Imaging Techniques for Detecting Prokaryotic Viruses in Environmental Samples
Abstract
:1. Introduction

| Microscopy Technique | Advantages | Disadvantages | Resolution | Coupling with Following Techniques Has Been Performed |
|---|---|---|---|---|
| Fluorescence microscopy |
|
| ~300 nm for conventional light microscopy techniques and 10 nm for super-resolution microscopy (SRM) [38] | |
| Nucleic acid staining |
| |||
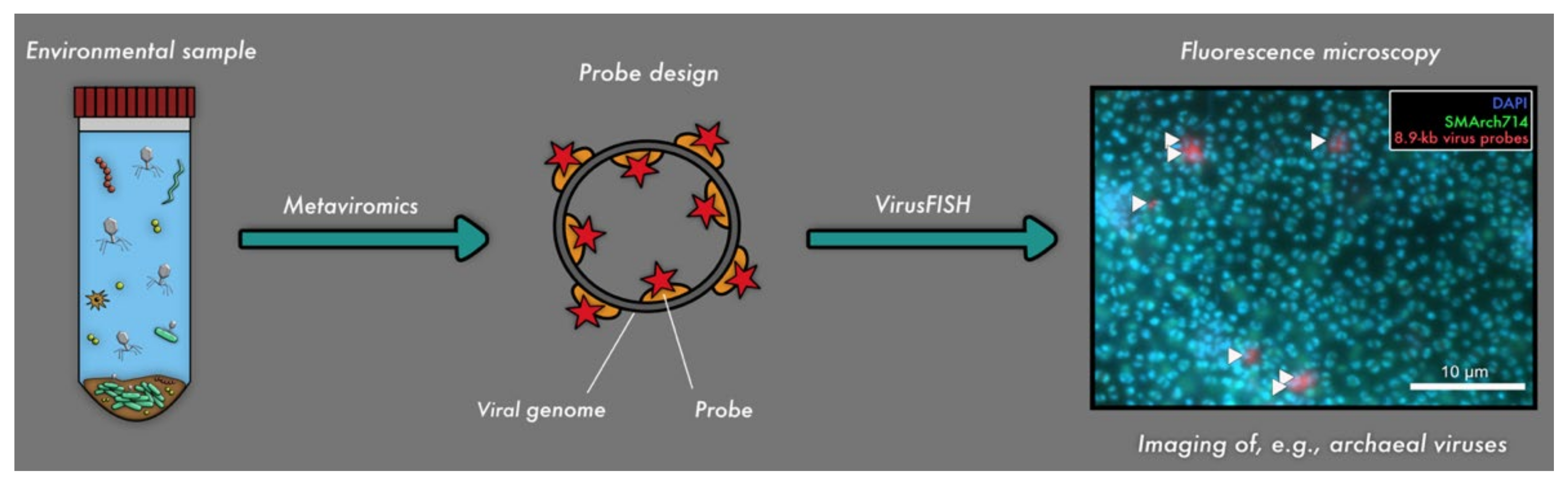
| Fluorescence in situ hybridization (FISH) (direct-geneFISH [45], phageFISH [46], virusFISH [10]) |
|
|
| |
| Electron microscopy (EM) |
| - Expensive equipment and time-consuming- Not possible in the field [48] |
|
|
| TEM, SEM and Cryo-EM |
|
| ||
| Helium-ion microscopy (HIM) |
|
| ||
| Atomic force microscopy (AFM) |
|
2. Retrieving Viral Fractions from Ecosystems for Microscopy Analyses
3. Using Electron Microscopy for Virus Quantification and for Discovery of Previously Unknown Viral Morphologies and Ultrastructures
3.1. Sample Preparation for Transmission Electron Microscopy
3.2. Estimating Viral Abundances in Environmental Samples Using TEMs
3.3. Determination of the Frequency of Visibly Infected Cells, Burst Sizes, and Spatial Distribution of Viruses
3.4. Observing (Novel) Viral Morphologies
3.5. Scanning Electron Microscopy for Studying Unique Viral Egress Mechanisms
3.6. Illustrating Virus–Host Associations by Using Cryo-Electron (Cryo-EM) Microscopy and Cryo-Electron Tomography (Cryo-ET)
4. Shedding Light on Viral Abundances in Ecosystems Using Epifluorescence Microscopy
5. Enhanced Surface Topography of Virus–Host Interactions Using Helium-Ion Microscopy
6. Atomic Force Microscopy for Cost-Effective Scanning of Viral Structures
7. Virus Discovery by (Meta)genomics and Microscopy
8. A Promising Technique for Linking Environmental Genomics to Fluorescence Microscopy of Viruses
8.1. Fluorescence In Situ Hybridization (FISH) for Tracking Virus–Host Interactions
8.2. Coupling of Metaviromics with Fluorescence In Situ Hybridization
9. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Fuhrman, J.A. Marine Viruses and Their Biogeochemical and Ecological Effects. Nature 1999, 399, 541–548. [Google Scholar] [CrossRef] [PubMed]
- Srinivasiah, S.; Bhavsar, J.; Thapar, K.; Liles, M.; Schoenfeld, T.; Wommack, K.E. Phages across the Biosphere: Contrasts of Viruses in Soil and Aquatic Environments. Res. Microbiol. 2008, 159, 349–357. [Google Scholar] [CrossRef]
- Suttle, C.A. Marine Viruses—Major Players in the Global Ecosystem. Nat. Rev. Microbiol. 2007, 5, 801–812. [Google Scholar] [CrossRef] [PubMed]
- Suttle, C.A. Viruses in the Sea. Nature 2005, 437, 356–361. [Google Scholar] [CrossRef]
- Thingstad, T.; Lignell, R. Theoretical Models for the Control of Bacterial Growth Rate, Abundance, Diversity and Carbon Demand. Aquat. Microb. Ecol. 1997, 13, 19–27. [Google Scholar] [CrossRef] [Green Version]
- Silveira, C.B.; Rohwer, F.L. Piggyback-the-Winner in Host-Associated Microbial Communities. Npj Biofilms Microbiomes 2016, 2, 16010. [Google Scholar] [CrossRef]
- Breitbart, M.; Thompson, L.; Suttle, C.; Sullivan, M. Exploring the Vast Diversity of Marine Viruses. Oceanography 2007, 20, 135–139. [Google Scholar] [CrossRef]
- Anantharaman, K.; Duhaime, M.B.; Breier, J.A.; Wendt, K.A.; Toner, B.M.; Dick, G.J. Sulfur Oxidation Genes in Diverse Deep-Sea Viruses. Science 2014, 344, 757–760. [Google Scholar] [CrossRef] [Green Version]
- Ahlgren, N.A.; Fuchsman, C.A.; Rocap, G.; Fuhrman, J.A. Discovery of Several Novel, Widespread, and Ecologically Distinct Marine Thaumarchaeota Viruses That Encode AmoC Nitrification Genes. ISME J. 2019, 13, 618–631. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rahlff, J.; Turzynski, V.; Esser, S.P.; Monsees, I.; Bornemann, T.L.V.; Figueroa-Gonzalez, P.A.; Schulz, F.; Woyke, T.; Klingl, A.; Moraru, C.; et al. Lytic Archaeal Viruses Infect Abundant Primary Producers in Earth’s Crust. Nat. Commun. 2021, 12, 4642. [Google Scholar] [CrossRef] [PubMed]
- Anderson, R.E.; Sogin, M.L.; Baross, J.A. Evolutionary Strategies of Viruses, Bacteria and Archaea in Hydrothermal Vent Ecosystems Revealed through Metagenomics. PLoS ONE 2014, 9, e109696. [Google Scholar] [CrossRef] [Green Version]
- Sharon, I.; Alperovitch, A.; Rohwer, F.; Haynes, M.; Glaser, F.; Atamna-Ismaeel, N.; Pinter, R.Y.; Partensky, F.; Koonin, E.V.; Wolf, Y.I.; et al. Photosystem I Gene Cassettes Are Present in Marine Virus Genomes. Nature 2009, 461, 258–262. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fridman, S.; Flores-Uribe, J.; Larom, S.; Alalouf, O.; Liran, O.; Yacoby, I.; Salama, F.; Bailleul, B.; Rappaport, F.; Ziv, T.; et al. A Myovirus Encoding Both Photosystem I and II Proteins Enhances Cyclic Electron Flow in Infected Prochlorococcus Cells. Nat. Microbiol. 2017, 2, 1350–1357. [Google Scholar] [CrossRef]
