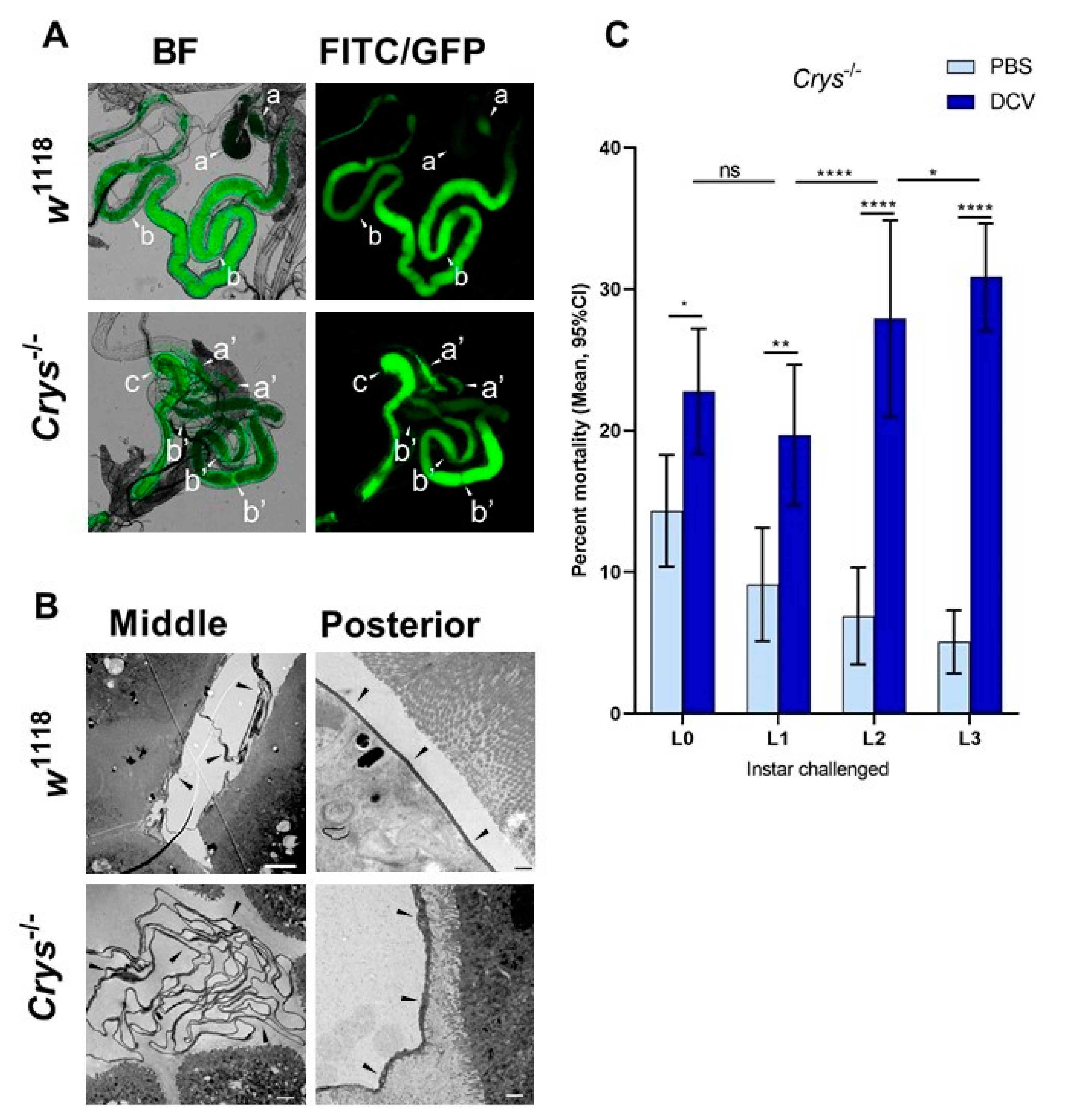
3.4. The Loss of Crys Leads to a Compromised PM Phenotype in Older Drosophila Larvae
3.5. A Compromised PM Is Associated with Increasing DCV-Induced Mortality of Late Instar Drosophila Larvae
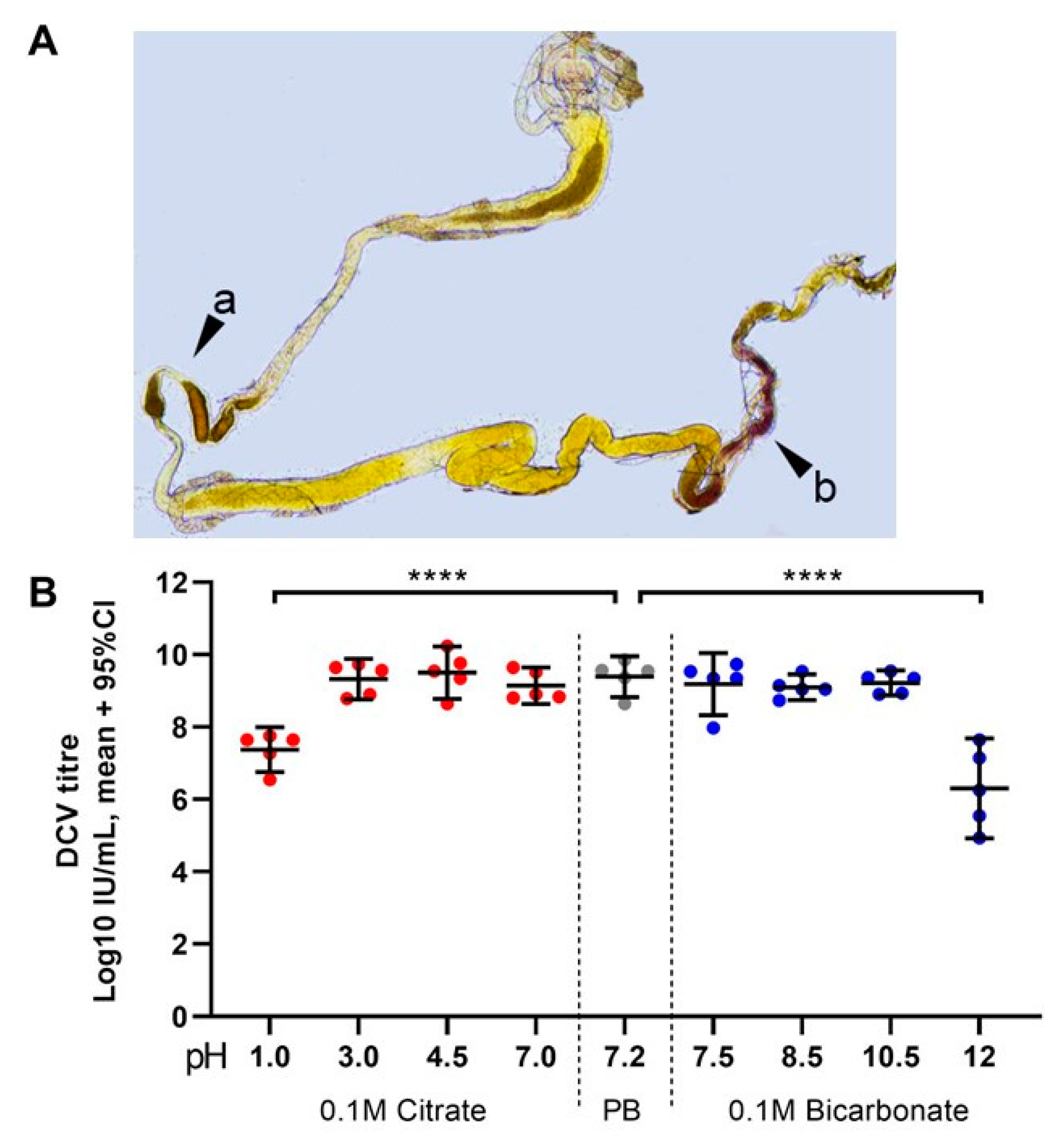
3.6. The pH of the Gut May Be Able to Inactivate Most DCV Particles Before They Can Escape the PM and Reach the Midgut Epithelium
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Acknowledgments
Conflicts of Interest
References
- Marques, J.T.; Imler, J.-L. The diversity of insect antiviral immunity: Insights from viruses. Curr. Opin. Microbiol. 2016, 32, 71–76. [Google Scholar] [CrossRef] [Green Version]
- Palmer, W.; Varghese, F.; van Rij, R. Natural variation in resistance to virus infection in Dipteran insects. Viruses 2018, 10, 118. [Google Scholar] [CrossRef] [Green Version]
- Evans, H.F. The influence of larval maturation on responses of Mamestra brassicae L. (Lepidoptera: Noctuidae) to nuclear polyhedrosis virus infection. Arch. Virol. 1983, 75, 163–170. [Google Scholar] [CrossRef]
- Li, S.Y.; Skinner, A.C. Influence of larval stage and virus inoculum on virus yield in insect host Neodiprion abietis (Hymenoptera: Diprionidae). J. Econ. Entomol. 2005, 98, 1876–1879. [Google Scholar] [CrossRef] [PubMed]
- Engelhard, E.K.; Volkman, L.E. Developmental resistance in fourth instar Trichoplusia ni orally inoculated with Autographa californica M nuclear polyhedrosis virus. Virology 1995, 209, 384–389. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Biji, C.P.; Sudheendrakumar, V.V.; Sajeev, T.V. Influence of virus inoculation method and host larval age on productivity of the nucleopolyhedrovirus of the teak defoliator, Hyblaea puera (Cramer). J. Virol. Methods 2006, 133, 100–104. [Google Scholar] [CrossRef] [PubMed]
- Ledermann, J.P.; Suchman, E.L.; Black, W.C.; Carlson, J.O. Infection and pathogenicity of the mosquito Densoviruses AeDNV, HeDNV, and APeDNV in Aedes aegypti Mosquitoes (Diptera: Culicidae). J. Econ. Entomol. 2004, 97, 1828–1835. [Google Scholar] [CrossRef]
- Evans, H.F.; Lomer, C.J.; Kelly, D.C. Growth of nuclear polyhedrosis virus in larvae of the cabbage moth, Mamestra brassicae L. Arch. Virol. 1981, 70, 207–214. [Google Scholar] [CrossRef]
- Gopal, M.; Gupta, A.; Sathiamma, B.; Nair, C.P.R. Control of the coconut pest Oryctes rhinoceros L. using the Oryctes virus. Int. J. Trop. Insect Sci. 2001, 21, 93–101. [Google Scholar] [CrossRef]
- Sporleder, M.; Rodriguez Cauti, E.M.; Huber, J.; Kroschel, J. Susceptibility of Phthorimaea operculella Zeller (Lepidoptera; Gelechiidae) to its granulovirus PoGV with larval age. Agric. For. Entomol. 2007, 9, 271–278. [Google Scholar] [CrossRef]
