Quantification of the Tradeoff between Test Sensitivity and Test Frequency in a COVID-19 Epidemic—A Multi-Scale Modeling Approach
Abstract
:1. Introduction
2. Methods
2.1. Within-Host Model
2.2. Between-Host Model
2.3. Daily Testing Rate
2.4. Between-Host Model with Testing
3. Results
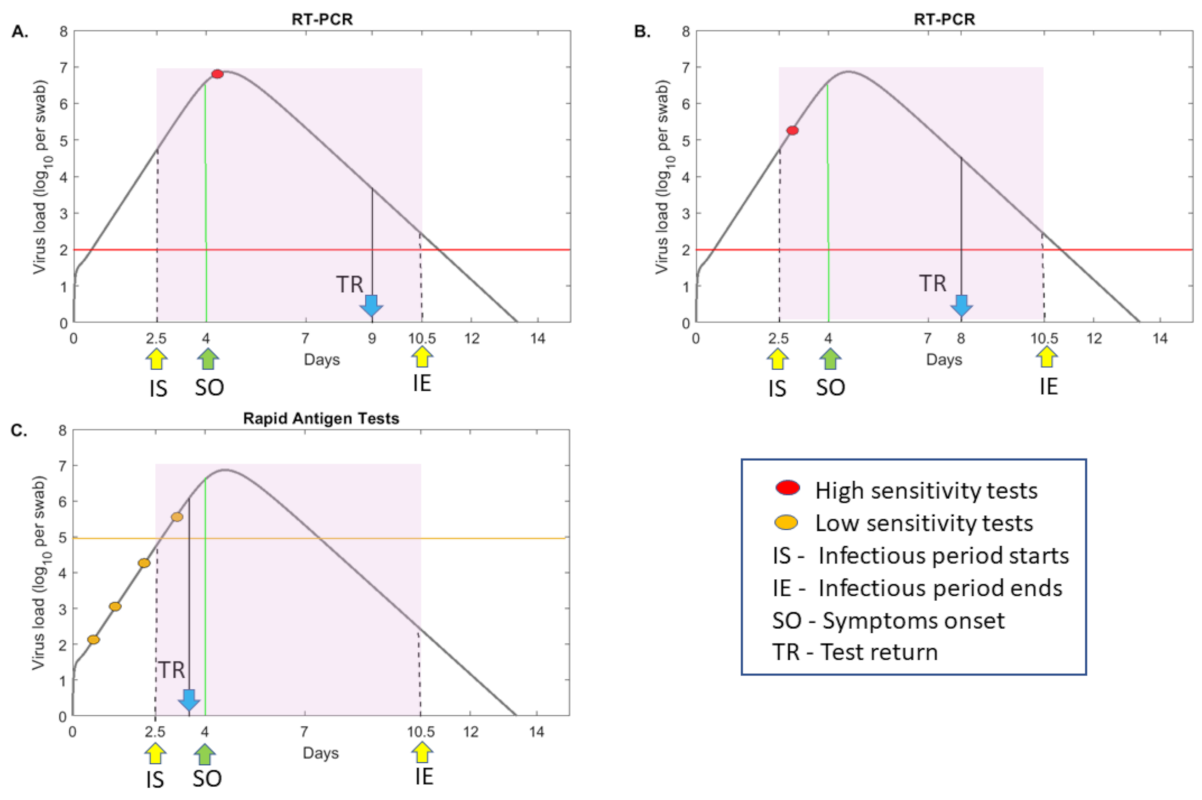
3.1. The Relationship between Test Sensitivity and Virus Titers
3.2. Mathematical Model of Testing during SARS-CoV-2 Transmission
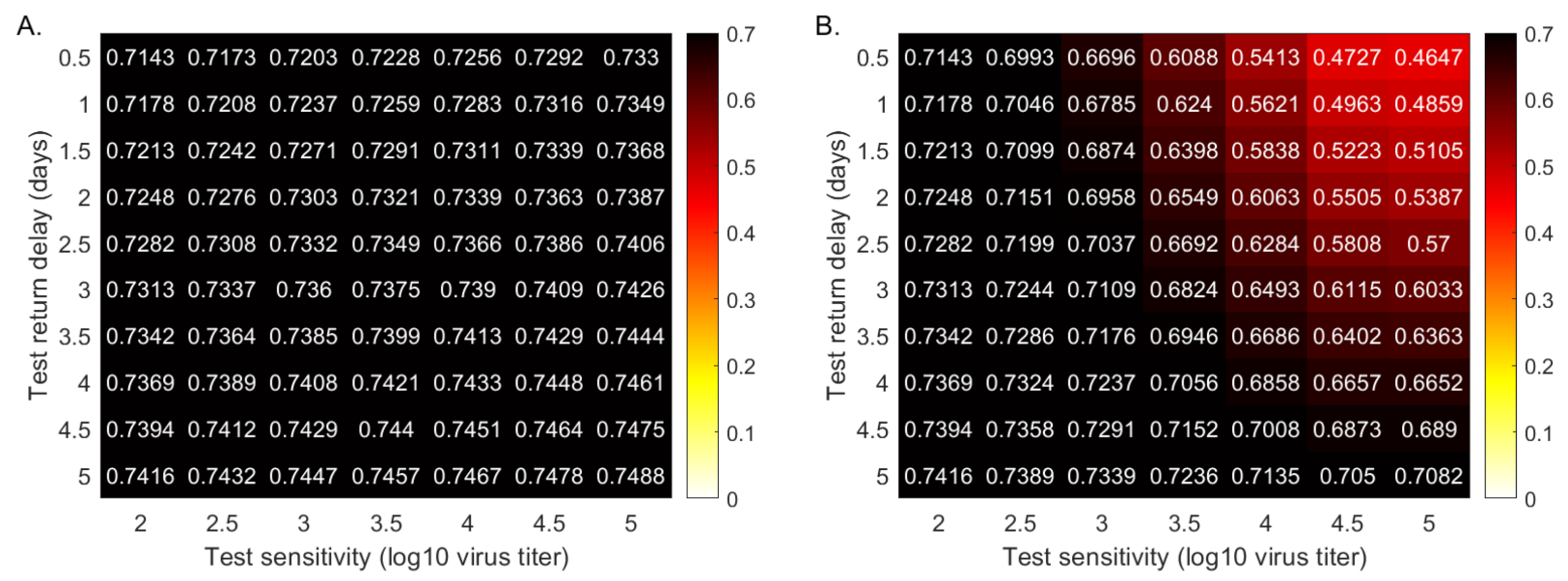
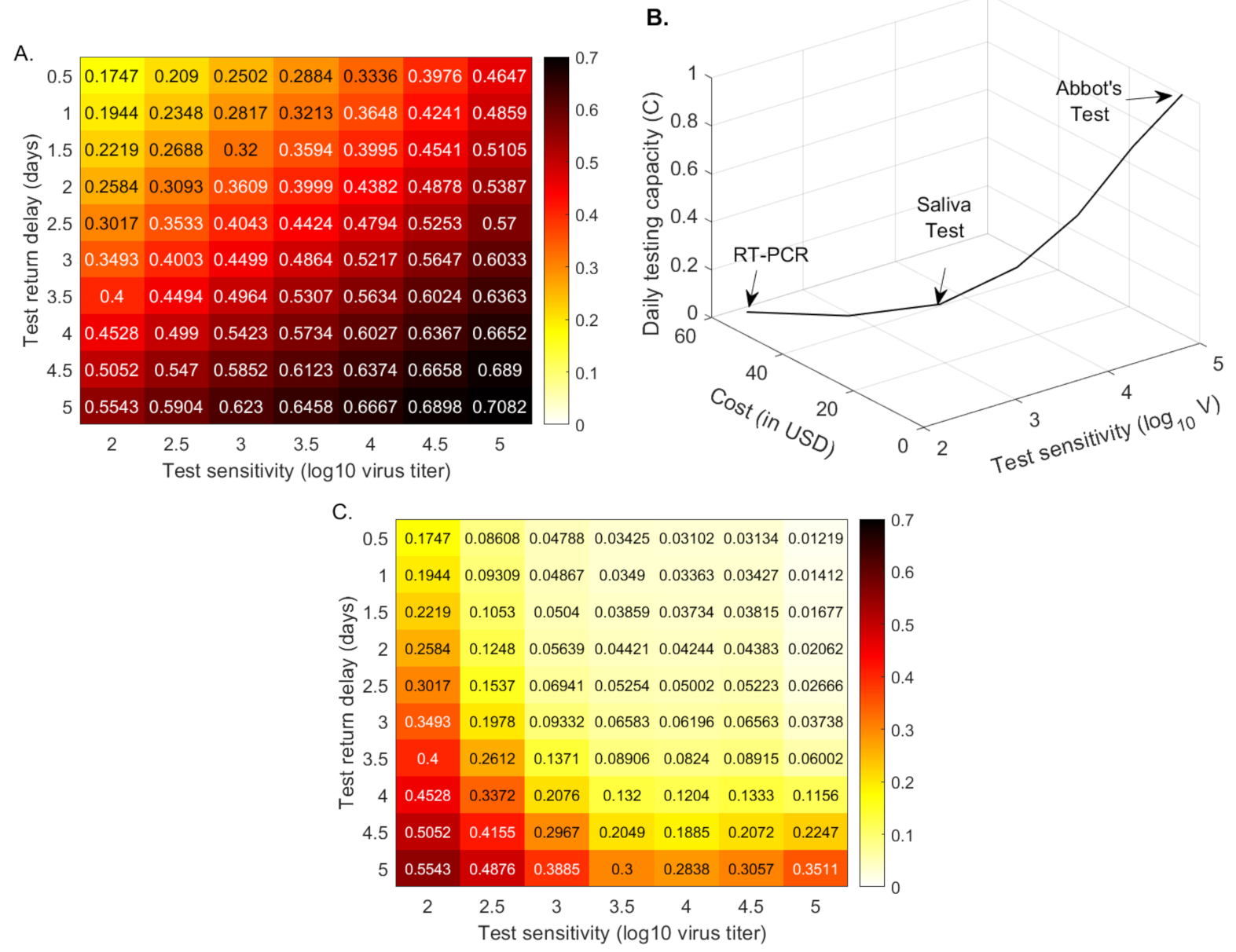
3.3. Quantifying the Tradeoff between Test Sensitivity and Return Delay
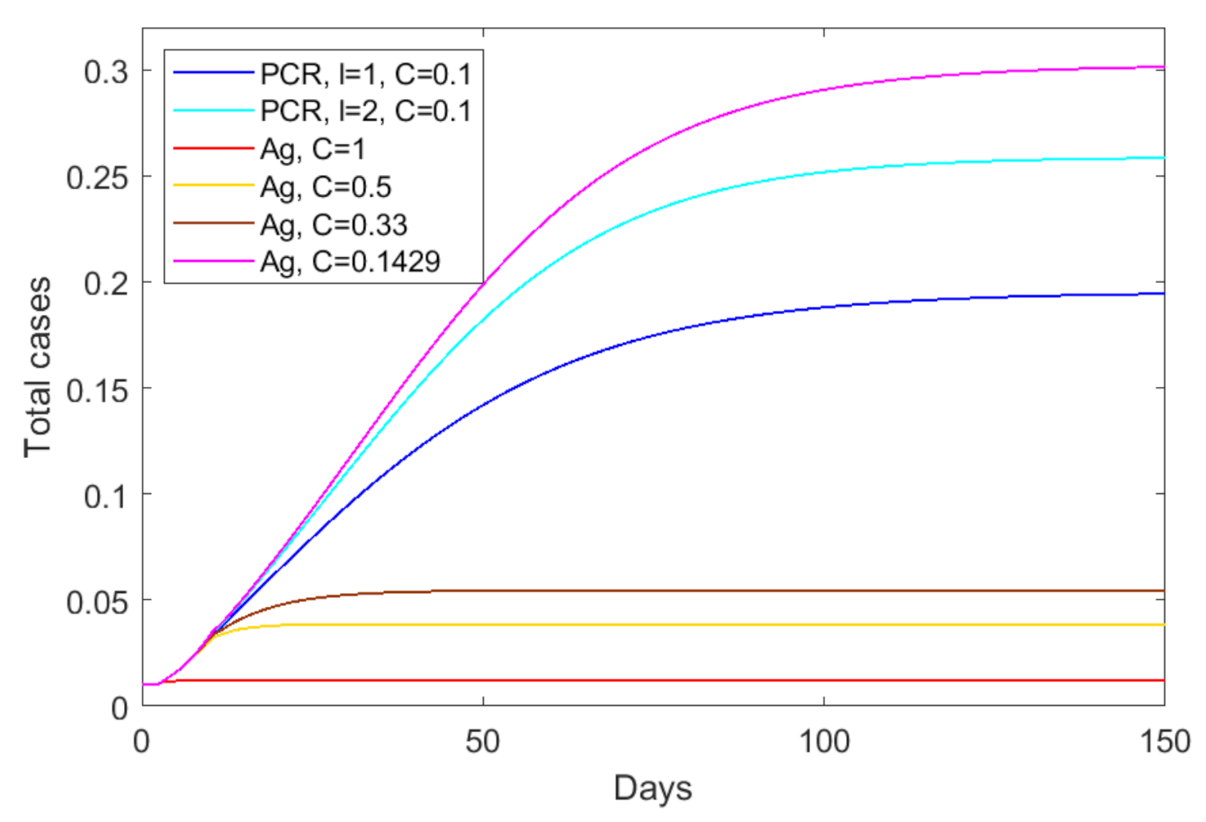
3.4. Quantifying the Tradeoff between Test Sensitivity and Test Frequency
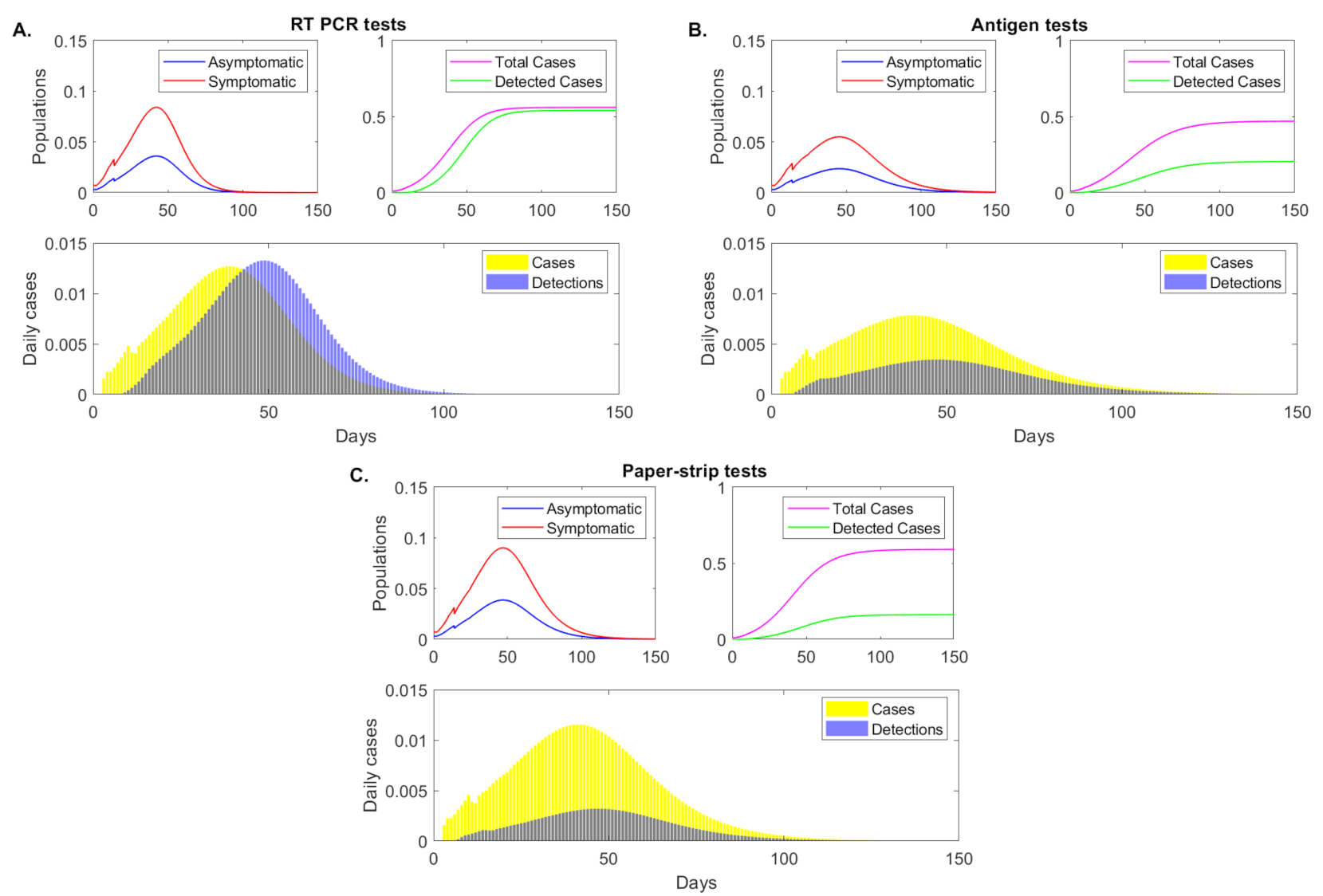
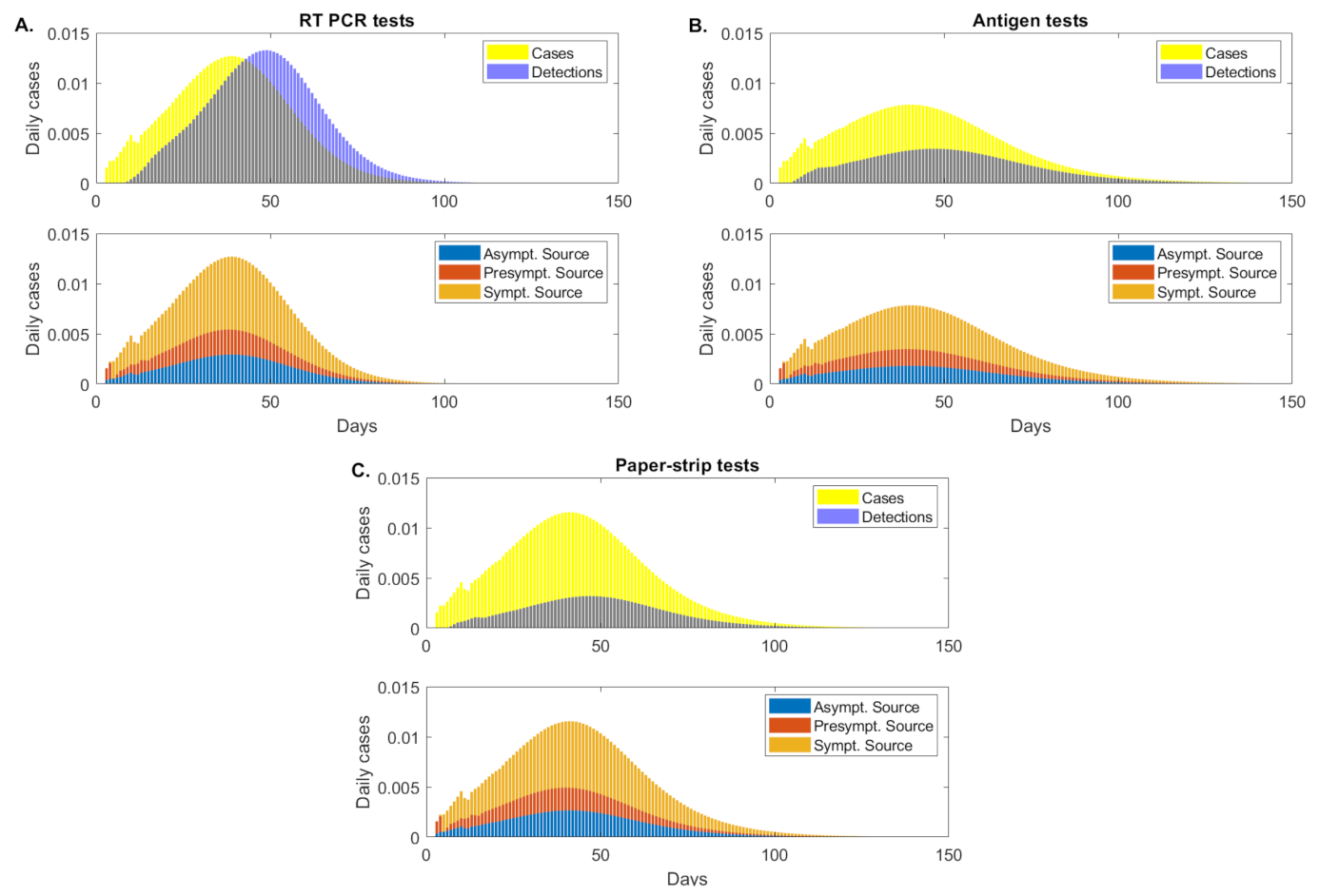
3.5. Transmission According to Infection Status
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A. Numerical Scheme
Appendix A.1. Initialization
Appendix A.2. Discretized Functions
Appendix A.3. Updating State Variables