- Zhang, H.; Ning, K. The Tara Oceans Project: New Opportunities and Greater Challenges Ahead. Genomics Proteomics Bioinformatics 2015, 13, 275–277. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brum, J.R.; Ignacio-Espinoza, J.C.; Roux, S.; Doulcier, G.; Acinas, S.G.; Alberti, A.; Chaffron, S.; Cruaud, C.; de Vargas, C.; Gasol, J.M.; et al. Patterns and Ecological Drivers of Ocean Viral Communities. Science 2015, 348, 1261498. [Google Scholar] [CrossRef] [Green Version]
- Hurwitz, B.L.; Deng, L.; Poulos, B.T.; Sullivan, M.B. Evaluation of Methods to Concentrate and Purify Ocean Virus Communities through Comparative, Replicated Metagenomics: Viral Community Concentration and Purification. Environ. Microbiol. 2013, 15, 1428–1440. [Google Scholar] [CrossRef] [Green Version]
- Duhaime, M.B.; Sullivan, M.B. Ocean Viruses: Rigorously Evaluating the Metagenomic Sample-to-Sequence Pipeline. Virology 2012, 434, 181–186. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roux, S.; Brum, J.R.; Dutilh, B.E.; Sunagawa, S.; Duhaime, M.B.; Loy, A.; Poulos, B.T.; Solonenko, N.; Lara, E.; Poulain, J.; et al. Ecogenomics and Potential Biogeochemical Impacts of Globally Abundant Ocean Viruses. Nature 2016, 537, 689–693. [Google Scholar] [CrossRef] [Green Version]
- Almeida, G.M.F.; Leppänen, M.; Maasilta, I.J.; Sundberg, L.-R. Bacteriophage Imaging: Past, Present and Future. Res. Microbiol. 2018, 169, 488–494. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weinbauer, M.G. Ecology of Prokaryotic Viruses. FEMS Microbiol. Rev. 2004, 28, 127–181. [Google Scholar] [CrossRef] [Green Version]
- Luef, B.; Neu, T.R.; Peduzzi, P. Imaging and Quantifying Virus Fluorescence Signals on Aquatic Aggregates: A New Method and Its Implication for Aquatic Microbial Ecology: Viruses on Riverine Aggregates. FEMS Microbiol. Ecol. 2009, 68, 372–380. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Danovaro, R.; Dell’Anno, A.; Trucco, A.; Serresi, M.; Vanucci, S. Determination of Virus Abundance in Marine Sediments. Appl. Environ. Microbiol. 2001, 67, 1384–1387. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Engelhardt, T.; Kallmeyer, J.; Cypionka, H.; Engelen, B. High Virus-to-Cell Ratios Indicate Ongoing Production of Viruses in Deep Subsurface Sediments. ISME J. 2014, 8, 1503–1509. [Google Scholar] [CrossRef] [PubMed]
- Williamson, K.E.; Wommack, K.E.; Radosevich, M. Sampling Natural Viral Communities from Soil for Culture-Independent Analyses. Appl. Environ. Microbiol. 2003, 69, 6628–6633. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Williamson, K.E.; Fuhrmann, J.J.; Wommack, K.E.; Radosevich, M. Viruses in Soil Ecosystems: An Unknown Quantity Within an Unexplored Territory. Annu. Rev. Virol. 2017, 4, 201–219. [Google Scholar] [CrossRef] [PubMed]
- Jahn, M.T.; Lachnit, T.; Markert, S.M.; Stigloher, C.; Pita, L.; Ribes, M.; Dutilh, B.E.; Hentschel, U. Lifestyle of Sponge Symbiont Phages by Host Prediction and Correlative Microscopy. ISME J. 2021. [Google Scholar] [CrossRef] [PubMed]
- Müller, T.; Sakin, V.; Müller, B. A Spotlight on Viruses—Application of Click Chemistry to Visualize Virus-Cell Interactions. Molecules 2019, 24, 481. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bize, A.; Karlsson, E.A.; Ekefjard, K.; Quax, T.E.F.; Pina, M.; Prevost, M.-C.; Forterre, P.; Tenaillon, O.; Bernander, R.; Prangishvili, D. A Unique Virus Release Mechanism in the Archaea. Proc. Natl. Acad. Sci. USA 2009, 106, 11306–11311. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Prangishvili, D.; Vestergaard, G.; Häring, M.; Aramayo, R.; Basta, T.; Rachel, R.; Garrett, R.A. Structural and Genomic Properties of the Hyperthermophilic Archaeal Virus ATV with an Extracellular Stage of the Reproductive Cycle. J. Mol. Biol. 2006, 359, 1203–1216. [Google Scholar] [CrossRef] [PubMed]
- DiMaio, F.; Yu, X.; Rensen, E.; Krupovic, M.; Prangishvili, D.; Egelman, E.H. A Virus That Infects a Hyperthermophile Encapsidates A-Form DNA. Science 2015, 348, 914–917. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guerrero-Ferreira, R.C.; Wright, E.R. Cryo-Electron Tomography of Bacterial Viruses. Virology 2013, 435, 179–186. [Google Scholar] [CrossRef] [Green Version]
- Leppänen, M.; Sundberg, L.-R.; Laanto, E.; de Freitas Almeida, G.M.; Papponen, P.; Maasilta, I.J. Imaging Bacterial Colonies and Phage-Bacterium Interaction at Sub-Nanometer Resolution Using Helium-Ion Microscopy. Adv. Biosyst. 2017, 1, 1700070. [Google Scholar] [CrossRef]