- Carlson, J.; Suchman, E.; Buchatsky, L. Densoviruses for control and genetic manipulation of mosquitoes. In Advances in Virus Research; Academic Press: Cambridge, MA, USA, 2006; Volume 68, pp. 361–392. ISBN 9780120398683. [Google Scholar]
- Hatem, A.E.-S.; Aldebis, H.K.; Vargas Osuna, E. Effects of the Spodoptera littoralis granulovirus on the development and reproduction of cotton leafworm S. littoralis. Biol. Control 2011, 59, 192–199. [Google Scholar] [CrossRef]
- Evans, H.F. Quantitative assessment of the relationships between dosage and response of the nuclear polyhedrosis virus of Mamestra brassicae. J. Invertebr. Pathol. 1981, 37, 101–109. [Google Scholar] [CrossRef]
- Kang, K.-D.; Kamita, S.G.; Suzuki, K.; Seong, S.-I. Effect of starvation upon baculovirus replication in larval Bombyx mori and Heliothis virescens. J. Invertebr. Pathol. 2011, 106, 205–210. [Google Scholar] [CrossRef]
- Plymale, R.; Grove, M.J.; Cox-Foster, D.; Ostiguy, N.; Hoover, K. Plant-mediated alteration of the peritrophic matrix and baculovirus infection in lepidopteran larvae. J. Insect Physiol. 2008, 54, 737–749. [Google Scholar] [CrossRef]
- Passarelli, A.L. Barriers to success: How baculoviruses establish efficient systemic infections. Virology 2011, 411, 383–392. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Beegle, C.C.; Oatman, E.R. Differential susceptibility of parasitized and nonparasitized larvae of Trichoplusia ni to a nuclear polyhedrosis virus. J. Invertebr. Pathol. 1974, 24, 188–195. [Google Scholar] [CrossRef]
- Wu, K.; Yang, B.; Huang, W.; Dobens, L.; Song, H.; Ling, E. Gut immunity in Lepidopteran insects. Dev. Comp. Immunol. 2016, 64, 65–74. [Google Scholar] [CrossRef] [PubMed]
- Mondotte, J.A.; Saleh, M.-C. Antiviral immune response and the route of infection in Drosophila melanogaster. In Advances in Virus Research; Academic Press: Cambridge, MA, USA, 2018; pp. 247–278. [Google Scholar]
- Franz, A.W.E.; Kantor, A.M.; Passarelli, A.L.; Clem, R.J. Tissue barriers to arbovirus infection in mosquitoes. Viruses 2015, 7, 3741–3767. [Google Scholar] [CrossRef] [PubMed]
- Lehane, M.J. Peritrophic matrix structure and function. Annu. Rev. Entomol. 1997, 42, 525–550. [Google Scholar] [CrossRef]
- Rodgers, F.H.; Gendrin, M.; Wyer, C.A.S.; Christophides, G.K. Microbiota-induced peritrophic matrix regulates midgut homeostasis and prevents systemic infection of malaria vector mosquitoes. PLoS Pathog. 2017, 13, e1006391. [Google Scholar] [CrossRef]
- Weiss, B.L.; Savage, A.F.; Griffith, B.C.; Wu, Y.; Aksoy, S. The peritrophic matrix mediates differential infection outcomes in the Tsetse fly gut following challenge with commensal, pathogenic, and parasitic microbes. J. Immunol. 2014, 193, 773–782. [Google Scholar] [CrossRef] [Green Version]