References
- World Health Organization Coronavirus Disease (COVID-19) Dashboard. Available online: https://covid19.who.int/ (accessed on 12 February 2021).
- Lee, D.; Lee, J. Testing on the Move South Korea’s rapid response to the COVID-19 pandemic. Transp. Res. Interdiscip. Perspect. 2020, 5, 100111. [Google Scholar] [CrossRef]
- Gudbjartsson, D.F.; Helgason, A.; Jonsson, H.; Magnusson, O.T.; Melsted, P.; Norddahl, G.L.; Saemundsdottir, J.; Sigurdsson, A.; Sulem, P.; Agustsdottir, A.B.; et al. Spread of SARS-CoV-2 in the Icelandic population. N. Engl. J. Med. 2020, 382, 2302–2315. [Google Scholar] [CrossRef]
- Rosenberg, E.S.; Holtgrave, D.R. Widespread and frequent testing is essential to controlling COVID-19 in the United States. Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am. 2020. [Google Scholar] [CrossRef] [PubMed]
- Oran, D.P.; Topol, E.J. Prevalence of asymptomatic SARS-CoV-2 infection: A narrative review. Ann. Intern. Med. 2020, 173, 362–367. [Google Scholar] [CrossRef] [PubMed]
- Paltiel, A.D.; Zheng, A.; Walensky, R.P. Assessment of SARS-CoV-2 screening strategies to permit the safe reopening of college campuses in the United States. JAMA Netw. Open 2020, 3, e2016818. [Google Scholar] [CrossRef]
- Zhang, K.; Shoukat, A.; Crystal, W.; Langley, J.M.; Galvani, A.P.; Moghadas, S.M. Routine saliva testing for the identification of silent COVID-19 infections in healthcare workers. Infect. Control. Hosp. Epidemiol. 2020, 1–17. [Google Scholar] [CrossRef]
- Tang, Y.W.; Schmitz, J.E.; Persing, D.H.; Stratton, C.W. Laboratory diagnosis of COVID-19: Current issues and challenges. J. Clin. Microbiol. 2020, 58. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mina, M.J.; Parker, R.; Larremore, D.B. Rethinking Covid-19 test sensitivity—A strategy for containment. N. Engl. J. Med. 2020, 383, e120. [Google Scholar] [CrossRef]
- He, D.; Zhao, S.; Lin, Q.; Zhuang, Z.; Cao, P.; Wang, M.H.; Yang, L. The relative transmissibility of asymptomatic COVID-19 infections among close contacts. Int. J. Infect. Dis. 2020, 94, 145–147. [Google Scholar] [CrossRef]