- Dubrovin, E.V.; Voloshin, A.G.; Kraevsky, S.V.; Ignatyuk, T.E.; Abramchuk, S.S.; Yaminsky, I.V.; Ignatov, S.G. Atomic Force Microscopy Investigation of Phage Infection of Bacteria. Langmuir 2008, 24, 13068–13074. [Google Scholar] [CrossRef]
- Hochstein, R.A.; Amenabar, M.J.; Munson-McGee, J.H.; Boyd, E.S.; Young, M.J. Acidianus Tailed Spindle Virus: A New Archaeal Large Tailed Spindle Virus Discovered by Culture-Independent Methods. J. Virol. 2016, 90, 3458–3468. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barrero-Canosa, J.; Moraru, C. PhageFISH for Monitoring Phage Infections at Single Cell Level. In Bacteriophages; Methods and Protocols; Humana Press: Totowa, NJ, USA, 2019; Vol. IV, pp. 1–26. ISBN 978-1-4939-8939-3. [Google Scholar]
- Prata, C.; Ribeiro, A.; Cunha, Â.; Gomes, N.C.; Almeida, A. Ultracentrifugation as a Direct Method to Concentrate Viruses in Environmental Waters: Virus-like Particle Enumeration as a New Approach to Determine the Efficiency of Recovery. J. Env. Monit. 2012, 14, 64–70. [Google Scholar] [CrossRef]
- Noble, R.; Fuhrman, J. Use of SYBR Green I for Rapid Epifluorescence Counts of Marine Viruses and Bacteria. Aquat. Microb. Ecol. 1998, 14, 113–118. [Google Scholar] [CrossRef] [Green Version]
- Hauser, M.; Wojcik, M.; Kim, D.; Mahmoudi, M.; Li, W.; Xu, K. Correlative Super-Resolution Microscopy: New Dimensions and New Opportunities. Chem. Rev. 2017, 117, 7428–7456. [Google Scholar] [CrossRef] [PubMed]
- Marie, D.; Brussaard, C.P.D.; Thyrhaug, R.; Bratbak, G.; Vaulot, D. Enumeration of Marine Viruses in Culture and Natural Samples by Flow Cytometry. Appl. Environ. Microbiol. 1999, 65, 45–52. [Google Scholar] [CrossRef] [Green Version]
- Thurber, R.V.; Haynes, M.; Breitbart, M.; Wegley, L.; Rohwer, F. Laboratory Procedures to Generate Viral Metagenomes. Nat. Protoc. 2009, 4, 470–483. [Google Scholar] [CrossRef]
- Weinbauer, M.; Suttle, C. Comparison of Epifluorescence and Transmission Electron Microscopy for Counting Viruses in Natural Marine Waters. Aquat. Microb. Ecol. 1997, 13, 225–232. [Google Scholar] [CrossRef] [Green Version]
- Wen, K.; Ortmann, A.C.; Suttle, C.A. Accurate Estimation of Viral Abundance by Epifluorescence Microscopy. Appl. Environ. Microbiol. 2004, 70, 3862–3867. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pina, S.; Creus, A.; Gonzaález, N.; Gironeés, R.; Felip, M.; Sommaruga, R. Abundance, Morphology and Distribution of Planktonic Virus-like Particles in Two High-Mountain Lakes. J. Plankton Res. 1998, 20, 2413–2421. [Google Scholar] [CrossRef]
- Bettarel, Y.; Sime-Ngando, T.; Amblard, C.; Laveran, H. A Comparison of Methods for Counting Viruses in Aquatic Systems. Appl. Environ. Microbiol. 2000, 66, 2283–2289. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barrero-Canosa, J.; Moraru, C.; Zeugner, L.; Fuchs, B.M.; Amann, R. Direct-geneFISH: A Simplified Protocol for the Simultaneous Detection and Quantification of Genes and RRNA in Microorganisms. Environ. Microbiol. 2017, 19, 70–82. [Google Scholar] [CrossRef]
- Allers, E.; Moraru, C.; Duhaime, M.B.; Beneze, E.; Solonenko, N.; Barrero-Canosa, J.; Amann, R.; Sullivan, M.B. Single-Cell and Population Level Viral Infection Dynamics Revealed by PhageFISH, a Method to Visualize Intracellular and Free Viruses: PhageFISH—Visualizing Intracellular and Free Viruses. Environ. Microbiol. 2013, 15, 2306–2318. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zimmerman, A.E.; Bachy, C.; Ma, X.; Roux, S.; Jang, H.B.; Sullivan, M.B.; Waldbauer, J.R.; Worden, A.Z. Closely Related Viruses of the Marine Picoeukaryotic Alga Ostreococcus Lucimarinus Exhibit Different Ecological Strategies. Environ. Microbiol. 2019, 21, 2148–2170. [Google Scholar] [CrossRef] [Green Version]
- Hennes, K.P.; Suttle, C.A. Direct Counts of Viruses in Natural Waters and Laboratory Cultures by Epifluorescence Microscopy. Limnol. Oceanogr. 1995, 40, 1050–1055. [Google Scholar] [CrossRef]
- Bachrach, U.; Friedmann, A. Practical Procedures for the Purification of Bacterial Viruses. Appl. Microbiol. 1971, 22, 706–715. [Google Scholar] [CrossRef]
- Häring, M.; Vestergaard, G.; Rachel, R.; Chen, L.; Garrett, R.A.; Prangishvili, D. Independent Virus Development Outside a Host. Nature 2005, 436, 1101–1102. [Google Scholar] [CrossRef]
- Bettstetter, M.; Peng, X.; Garrett, R.A.; Prangishvili, D. AFV1, a Novel Virus Infecting Hyperthermophilic Archaea of the Genus Acidianus. Virology 2003, 315, 68–79. [Google Scholar] [CrossRef] [Green Version]