- Kuraishi, T.; Binggeli, O.; Opota, O.; Buchon, N.; Lemaitre, B. Genetic evidence for a protective role of the peritrophic matrix against intestinal bacterial infection in Drosophila melanogaster. Proc. Natl. Acad. Sci. USA 2011, 108, 15966–15971. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nguyen, S.; Baker, K.; Padman, B.S.; Patwa, R.; Dunstan, R.A.; Weston, T.A.; Schlosser, K.; Bailey, B.; Lithgow, T.; Lazarou, M.; et al. Bacteriophage transcytosis provides a mechanism to cross epithelial cell layers. MBio 2017, 8. [Google Scholar] [CrossRef] [Green Version]
- Hegedus, D.; Erlandson, M.; Gillott, C.; Toprak, U. New insights into peritrophic matrix synthesis, architecture, and function. Annu. Rev. Entomol. 2009, 54, 285–302. [Google Scholar] [CrossRef]
- Overend, G.; Luo, Y.; Henderson, L.; Douglas, A.E.; Davies, S.A.; Dow, J.A.T. Molecular mechanism and functional significance of acid generation in the Drosophila midgut. Sci. Rep. 2016, 6, 27242. [Google Scholar] [CrossRef] [Green Version]
- Miguel-Aliaga, I.; Jasper, H.; Lemaitre, B. Anatomy and physiology of the digestive tract of Drosophila melanogaster. Genetics 2018, 210, 357–396. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Buchon, N.; Osman, D.; David, F.P.A.; Yu Fang, H.; Boquete, J.-P.; Deplancke, B.; Lemaitre, B. Morphological and molecular characterization of adult midgut compartmentalization in Drosophila. Cell Rep. 2013, 3, 1725–1738. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Naikkhwah, W.; O’Donnell, M.J. Phenotypic plasticity in response to dietary salt stress: Na+ and K+ transport by the gut of Drosophila melanogaster larvae. J. Exp. Biol. 2012, 215, 461–470. [Google Scholar] [CrossRef] [Green Version]
- Edwards, M.J.; Jacobs-Lorena, M. Permeability and disruption of the peritrophic matrix and caecal membrane from Aedes aegypti and Anopheles gambiae mosquito larvae. J. Insect Physiol. 2000, 46, 1313–1320. [Google Scholar] [CrossRef]
- Kelkenberg, M.; Odman-Naresh, J.; Muthukrishnan, S.; Merzendorfer, H. Chitin is a necessary component to maintain the barrier function of the peritrophic matrix in the insect midgut. Insect Biochem. Mol. Biol. 2015, 56, 21–28. [Google Scholar] [CrossRef]
- Agrawal, S.; Kelkenberg, M.; Begum, K.; Steinfeld, L.; Williams, C.E.; Kramer, K.J.; Beeman, R.W.; Park, Y.; Muthukrishnan, S.; Merzendorfer, H. Two essential peritrophic matrix proteins mediate matrix barrier functions in the insect midgut. Insect Biochem. Mol. Biol. 2014, 49, 24–34. [Google Scholar] [CrossRef]