- Huang, C.G.; Lee, K.M.; Hsiao, M.J.; Yang, S.L.; Huang, P.N.; Gong, Y.N.; Hsieh, T.H.; Huang, P.W.; Lin, Y.J.; Liu, Y.C.; et al. Culture-based virus isolation to evaluate potential infectivity of clinical specimens tested for COVID-19. J. Clin. Microbiol. 2020, 58. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Lau, E.H.; Wu, P.; Deng, X.; Wang, J.; Hao, X.; Lau, Y.C.; Wong, J.Y.; Guan, Y.; Tan, X.; et al. Temporal dynamics in viral shedding and transmissibility of COVID-19. Nat. Med. 2020, 26, 672–675. [Google Scholar] [CrossRef] [Green Version]
- McIntosh, K.; Hirsch, M.; Bloom, A. Coronavirus disease 2019 (COVID-19): Epidemiology, virology, and prevention. Lancet Infect. Dis. 2020, 1, 2019–2020. [Google Scholar]
- Bullard, J.; Dust, K.; Funk, D.; Strong, J.E.; Alexander, D.; Garnett, L.; Boodman, C.; Bello, A.; Hedley, A.; Schiffman, Z.; et al. Predicting infectious severe acute respiratory syndrome coronavirus 2 from diagnostic samples. Clin. Infect. Dis. 2020, 71, 2663–2666. [Google Scholar] [CrossRef] [PubMed]