- Hara, S.; Terauchi, K.; Koike, I. Abundance of Viruses in Marine Waters: Assessment by Epifluorescence and Transmission Electron Microscopy. Appl. Environ. Microbiol. 1991, 57, 2731–2734. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kuznetsov, Y.G.; McPherson, A. Atomic Force Microscopy in Imaging of Viruses and Virus-Infected Cells. Microbiol. Mol. Biol. Rev. 2011, 75, 268–285. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Allison, D.P.; Mortensen, N.P.; Sullivan, C.J.; Doktycz, M.J. Atomic Force Microscopy of Biological Samples. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2010, 2, 618–634. [Google Scholar] [CrossRef] [PubMed]
- Dubrovin, E.V.; Kirikova, M.N.; Novikov, V.K.; Drygin, Y.F.; Yaminsky, I.V. Study of the Peculiarities of Adhesion of Tobacco Mosaic Virus by Atomic Force Microscopy. Colloid J. 2004, 66, 673–678. [Google Scholar] [CrossRef]
- Andany, S.H.; Hlawacek, G.; Hummel, S.; Brillard, C.; Kangül, M.; Fantner, G.E. An Atomic Force Microscope Integrated with a Helium Ion Microscope for Correlative Nanoscale Characterization. Beilstein J. Nanotechnol. 2020, 11, 1272–1279. [Google Scholar] [CrossRef] [PubMed]
- Munson-McGee, J.; Snyder, J.; Young, M. Archaeal Viruses from High-Temperature Environments. Genes 2018, 9, 128. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Anderson, R.E.; Brazelton, W.J.; Baross, J.A. The Deep Viriosphere: Assessing the Viral Impact on Microbial Community Dynamics in the Deep Subsurface. Rev. Mineral. Geochem. 2013, 75, 649–675. [Google Scholar] [CrossRef] [Green Version]
- Budinoff, C.R.; Loar, S.N.; LeCleir, G.R.; Wilhelm, S.W.; Buchan, A. A Protocol for Enumeration of Aquatic Viruses by Epifluorescence Microscopy Using AnodiscTM 13 Membranes. BMC Microbiol. 2011, 11, 168. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wommack, K.E.; Sime-Ngando, T.; Winget, D.; Jamindar, S.; Helton, R. Filtration-Based Methods for the Collection of Viral Concentrates from Large Water Samples. Man. Aquat. Viral Ecol. MAVE 2010, 12, 110–117. [Google Scholar] [CrossRef] [Green Version]
- Alonso, M.C.; Rodríguez, J.; Borrego, J.J. Enumeration and Isolation of Viral Particles from Oligotrophic Marine Environments by Tangential Flow Filtration. Int. Microbiol. Off. J. Span. Soc. Microbiol. 1999, 2, 227–232. [Google Scholar]
- Corinaldesi, C.; Dell’Anno, A.; Magagnini, M.; Danovaro, R. Viral Decay and Viral Production Rates in Continental-Shelf and Deep-Sea Sediments of the Mediterranean Sea. FEMS Microbiol. Ecol. 2010, 72, 208–218. [Google Scholar] [CrossRef]
- Nasukawa, T.; Uchiyama, J.; Taharaguchi, S.; Ota, S.; Ujihara, T.; Matsuzaki, S.; Murakami, H.; Mizukami, K.; Sakaguchi, M. Virus Purification by CsCl Density Gradient Using General Centrifugation. Arch. Virol. 2017, 162, 3523–3528. [Google Scholar] [CrossRef]
- John, S.G.; Mendez, C.B.; Deng, L.; Poulos, B.; Kauffman, A.K.M.; Kern, S.; Brum, J.; Polz, M.F.; Boyle, E.A.; Sullivan, M.B. A Simple and Efficient Method for Concentration of Ocean Viruses by Chemical Flocculation: Virus Concentration by Flocculation with Iron. Environ. Microbiol. Rep. 2011, 3, 195–202. [Google Scholar] [CrossRef] [Green Version]
- Fouladvand, F.; Bemani, P.; Mohammadi, M.; Amini, R.; Azizi Jalilian, F. A Review of the Methods for Concentrating M13 Phage. J. Appl. Biotechnol. Rep. 2020, 7. [Google Scholar] [CrossRef]
- Ford, T.; Graham, J.; Rickwood, D. Iodixanol: A Nonionic Iso-Osmotic Centrifugation Medium for the Formation of Self-Generated Gradients. Anal. Biochem. 1994, 220, 360–366. [Google Scholar] [CrossRef]
- Kausche, G.A.; Pfankuch, E.; Ruska, H. Die Sichtbarmachung von pflanzlichem Virus im Übermikroskop. Naturwissenschaften 1939, 27, 292–299. [Google Scholar] [CrossRef]
- Barreto-Vieira, D.F.; Barth, O.M. Negative and Positive Staining in Transmission Electron Microscopy for Virus Diagnosis. In Microbiology in Agriculture and Human Health; Shah, M.M., Ed.; InTech: London UK, 2015. [Google Scholar] [CrossRef] [Green Version]
- Reynolds, E.S. The use of lead citrate at high ph as an electron-opaque stain in electron microscopy. J. Cell Biol. 1963, 17, 208–212. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Milne, R.G.; Trautner, T.A. Thin Sectioning and Electron Microscopy of SP50 Bacteriophage Adsorbed to Bacillus Subtilis. J. Ultrastruct. Res. 1967, 20, 267–276. [Google Scholar] [CrossRef]
- Lundstrom, K.H.; Bamford, D.H.; Palva, E.T.; Lounatmaa, K. Lipid-Containing Bacteriophage PR4: Structure and Life Cycle. J. Gen. Virol. 1979, 43, 583–592. [Google Scholar] [CrossRef] [PubMed]