- Shibata, T.; Hadano, J.; Kawasaki, D.; Dong, X.; Kawabata, S.-I. Drosophila TG-A transglutaminase is secreted via an unconventional Golgi-independent mechanism involving exosomes and two types of fatty acylations. J. Biol. Chem. 2017, 292, 10723–10734. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, H.; Hultmark, D. Tissue communication in a systemic immune response of Drosophila. Fly 2016, 10, 115–122. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jousset, F.-X.; Bergoin, M.; Revet, B. Characterization of the Drosophila C Virus. J. Gen. Virol. 1977, 34, 269–283. [Google Scholar] [CrossRef] [PubMed]
- Johnson, K.N.; Christian, P.D. The novel genome organization of the insect picorna-like virus Drosophila C virus suggests this virus belongs to a previously undescribed virus family. J. Gen. Virol. 1998, 79, 191–203. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Valles, S.M.; Chen, Y.; Firth, A.E.; Guérin, D.M.A.; Hashimoto, Y.; Herrero, S.; de Miranda, J.R.; Ryabov, E.; Consortium, I.R. ICTV Virus Taxonomy Profile: Dicistroviridae. J. Gen. Virol. 2017, 98, 355–356. [Google Scholar] [CrossRef]
- Jousset, F.X.; Plus, N.; Croizier, G.; Thomas, M. Existence in Drosophila of 2 groups of picornavirus with different biological and serological properties. Comptes Rendus Acad. Sci. Hebd. Seances Acad. Sci. D 1972, 275, 3043–3046. [Google Scholar]
- Jousset, F.X.; Plus, N. Study of the vertical transmission and horizontal transmission of Drosophila melanogaster and Drosophila immigrans picornavirus. Ann. Microbiol. 1975, 126, 231–249. [Google Scholar]
- Stevanovic, A.L.; Johnson, K.N. Infectivity of Drosophila C virus following oral delivery in Drosophila larvae. J. Gen. Virol. 2015, 96, 1490–1496. [Google Scholar] [CrossRef] [Green Version]
- Mondotte, J.A.; Gausson, V.; Frangeul, L.; Blanc, H.; Lambrechts, L.; Saleh, M.-C. Immune priming and clearance of orally acquired RNA viruses in Drosophila. Nat. Microbiol. 2018, 3, 1394–1403. [Google Scholar] [CrossRef]
- Muscio, O.A.; La Torre, J.; Bonder, M.A.; Scodeller, E.A. Triatoma virus pathogenicity in laboratory colonies of Triatoma infestans (Hemiptera: Reduviidae). J. Med. Entomol. 1997, 34, 253–256. [Google Scholar] [CrossRef]
- Lee, K.-Z.; Vilcinskas, A. Analysis of virus susceptibility in the invasive insect pest Drosophila suzukii. J. Invertebr. Pathol. 2017, 148, 138–141. [Google Scholar] [CrossRef]
- Valles, S.M.; Porter, S.D.; Firth, A.E. Solenopsis invicta virus 3: Pathogenesis and stage specificity in red imported fire ants. Virology 2014, 460–461, 66–71. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brødsgaard, C.J.; Ritter, W.; Hansen, H.; Brødsgaard, H.F. Interactions among Varroa jacobsoni mites, acute paralysis virus, and Paenibacillus larvae larvae and their influence on mortality of larval honeybees in vitro. Apidologie 2000, 31, 543–554. [Google Scholar] [CrossRef] [Green Version]