- Lanser, L.; Bellmann-Weiler, R.; Öttl, K.W.; Huber, L.; Griesmacher, A.; Theurl, I.; Weiss, G. Evaluating the clinical utility and sensitivity of SARS-CoV-2 antigen testing in relation to RT-PCR Ct values. Infection 2020, 1–3. [Google Scholar] [CrossRef]
- Vogels, C.B.; Brito, A.F.; Wyllie, A.L.; Fauver, J.R.; Ott, I.M.; Kalinich, C.C.; Petrone, M.E.; Casanovas-Massana, A.; Muenker, M.C.; Moore, A.J.; et al. Analytical sensitivity and efficiency comparisons of SARS-CoV-2 RT–qPCR primer–probe sets. Nat. Microbiol. 2020, 5, 1299–1305. [Google Scholar] [CrossRef]
- Etievant, S.; Bal, A.; Escurret, V.; Brengel-Pesce, K.; Bouscambert, M.; Cheynet, V.; Generenaz, L.; Oriol, G.; Destras, G.; Billaud, G.; et al. Sensitivity assessment of SARS-CoV-2 PCR assays developed by WHO referral laboratories. medRxiv 2020. [Google Scholar] [CrossRef]
- Böhmer, M.M.; Buchholz, U.; Corman, V.M.; Hoch, M.; Katz, K.; Marosevic, D.V.; Böhm, S.; Woudenberg, T.; Ackermann, N.; Konrad, R.; et al. Investigation of a COVID-19 outbreak in Germany resulting from a single travel-associated primary case: A case series. Lancet Infect. Dis. 2020, 20, 920–928. [Google Scholar] [CrossRef]
- Singanayagam, A.; Patel, M.; Charlett, A.; Bernal, J.L.; Saliba, V.; Ellis, J.; Ladhani, S.; Zambon, M.; Gopal, R. Duration of infectiousness and correlation with RT-PCR cycle threshold values in cases of COVID-19, England, January to May 2020. Eurosurveillance 2020, 25, 2001483. [Google Scholar] [CrossRef]
- Bryan, A.; Fink, S.L.; Gattuso, M.A.; Pepper, G.; Chaudhary, A.; Wener, M.H.; Morishima, C.; Jerome, K.R.; Mathias, P.C.; Greninger, A.L. SARS-CoV-2 viral load on admission is associated with 30-day mortality. In Open Forum Infectious Diseases; Oxford University Press: Oxford, UK, 2020; Volume 7, p. ofaa535. [Google Scholar]
- FDA. EUA Authorizations. Available online: https://www.fda.gov/medical-devices/coronavirus-disease-2019-covid-19-emergency-use-authorizations-medical-devices/vitro-diagnostics-euas individual-molecular (accessed on 30 November 2020).