- Watson, M.L. Staining of Tissue Sections for Electron Microscopy with Heavy Metals. J. Biophys. Biochem. Cytol. 1958, 4, 475–478. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Proctor, L.M. Advances in the Study of Marine Viruses. Microsc. Res. Tech. 1997, 37, 136–161. [Google Scholar] [CrossRef]
- Belnap, D.M. Detection of Bacteriophages: Electron Microscopy and Visualization. In Bacteriophages; Harper, D.R., Abedon, S.T., Burrowes, B.H., McConville, M.L., Eds.; Springer International Publishing: Cham, Switzerland, 2021; pp. 561–620. ISBN 978-3-319-41985-5. [Google Scholar]
- Ackermann, H.-W. Basic Phage Electron Microscopy. In Bacteriophages; Clokie, M.R.J., Kropinski, A.M., Eds.; Methods in Molecular Biology; Humana Press: Totowa, NJ, USA, 2009; Volume 501, pp. 113–126. ISBN 978-1-58829-682-5. [Google Scholar]
- Bergh, Ø.; BØrsheim, K.Y.; Bratbak, G.; Heldal, M. High Abundance of Viruses Found in Aquatic Environments. Nature 1989, 340, 467–468. [Google Scholar] [CrossRef]
- Torrella, F.; Morita, R.Y. Evidence by Electron Micrographs for a High Incidence of Bacteriophage Particles in the Waters of Yaquina Bay, Oregon: Ecological and Taxonomical Implicationst. Appl. Environ. Microbiol. 1979, 37, 5. [Google Scholar] [CrossRef] [Green Version]
- Bratbak, G.; Heldal, M.; Norland, S.; Thingstad, T.F. Viruses as Partners in Spring Bloom Microbial Trophodynamics. Appl. Environ. Microbiol. 1990, 56, 1400–1405. [Google Scholar] [CrossRef] [Green Version]
- Peduzzi, P.; Weinbauer, M.G. Effect of Concentrating the Virus-Rich 2-2nm Size Fraction of Seawater on the Formation of Algal Flocs (Marine Snow). Limnol. Oceanogr. 1993, 38, 1562–1565. [Google Scholar] [CrossRef]
- Williamson, S.J.; Cary, S.C.; Williamson, K.E.; Helton, R.R.; Bench, S.R.; Winget, D.; Wommack, K.E. Lysogenic Virus–Host Interactions Predominate at Deep-Sea Diffuse-Flow Hydrothermal Vents. ISME J. 2008, 2, 1112–1121. [Google Scholar] [CrossRef]
- Kyle, J.E.; Eydal, H.S.C.; Ferris, F.G.; Pedersen, K. Viruses in Granitic Groundwater from 69 to 450 m Depth of the Äspö Hard Rock Laboratory, Sweden. ISME J. 2008, 2, 571–574. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pan, D.; Nolan, J.; Williams, K.H.; Robbins, M.J.; Weber, K.A. Abundance and Distribution of Microbial Cells and Viruses in an Alluvial Aquifer. Front. Microbiol. 2017, 8, 1199. [Google Scholar] [CrossRef] [PubMed]
- Brum, J.R.; Schenck, R.O.; Sullivan, M.B. Global Morphological Analysis of Marine Viruses Shows Minimal Regional Variation and Dominance of Non-Tailed Viruses. ISME J. 2013, 7, 1738–1751. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bellas, C.M.; Anesio, A.M.; Telling, J.; Stibal, M.; Tranter, M.; Davis, S. Viral Impacts on Bacterial Communities in Arctic Cryoconite. Environ. Res. Lett. 2013, 8, 045021. [Google Scholar] [CrossRef]
- Peduzzi, P. Virus Ecology of Fluvial Systems: A Blank Spot on the Map? Biol. Rev. 2016, 91, 937–949. [Google Scholar] [CrossRef]
- Jasna, V.; Pradeep Ram, A.S.; Parvathi, A.; Sime-Ngando, T. Differential Impact of Lytic Viruses on Prokaryotic Morphopopulations in a Tropical Estuarine System (Cochin Estuary, India). PLoS ONE 2018, 13, e0194020. [Google Scholar] [CrossRef] [Green Version]
- Weinbauer, M.; Peduzzi, P. Frequency, Size and Distribution of Bacteriophages in Different Marine Bacterial Morphotypes. Mar. Ecol. Prog. Ser. 1994, 108, 11–20. [Google Scholar] [CrossRef]
- Castillo, Y.M.; Sebastián, M.; Forn, I.; Grimsley, N.; Yau, S.; Moraru, C.; Vaqué, D. Visualization of Viral Infection Dynamics in a Unicellular Eukaryote and Quantification of Viral Production Using Virus Fluorescence in Situ Hybridization. Front. Microbiol. 2020, 11, 1559. [Google Scholar] [CrossRef]
- Brum, J.; Steward, G.; Jiang, S.; Jellison, R. Spatial and Temporal Variability of Prokaryotes, Viruses, and Viral Infections of Prokaryotes in an Alkaline, Hypersaline Lake. Aquat. Microb. Ecol. 2005, 41, 247–260. [Google Scholar] [CrossRef] [Green Version]
- Norrby, E. The morphology of virus particles. Classification of viruses. In Textbook of Medical Virology; Elsevier: Amsterdam, The Netherlands, 1983; pp. 4–16. ISBN 978-0-407-00253-1. [Google Scholar]
- Krupovic, M.; Prangishvili, D.; Hendrix, R.W.; Bamford, D.H. Genomics of Bacterial and Archaeal Viruses: Dynamics within the Prokaryotic Virosphere. Microbiol. Mol. Biol. Rev. 2011, 75, 610–635. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, J.; Zheng, X.; Wang, H.; Jiang, H.; Dong, H.; Huang, L. Novel Sulfolobus Fuselloviruses with Extensive Genomic Variations. J. Virol. 2019, 94, e01624-19. [Google Scholar] [CrossRef] [PubMed]