- Maori, E.; Lavi, S.; Mozes-Koch, R.; Gantman, Y.; Peretz, Y.; Edelbaum, O.; Tanne, E.; Sela, I. Isolation and characterization of Israeli acute paralysis virus, a dicistrovirus affecting honeybees in Israel: Evidence for diversity due to intra- and inter-species recombination. J. Gen. Virol. 2007, 88, 3428–3438. [Google Scholar] [CrossRef]
- Villegas-Ospina, S.; Johnson, K.N.; School of Biological Sciences, Faculty of Science, The University of Queensland, Australia. Titration of viral stocks and lysates, Fluorescence and bright field microscope imaging of control and negative samples, Standardising, controls and negative samples for qPCR. Unpublished work. 2020. [Google Scholar]
- Hedges, L.M.; Brownlie, J.C.; O’Neill, S.L.; Johnson, K.N. Wolbachia and virus protection in insects. Science 2008, 322, 702. [Google Scholar] [CrossRef]
- Osborne, S.E.; Iturbe-Ormaetxe, I.; Brownlie, J.C.; O’Neill, S.L.; Johnson, K.N. Antiviral protection and the importance of Wolbachia density and tissue tropism in Drosophila simulans. Appl. Environ. Microbiol. 2012, 78, 6922–6929. [Google Scholar] [CrossRef] [Green Version]
- Ashburner, M.; Golic, K.G.; Hawley, R.S. Drosophila: A Laboratory Handbook, 2nd ed.; Cold Spring Harbor Laboratory Press: Cold Spring Harbor, NY, USA, 2005; ISBN 0-87969-706-7. [Google Scholar]
- Osborne, S.E.; Leong, Y.S.; O’Neill, S.L.; Johnson, K.N. Variation in antiviral protection mediated by different Wolbachia strains in Drosophila simulans. PLoS Pathog. 2009, 5, e1000656. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aimar, P.; Meireles, M.; Sanchez, V. A contribution to the translation of retention curves into pore size distributions for sieving membranes. J. Membr. Sci. 1990, 54, 321–338. [Google Scholar] [CrossRef]
- Granath, K.A.; Kvist, B.E. Molecular weight distribution analysis by gel chromatography on Sephadex. J. Chromatogr. 1967, 28, 69–81. [Google Scholar] [CrossRef]
- Reed, L.J.; Muench, H. A Simple method of estimating fifty per cent endpoints. Am. J. Epidemiol. 1938, 27, 493–497. [Google Scholar] [CrossRef]
- Therneau, T. Mixed Effects Cox Models. CRAN Repos. Available online: https://cran.r-project.org/web/packages/coxme/coxme.pdf (accessed on 14 January 2020).
- Therneau, T.M.; Grambsch, P.M.; Pankratz, V.S. Penalized Survival Models and Frailty. J. Comput. Graph. Stat. 2003, 12, 156–175. [Google Scholar] [CrossRef]
- Derksen, A.C.G.; Granados, R.R. Alteration of a lepidopteran peritrophic membrane by baculoviruses and enhancement of viral infectivity. Virology 1988, 167, 242–250. [Google Scholar] [CrossRef]