- COVID-19 Update: FDA Authorizes First Diagnostic Test Where Results Can Be Read Directly From Testing Card. Available online: https://www.fda.gov/news-events/press-announcements/covid-19-update-fda-authorizes-first-diagnostic-test-where-results-can-be-read-directly-testing-card (accessed on 1 February 2021).
- Prince-Guerra, J.L.; Almendares, O.; Nolen, L.D.; Gunn, J.K.; Dale, A.P.; Buono, S.A.; Deutsch-Feldman, M.; Suppiah, S.; Hao, L.; Zeng, Y.; et al. Evaluation of Abbott BinaxNOW Rapid Antigen Test for SARS-CoV-2 Infection at Two Community-Based Testing Sites—Pima County, Arizona, 3–17 November 2020. Morb. Mortal. Wkly. Rep. 2021, 70, 100. [Google Scholar] [CrossRef] [PubMed]
- Harritshoej, L.H.; Gybel-Brask, M.; Afzal, S.; Kamstrup, P.R.; Joergensen, C.S.; Thomsen, M.K.; Hilsted, L.M.; Friis-Hansen, L.J.; Szecsi, P.B.; Pedersen, L.; et al. Comparison of sixteen serological SARS-CoV-2 immunoassays in sixteen clinical laboratories. medRxiv 2020. [Google Scholar] [CrossRef]
- Larremore, D.B.; Wilder, B.; Lester, E.; Shehata, S.; Burke, J.M.; Hay, J.A.; Tambe, M.; Mina, M.J.; Parker, R. Test sensitivity is secondary to frequency and turnaround time for COVID-19 screening. Sci. Adv. 2021, 7, eabd5393. [Google Scholar] [CrossRef]
- Ke, R.; Zitzmann, C.; Ribeiro, R.M.; Perelson, A.S. Kinetics of SARS-CoV-2 infection in the human upper and lower respiratory tracts and their relationship with infectiousness. medRxiv 2020. [Google Scholar] [CrossRef]
- Wölfel, R.; Corman, V.M.; Guggemos, W.; Seilmaier, M.; Zange, S.; Müller, M.A.; Niemeyer, D.; Jones, T.C.; Vollmar, P.; Rothe, C.; et al. Virological assessment of hospitalized patients with COVID-2019. Nature 2020, 581, 465–469. [Google Scholar] [CrossRef] [Green Version]
- Baccam, P.; Beauchemin, C.; Macken, C.A.; Hayden, F.G.; Perelson, A.S. Kinetics of influenza A virus infection in humans. J. Virol. 2006, 80, 7590–7599. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Beauchemin, C.A.; Handel, A. A review of mathematical models of influenza A infections within a host or cell culture: Lessons learned and challenges ahead. BMC Public Health 2011, 11, S7. [Google Scholar] [CrossRef] [Green Version]
- Smith, A.M.; Perelson, A.S. Influenza A virus infection kinetics: Quantitative data and models. Wiley Interdiscip. Rev. Syst. Biol. Med. 2011, 3, 429–445. [Google Scholar] [CrossRef] [Green Version]
- Nikin-Beers, R.; Ciupe, S.M. Modelling original antigenic sin in dengue viral infection. Math. Med. Biol. A J. IMA 2017, 35, 257–272. [Google Scholar] [CrossRef] [PubMed]
- Nikin-Beers, R.; Ciupe, S.M. The role of antibody in enhancing dengue virus infection. Math. Biosci. 2015, 263, 83–92. [Google Scholar] [CrossRef]
- Ben-Shachar, R.; Schmidler, S.; Koelle, K. Drivers of inter-individual variation in dengue viral load dynamics. PLoS Comput. Biol. 2016, 12, e1005194. [Google Scholar] [CrossRef] [PubMed]
- Best, K.; Guedj, J.; Madelain, V.; de Lamballerie, X.; Lim, S.Y.; Osuna, C.E.; Whitney, J.B.; Perelson, A.S. Zika plasma viral dynamics in nonhuman primates provides insights into early infection and antiviral strategies. Proc. Natl. Acad. Sci. USA 2017, 114, 8847–8852. [Google Scholar] [CrossRef] [Green Version]
- Banerjee, S.; Guedj, J.; Ribeiro, R.M.; Moses, M.; Perelson, A.S. Estimating biologically relevant parameters under uncertainty for experimental within-host murine West Nile virus infection. J. R. Soc. Interface 2016, 13, 20160130. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ogando, N.S.; Dalebout, T.J.; Zevenhoven-Dobbe, J.C.; Limpens, R.W.; van der Meer, Y.; Caly, L.; Druce, J.; de Vries, J.J.; Kikkert, M.; Bárcena, M.; et al. SARS-coronavirus-2 replication in Vero E6 cells: Replication kinetics, rapid adaptation and cytopathology. J. Gen. Virol. 2020, 101, 925. [Google Scholar] [CrossRef]
- Duke Covid Testing Tracker. Available online: https://coronavirus.duke.edu/covid-testing/ (accessed on 1 March 2021).