- Zillig, W.; Kletzin, A.; Schleper, C.; Holz, I.; Janekovic, D.; Hain, J.; Lanzendörfer, M.; Kristjansson, J.K. Screening for Sulfolobales, Their Plasmids and Their Viruses in Icelandic Solfataras. Syst. Appl. Microbiol. 1993, 16, 609–628. [Google Scholar] [CrossRef]
- Rachel, R.; Bettstetter, M.; Hedlund, B.P.; Häring, M.; Kessler, A.; Stetter, K.O.; Prangishvili, D. Remarkable Morphological Diversity of Viruses and Virus-like Particles in Hot Terrestrial Environments. Arch. Virol. 2002, 147, 2419–2429. [Google Scholar] [CrossRef] [PubMed]
- Prangishvili, D.; Arnold, H.P.; Götz, D.; Ziese, U.; Holz, I.; Kristjansson, J.K.; Zillig, W. A Novel Virus Family, the Rudiviridae: Structure, Virus-Host Interactions and Genome Variability of the Sulfolobus Viruses SIRV1 and SIRV2. Genetics 1999, 152, 1387–1396. [Google Scholar] [CrossRef] [PubMed]
- Brumfield, S.K.; Ortmann, A.C.; Ruigrok, V.; Suci, P.; Douglas, T.; Young, M.J. Particle Assembly and Ultrastructural Features Associated with Replication of the Lytic Archaeal Virus Sulfolobus Turreted Icosahedral Virus. J. Virol. 2009, 83, 5964–5970. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mochizuki, T.; Krupovic, M.; Pehau-Arnaudet, G.; Sako, Y.; Forterre, P.; Prangishvili, D. Archaeal Virus with Exceptional Virion Architecture and the Largest Single-Stranded DNA Genome. Proc. Natl. Acad. Sci. USA 2012, 109, 13386–13391. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, G.; Xiao, J.; Wang, H.; Gong, C.; Pan, Y.; Yan, S.; Wang, Y. Efficient Purification and Concentration of Viruses from a Large Body of High Turbidity Seawater. MethodsX 2014, 1, 197–206. [Google Scholar] [CrossRef]
- Dewey, J.S.; Savva, C.G.; White, R.L.; Vitha, S.; Holzenburg, A.; Young, R. Micron-Scale Holes Terminate the Phage Infection Cycle. Proc. Natl. Acad. Sci. USA 2010, 107, 2219–2223. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chang, J.; Liu, X.; Rochat, R.H.; Baker, M.L.; Chiu, W. Reconstructing Virus Structures from Nanometer to Near-Atomic Resolutions with Cryo-Electron Microscopy and Tomography. In Viral Molecular Machines; Rossmann, M.G., Rao, V.B., Eds.; Advances in Experimental Medicine and Biology; Springer US: Boston, MA, USA, 2012; Volume 726, pp. 49–90. ISBN 978-1-4614-0979-3. [Google Scholar]
- Luque, D.; Castón, J.R. Cryo-Electron Microscopy for the Study of Virus Assembly. Nat. Chem. Biol. 2020, 16, 231–239. [Google Scholar] [CrossRef]
- Briggs, J.A. Structural Biology in Situ—the Potential of Subtomogram Averaging. Curr. Opin. Struct. Biol. 2013, 23, 261–267. [Google Scholar] [CrossRef] [PubMed]
- Tu, J.; Park, T.; Morado, D.R.; Hughes, K.T.; Molineux, I.J.; Liu, J. Dual Host Specificity of Phage SP6 Is Facilitated by Tailspike Rotation. Virology 2017, 507, 206–215. [Google Scholar] [CrossRef] [PubMed]
- Chang, J.T.; Schmid, M.F.; Haase-Pettingell, C.; Weigele, P.R.; King, J.A.; Chiu, W. Visualizing the Structural Changes of Bacteriophage Epsilon15 and Its Salmonella Host during Infection. J. Mol. Biol. 2010, 402, 731–740. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Farley, M.M.; Tu, J.; Kearns, D.B.; Molineux, I.J.; Liu, J. Ultrastructural Analysis of Bacteriophage Φ29 during Infection of Bacillus Subtilis. J. Struct. Biol. 2017, 197, 163–171. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hu, B.; Margolin, W.; Molineux, I.J.; Liu, J. The Bacteriophage T7 Virion Undergoes Extensive Structural Remodeling during Infection. Science 2013, 339, 576–579. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hu, B.; Margolin, W.; Molineux, I.J.; Liu, J. Structural Remodeling of Bacteriophage T4 and Host Membranes during Infection Initiation. Proc. Natl. Acad. Sci. USA 2015, 112, E4919–E4928. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, X.; Zhang, Q.; Murata, K.; Baker, M.L.; Sullivan, M.B.; Fu, C.; Dougherty, M.T.; Schmid, M.F.; Osburne, M.S.; Chisholm, S.W.; et al. Structural Changes in a Marine Podovirus Associated with Release of Its Genome into Prochlorococcus. Nat. Struct. Mol. Biol. 2010, 17, 830–836. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stedman, K.M.; DeYoung, M.; Saha, M.; Sherman, M.B.; Morais, M.C. Structural Insights into the Architecture of the Hyperthermophilic Fusellovirus SSV1. Virology 2015, 474, 105–109. [Google Scholar] [CrossRef] [Green Version]
- Quemin, E.R.J.; Chlanda, P.; Sachse, M.; Forterre, P.; Prangishvili, D.; Krupovic, M. Eukaryotic-Like Virus Budding in Archaea. mBio 2016, 7, e01439-16. [Google Scholar] [CrossRef] [Green Version]