- Shibata, T.; Maki, K.; Hadano, J.; Fujikawa, T.; Kitazaki, K.; Koshiba, T.; Kawabata, S. Crosslinking of a peritrophic matrix protein protects gut epithelia from bacterial exotoxins. PLoS Pathog. 2015, 11, e1005244. [Google Scholar] [CrossRef] [Green Version]
- Sekihara, S.; Shibata, T.; Hyakkendani, M.; Kawabata, S.-I. RNA Interference directed against the transglutaminase gene triggers dysbiosis of Gut Microbiota in Drosophila. J. Biol. Chem. 2016, 291, 25077–25087. [Google Scholar] [CrossRef] [Green Version]
- Metaxakis, A.; Oehler, S.; Klinakis, A.; Savakis, C. Minos as a genetic and genomic tool in Drosophila melanogaster. Genetics 2005, 171, 571–581. [Google Scholar] [CrossRef] [Green Version]
- Bellen, H.J.; Levis, R.W.; He, Y.; Carlson, J.W.; Evans-Holm, M.; Bae, E.; Kim, J.; Metaxakis, A.; Savakis, C.; Schulze, K.L.; et al. The Drosophila gene disruption project: Progress using transposons with distinctive site specificities. Genetics 2011, 188, 731–743. [Google Scholar] [CrossRef] [Green Version]
- Chtarbanova, S.; Lamiable, O.; Lee, K.-Z.; Galiana, D.; Troxler, L.; Meignin, C.; Hetru, C.; Hoffmann, J.A.; Daeffler, L.; Imler, J.-L. Drosophila C virus systemic infection leads to intestinal obstruction. J. Virol. 2014, 88, 14057–14069. [Google Scholar] [CrossRef] [Green Version]
- Horn, C.; Jaunich, B.; Wimmer, E.A. Highly sensitive, fluorescent transformation marker for Drosophila transgenesis. Dev. Genes Evol. 2000, 210, 623–629. [Google Scholar] [CrossRef]
- Stein, L.D. Using GBrowse 2.0 to visualize and share next-generation sequence data. Brief. Bioinform. 2013, 14, 162–171. [Google Scholar] [CrossRef] [PubMed]
- Shibata, T.; Ariki, S.; Shinzawa, N.; Miyaji, R.; Suyama, H.; Sako, M.; Inomata, N.; Koshiba, T.; Kanuka, H.; Kawabata, S. Protein crosslinking by transglutaminase controls cuticle morphogenesis in Drosophila. PLoS ONE 2010, 5, e13477. [Google Scholar] [CrossRef] [Green Version]
- Snijder, J.; Uetrecht, C.; Rose, R.J.; Sanchez-Eugenia, R.; Marti, G.A.; Agirre, J.; Guérin, D.M.A.; Wuite, G.J.L.; Heck, A.J.R.; Roos, W.H. Probing the biophysical interplay between a viral genome and its capsid. Nat. Chem. 2013, 5, 502–509. [Google Scholar] [CrossRef]
- Hashimoto, Y.; Valles, S.M. Infection characteristics of Solenopsis invicta virus 2 in the red imported fire ant, Solenopsis invicta. J. Invertebr. Pathol. 2008, 99, 136–140. [Google Scholar] [CrossRef] [PubMed]
- de Miranda, J.R.; Cordoni, G.; Budge, G. The Acute bee paralysis virus–Kashmir bee virus–Israeli acute paralysis virus complex. J. Invertebr. Pathol. 2010, 103, S30–S47. [Google Scholar] [CrossRef] [PubMed]