- Coronavirus Information. Available online: https://coronavirus.virginia.edu/covid-tracker (accessed on 1 March 2021).
- COVID-19 Tracking. Available online: https://covid.cornell.edu/testing/dashboard/ (accessed on 1 March 2021).
- Neilan, A.M.; Losina, E.; Bangs, A.C.; Flanagan, C.; Panella, C.; Eskibozkurt, G.E.; Mohareb, A.; Hyle, E.P.; Scott, J.A.; Weinstein, M.C.; et al. Clinical Impact, Costs, and Cost-Effectiveness of Expanded SARS-CoV-2 Testing in Massachusetts. medrxiv 2020. [Google Scholar] [CrossRef]
- Wyllie, A.L.; Fournier, J.; Casanovas-Massana, A.; Campbell, M.; Tokuyama, M.; Vijayakumar, P.; Warren, J.L.; Geng, B.; Muenker, M.C.; Moore, A.J.; et al. Saliva or nasopharyngeal swab specimens for detection of SARS-CoV-2. N. Engl. J. Med. 2020, 383, 1283–1286. [Google Scholar] [CrossRef] [PubMed]
- Brook, C.E.; Northrup, G.R.; Ehrenberg, A.J.; Doudna, J.A.; Boots, M.; IGI Testing Consortium. Optimizing COVID-19 control with asymptomatic surveillance testing in a university environment. medRxiv 2020. [Google Scholar] [CrossRef]
- Bergstrom, T.; Bergstrom, C.T.; Li, H. Frequency and accuracy of proactive testing for COVID-19. medRxiv 2020. [Google Scholar] [CrossRef]
| Fixed Parameters | Description | Value | Source |
|---|---|---|---|
| Eclipse phase duration | 4/day | [36] | |
| c | Viral clearance | 10/day | [28] |
| Transport between tracts | 0 | [26] | |
| Estimated Parameters | Description | Value | Source |
| Infection rate in URT | 5.1/swab× day | [26] | |
| Infection rate in LRT | /mL× day | [26] | |
| URT virus production | 50/day | [26] | |
| LRT virus production | /day | [26] | |
| URT cell death | 2/day | [26] | |
| LRT cell death | /day | [26] | |
| - | 0.01 | [26] | |
| Initial Conditions | Description | Value | Source |
| Epithelial cells in URT | /mL | [26] | |
| Epithelial cells in LRT | /mL | [28] | |
| Exposed epithelial cells | 0 | [26] | |
| Infectious epithelial cells in URT | 10 | [26] | |
| Infectious epithelial cells in LRT | 1 | [26] | |
| Virus | 0 | [26] |
| Fixed Parameters | Description | Value | Source |
|---|---|---|---|
| Transmission rate | /day | ||
| b | Birth rate | /day | |
| Death rate | /day | ||
| Disease induced mortality rate | /day | ||
| f | Fraction of symptomatic infections | [10] | |
| Relative asymp. infectiousness | 0.7 | ||
| ℓ | Test return delay | varied | |
| Age of onset of virus detectability | varied (days) | ||
| Age of onset of infectiousness | days | [27] | |
| Age of end of infectiousness | days | [10,12] | |
| Age of loss of virus detectability | varied (days) | ||
| Initial Conditions | Description | Value | Source |
| Susceptible population | 0.99 | ||
| Infected symptomatic population | |||
| Infected asymptomatic population |
| Test Type | Infectious Subgroup | ||||
|---|---|---|---|---|---|
| Antigen | Symptomatic | ||||
| Presymptomatic | |||||
| Asymptomatic | |||||
| Total | |||||
| Paper-strip | Symptomatic | ||||
| Presymptomatic | |||||
| Asymptomatic | |||||
| Total |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Forde, J.E.; Ciupe, S.M. Quantification of the Tradeoff between Test Sensitivity and Test Frequency in a COVID-19 Epidemic—A Multi-Scale Modeling Approach. Viruses 2021, 13, 457. https://doi.org/10.3390/v13030457
Forde JE, Ciupe SM. Quantification of the Tradeoff between Test Sensitivity and Test Frequency in a COVID-19 Epidemic—A Multi-Scale Modeling Approach. Viruses. 2021; 13(3):457. https://doi.org/10.3390/v13030457
Chicago/Turabian StyleForde, Jonathan E., and Stanca M. Ciupe. 2021. "Quantification of the Tradeoff between Test Sensitivity and Test Frequency in a COVID-19 Epidemic—A Multi-Scale Modeling Approach" Viruses 13, no. 3: 457. https://doi.org/10.3390/v13030457