- Vestergaard, G.; Aramayo, R.; Basta, T.; Häring, M.; Peng, X.; Brügger, K.; Chen, L.; Rachel, R.; Boisset, N.; Garrett, R.A.; et al. Structure of the Acidianus Filamentous Virus 3 and Comparative Genomics of Related Archaeal Lipothrixviruses. J. Virol. 2008, 82, 371–381. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hong, C.; Pietilä, M.K.; Fu, C.J.; Schmid, M.F.; Bamford, D.H.; Chiu, W. Lemon-Shaped Halo Archaeal Virus His1 with Uniform Tail but Variable Capsid Structure. Proc. Natl. Acad. Sci. USA 2015, 112, 2449–2454. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Daum, B.; Quax, T.E.F.; Sachse, M.; Mills, D.J.; Reimann, J.; Yildiz, Ö.; Häder, S.; Saveanu, C.; Forterre, P.; Albers, S.-V.; et al. Self-Assembly of the General Membrane-Remodeling Protein PVAP into Sevenfold Virus-Associated Pyramids. Proc. Natl. Acad. Sci. USA 2014, 111, 3829–3834. [Google Scholar] [CrossRef] [Green Version]
- Tokuyasu, K.T. A Technique for Ultracryotomy of Cell Suspensions and Tissues. J. Cell Biol. 1973, 57, 551–565. [Google Scholar] [CrossRef]
- Vijayakrishnan, S.; McElwee, M.; Loney, C.; Rixon, F.; Bhella, D. In Situ Structure of Virus Capsids within Cell Nuclei by Correlative Light and Cryo-Electron Tomography. Sci. Rep. 2020, 10, 17596. [Google Scholar] [CrossRef]
- Breitbart, M.; Rohwer, F. Here a Virus, There a Virus, Everywhere the Same Virus? Trends Microbiol. 2005, 13, 278–284. [Google Scholar] [CrossRef]
- Proctor, L.; Fuhrman, J. Mortality of Marine Bacteria in Response to Enrichments of the Virus Size Fraction from Seawater. Mar. Ecol. Prog. Ser. 1992, 87, 283–293. [Google Scholar] [CrossRef]
- Chen, F.; Lu, J.-r.; Binder, B.J.; Liu, Y.-c.; Hodson, R.E. Application of Digital Image Analysis and Flow Cytometry To Enumerate Marine Viruses Stained with SYBR Gold. Appl. Environ. Microbiol. 2001, 67, 539–545. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peduzzi, P.; Agis, M.; Luef, B. Evaluation of Confocal Laser Scanning Microscopy for Enumeration of Virus-like Particles in Aquatic Systems. Environ. Monit. Assess. 2013, 185, 5411–5418. [Google Scholar] [CrossRef] [PubMed]
- Forterre, P.; Soler, N.; Krupovic, M.; Marguet, E.; Ackermann, H.-W. Fake Virus Particles Generated by Fluorescence Microscopy. Trends Microbiol. 2013, 21, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Soler, N.; Krupovic, M.; Marguet, E.; Forterre, P. Membrane Vesicles in Natural Environments: A Major Challenge in Viral Ecology. ISME J. 2015, 9, 793–796. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Holmfeldt, K.; Odić, D.; Sullivan, M.B.; Middelboe, M.; Riemann, L. Cultivated Single-Stranded DNA Phages That Infect Marine Bacteroidetes Prove Difficult to Detect with DNA-Binding Stains. Appl. Environ. Microbiol. 2012, 78, 892–894. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ward, B.W.; Notte, J.A.; Economou, N.P. Helium Ion Microscope: A New Tool for Nanoscale Microscopy and Metrology. J. Vac. Sci. Technol. B Microelectron. Nanometer Struct. 2006, 24, 2871. [Google Scholar] [CrossRef] [Green Version]
- Sharma, A.; Schmidt, M.; Kiesel, B.; Mahato, N.K.; Cralle, L.; Singh, Y.; Richnow, H.H.; Gilbert, J.A.; Arnold, W.; Lal, R. Bacterial and Archaeal Viruses of Himalayan Hot Springs at Manikaran Modulate Host Genomes. Front. Microbiol. 2018, 9, 3095. [Google Scholar] [CrossRef] [PubMed]
- Smith, D.M.; Goodwin, T.W.; Schillinger, J.A. Challenges to the Worldwide Supply of Helium in the Next Decade. In AIP Conference Proceedings; American Institute of Physics: College Park, MD, USA, 2004; Vol. 710, pp. 119–138. [Google Scholar]
- Binnig, G.; Quate, C.F.; Gerber, C. Atomic Force Microscope. Phys. Rev. Lett. 1986, 56, 930–933. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hansma, P.; Drake, B.; Marti, O.; Gould, S.; Prater, C. The Scanning Ion-Conductance Microscope. Science 1989, 243, 641–643. [Google Scholar] [CrossRef] [PubMed]
- Bard, A.J.; Fan, F.-R.F.; Pierce, D.T.; Unwin, P.R.; Wipf, D.O.; Zhou, F. Chemical Imaging of Surfaces with the Scanning Electrochemical Microscope. Science 1991, 254, 68–74. [Google Scholar] [CrossRef]
- Nonnenmacher, M.; O’Boyle, M.P.; Wickramasinghe, H.K. Kelvin Probe Force Microscopy. Appl. Phys. Lett. 1991, 58, 2921–2923. [Google Scholar] [CrossRef] [Green Version]
- Shekhawat, G.S. Nanoscale Imaging of Buried Structures via Scanning Near-Field Ultrasound Holography. Science 2005, 310, 89–92. [Google Scholar] [CrossRef] [PubMed]
- Dufrene, Y.F. Atomic Force Microscopy, a Powerful Tool in Microbiology. J. Bacteriol. 2002, 184, 5205–5213. [Google Scholar] [CrossRef] [Green Version]
- Kuznetsov, Y.G.; McPherson, A. Atomic Force Microscopy Investigation of Turnip Yellow Mosaic Virus Capsid Disruption and RNA Extrusion. Virology 2006, 352, 329–337. [Google Scholar] [CrossRef] [Green Version]