- Crawford, A.M.; Glare, T.R. Oryctes virus—Time for a new look at a useful biocontrol agent. J. Invertebr. Pathol. 2005, 89, 91–94. [Google Scholar] [CrossRef]
- Liu, S.; Li, H.; Sivakumar, S.; Bonning, B.C. Virus-Derived Genes for Insect-Resistant Transgenic Plants. Adv. Virus Res. 2006, 68, 427–457. [Google Scholar] [CrossRef]
- Afolami, O.; Oladunmoye, M. Baculoviruses: Emerging frontiers for viral biocontrol of insect pests of agricultural importance. J. Adv. Microbiol. 2017, 5, 1–7. [Google Scholar] [CrossRef]
- Yang, B.; Huang, W.; Zhang, J.; Xu, Q.; Zhu, S.; Zhang, Q.; Beerntsen, B.T.; Song, H.; Ling, E. Analysis of gene expression in the midgut of Bombyx mori during the larval molting stage. BMC Genom. 2016, 17, 866. [Google Scholar] [CrossRef] [Green Version]
- Simhadri, R.K.; Fast, E.M.; Guo, R.; Schultz, M.J.; Vaisman, N.; Ortiz, L.; Bybee, J.; Slatko, B.E.; Frydman, H.M. The gut commensal microbiome of Drosophila melanogaster is modified by the endosymbiont Wolbachia. mSphere 2017, 2, e00287-17. [Google Scholar] [CrossRef] [Green Version]
- Shanbhag, S.; Tripathi, S.; Lehman, H.K.; Ochrietor, J.D.; Kohn, A.; Tu, C.; Linser, P.J. Epithelial ultrastructure and cellular mechanisms of acid and base transport in the Drosophila midgut. J. Exp. Biol. 2009, 212, 1731–1744. [Google Scholar] [CrossRef] [Green Version]
- Cheng, X.; Lynn, D.E. Chapter 5 Baculovirus interactions: In vitro and in vivo. Adv. Appl. Microbiol. 2009, 68, 217–239. [Google Scholar] [CrossRef] [PubMed]
- Cherry, S.; Perrimon, N. Entry is a rate-limiting step for viral infection in a Drosophila melanogaster model of pathogenesis. Nat. Immunol. 2004, 5, 81–87. [Google Scholar] [CrossRef]
- Stevanovic, A.L.; Arnold, P.A.; Johnson, K.N. Wolbachia-mediated antiviral protection in Drosophila larvae and adults following oral infection. Appl. Environ. Microbiol. 2015, 81, 8215–8223. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ferreira, Á.G.; Naylor, H.; Esteves, S.S.; Pais, I.S.; Martins, N.E.; Teixeira, L. The Toll-Dorsal pathway is required for resistance to viral oral infection in Drosophila. PLoS Pathog. 2014, 10, e1004507. [Google Scholar] [CrossRef] [Green Version]
- Clem, R.J.; Passarelli, A.L. Baculoviruses: Sophisticated pathogens of insects. PLoS Pathog. 2013, 9, e1003729. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; Vilcinskas, A.; Kanost, M.R. Immunity in lepidopteran insects. Adv. Exp. Med. Biol. 2010, 708, 181–204. [Google Scholar]
- Rao, R.; Fiandra, L.; Giordana, B.; de Eguileor, M.; Congiu, T.; Burlini, N.; Arciello, S.; Corrado, G.; Pennacchio, F. AcMNPV ChiA protein disrupts the peritrophic membrane and alters midgut physiology of Bombyx mori larvae. Insect Biochem. Mol. Biol. 2004, 34, 1205–1213. [Google Scholar] [CrossRef]
- Palmer, W.H.; Medd, N.C.; Beard, P.M.; Obbard, D.J. Isolation of a natural DNA virus of Drosophila melanogaster, and characterisation of host resistance and immune responses. PLoS Pathog. 2018, 14, e1007050. [Google Scholar] [CrossRef]
| Sequence | Product Length | |
|---|---|---|
| Conventional PCR | ||
| 12SA1 | 5′-AAACTAGGATTAGATACCCTATTAT-3′ | 400 bp |
| 12SB1 | 5′-AAGAGCGACGGGCGATGTGT-3′ | |
| Wsp-81F | 5′-TGGTCCAATAAGTGATGAAGAAAC-3′ | 610 bp |
| Wsp-691R | 5′-AAAAATTAAACGCTACTCCA-3′ | |
| Crys-Fw | 5′-CATCGGCAGCAAACGGAAAA-3′ | 897 bp a |
| Crys-MB08319-Fw | 5′-ATGAAAGGTTGGGCTTCGGA-3′ | 642 bp b |
| Crys-Rv | 5′-GACGCAGGTATGCCGAATTG-3′ | |
| RT-qPCR | ||
| Rpl32-Fw | 5′-GACGCTTCAAGGGACAGTATCTG-3′ | 141 bp |
| RpL32-Rv | 5′-AAACGCGGTTCTGCATGAG-3′ | |
| Crys-Fw | 5′-CATCGGCAGCAAACGGAAAA-3′ | 150 bp c |
| Crys-Rv | 5′-GACGCAGGTATGCCGAATTG-3′ | |
| FITC Outside PM | Anterior Midgut | Middle Midgut | Posterior Midgut | |
|---|---|---|---|---|
| L1 | w1118 | 4.9% (2/41) | 19.5% (8/41) | 92.7% (38/41) |
| Crys−/− | 6.1% (2/33) | 30.3% (10/33) | 93.9% (31/33) | |
| L3 | w1118 | 7.9% (3/38) | 31.5% (12/38) | 94.7% (36/38) |
| Crys−/− | 16.3% (7/43) | 60.5% (26/43) | 97.7% (42/43) | |
| χ2 | z | p | ||
| L1 vs. L3, w1118 | ||||
| Anterior | 0.303 | 0.550 | 0.582 | |
| Middle | 1.519 | 1.232 | 0.218 | |
| Posterior | 0.140 | 0.374 | 0.708 | |
| L1 vs. L3, Crys−/− | ||||
| Anterior | 1.867 | 1.367 | 0.172 | |
| Middle | 6.813 | 2.610 | 0.009 * | |
| Posterior | 0.687 | 0.829 | 0.407 | |
| Crys−/− vs. w1118, L1 | ||||
| Anterior | 0.050 | 0.223 | 0.823 | |
| Middle | 1.157 | 1.075 | 0.282 | |
| Posterior | 0.046 | 0.214 | 0.831 | |
| Crys−/− vs. w1118, L3 | ||||
| Anterior | 1.310 | 1.145 | 0.252 | |
| Middle | 6.759 | 2.600 | 0.009* | |
| Posterior | 0.488 | 0.698 | 0.485 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Villegas-Ospina, S.; Merritt, D.J.; Johnson, K.N. Physical and Chemical Barriers in the Larval Midgut Confer Developmental Resistance to Virus Infection in Drosophila. Viruses 2021, 13, 894. https://doi.org/10.3390/v13050894
Villegas-Ospina S, Merritt DJ, Johnson KN. Physical and Chemical Barriers in the Larval Midgut Confer Developmental Resistance to Virus Infection in Drosophila. Viruses. 2021; 13(5):894. https://doi.org/10.3390/v13050894
Chicago/Turabian StyleVillegas-Ospina, Simon, David J. Merritt, and Karyn N. Johnson. 2021. "Physical and Chemical Barriers in the Larval Midgut Confer Developmental Resistance to Virus Infection in Drosophila" Viruses 13, no. 5: 894. https://doi.org/10.3390/v13050894