- Kuznetsov, Y.G.; Dowell, J.J.; Gavira, J.A.; Ng, J.D.; McPherson, A. Biophysical and Atomic Force Microscopy Characterization of the RNA from Satellite Tobacco Mosaic Virus. Nucleic Acids Res. 2010, 38, 8284–8294. [Google Scholar] [CrossRef]
- Kuznetsov, Y.G.; Gurnon, J.R.; Van Etten, J.L.; McPherson, A. Atomic Force Microscopy Investigation of a Chlorella Virus, PBCV-1. J. Struct. Biol. 2005, 149, 256–263. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xiao, C.; Kuznetsov, Y.G.; Sun, S.; Hafenstein, S.L.; Kostyuchenko, V.A.; Chipman, P.R.; Suzan-Monti, M.; Raoult, D.; McPherson, A.; Rossmann, M.G. Structural Studies of the Giant Mimivirus. PLoS Biol. 2009, 7, e1000092. [Google Scholar] [CrossRef]
- Kuznetsov, Y.G.; Xiao, C.; Sun, S.; Raoult, D.; Rossmann, M.; McPherson, A. Atomic Force Microscopy Investigation of the Giant Mimivirus. Virology 2010, 404, 127–137. [Google Scholar] [CrossRef] [Green Version]
- Matsko, N.; Klinov, D.; Manykin, A.; Demin, V.; Klimenko, S. Atomic Force Microscopy Analysis of Bacteriophages ΦKZ and T4. Microscopy 2001, 50, 417–422. [Google Scholar] [CrossRef]
- Kolbe, W.F.; Ogletree, D.F.; Salmeron, M.B. Atomic Force Microscopy Imaging of T4 Bacteriophages on Silicon Substrates. Ultramicroscopy 1992, 42–44, 1113–1117. [Google Scholar] [CrossRef] [Green Version]
- Kuznetsov, Y.G.; Daijogo, S.; Zhou, J.; Semler, B.L.; McPherson, A. Atomic Force Microscopy Analysis of Icosahedral Virus RNA. J. Mol. Biol. 2005, 347, 41–52. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kuznetsov, Y.G.; Malkin, A.J.; Lucas, R.W.; Plomp, M.; McPherson, A. Imaging of Viruses by Atomic Force Microscopy. J. Gen. Virol. 2001, 82, 2025–2034. [Google Scholar] [CrossRef] [PubMed]
- Chang, K.-C.; Chiang, Y.-W.; Yang, C.-H.; Liou, J.-W. Atomic Force Microscopy in Biology and Biomedicine. Tzu Chi Med. J. 2012, 24, 162–169. [Google Scholar] [CrossRef] [Green Version]
- Amann, R.; Springer, N.; Ludwig, W.; Görtz, H.D.; Schleifer, K.H. Identification in Situ and Phylogeny of Uncultured Bacterial Endosymbionts. Nature 1991, 351, 161–164. [Google Scholar] [CrossRef] [PubMed]
- Tyson, G.W.; Banfield, J.F. Cultivating the Uncultivated: A Community Genomics Perspective. Trends Microbiol. 2005, 13, 411–415. [Google Scholar] [CrossRef]
- Alneberg, J.; Bennke, C.; Beier, S.; Bunse, C.; Quince, C.; Ininbergs, K.; Riemann, L.; Ekman, M.; Jürgens, K.; Labrenz, M.; et al. Ecosystem-Wide Metagenomic Binning Enables Prediction of Ecological Niches from Genomes. Commun. Biol. 2020, 3, 1–10. [Google Scholar] [CrossRef]
- Wegley, L.; Edwards, R.; Rodriguez-Brito, B.; Liu, H.; Rohwer, F. Metagenomic Analysis of the Microbial Community Associated with the Coral Porites Astreoides. Environ. Microbiol. 2007, 9, 2707–2719. [Google Scholar] [CrossRef]
- Zablocki, O.; van Zyl, L.; Kirby, B.; Trindade, M. Diversity of DsDNA Viruses in a South African Hot Spring Assessed by Metagenomics and Microscopy. Viruses 2017, 9, 348. [Google Scholar] [CrossRef] [Green Version]
- Malki, K.; Kula, A.; Bruder, K.; Sible, E.; Hatzopoulos, T.; Steidel, S.; Watkins, S.C.; Putonti, C. Bacteriophages Isolated from Lake Michigan Demonstrate Broad Host-Range across Several Bacterial Phyla. Virol. J. 2015, 12, 164. [Google Scholar] [CrossRef] [Green Version]
- Daly, R.A.; Roux, S.; Borton, M.A.; Morgan, D.M.; Johnston, M.D.; Booker, A.E.; Hoyt, D.W.; Meulia, T.; Wolfe, R.A.; Hanson, A.J.; et al. Viruses Control Dominant Bacteria Colonizing the Terrestrial Deep Biosphere after Hydraulic Fracturing. Nat. Microbiol. 2019, 4, 352–361. [Google Scholar] [CrossRef] [PubMed]
- Danovaro, R.; Dell’Anno, A.; Corinaldesi, C.; Rastelli, E.; Cavicchioli, R.; Krupovic, M.; Noble, R.T.; Nunoura, T.; Prangishvili, D. Virus-Mediated Archaeal Hecatomb in the Deep Seafloor. Sci. Adv. 2016, 2, e1600492. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Teira, E.; Reinthaler, T.; Pernthaler, A.; Pernthaler, J.; Herndl, G.J. Combining Catalyzed Reporter Deposition-Fluorescence In Situ Hybridization and Microautoradiography To Detect Substrate Utilization by Bacteria and Archaea in the Deep Ocean. Appl. Environ. Microbiol. 2004, 70, 4411–4414. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Amann, R.; Fuchs, B.M.; Behrens, S. The Identification of Microorganisms by Fluorescence in Situ Hybridisation. Curr. Opin. Biotechnol. 2001, 12, 231–236. [Google Scholar] [CrossRef]
- Kenzaka, T.; Tani, K.; Nasu, M. High-Frequency Phage-Mediated Gene Transfer in Freshwater Environments Determined at Single-Cell Level. ISME J. 2010, 4, 648–659. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Knierim, B.; Luef, B.; Wilmes, P.; Webb, R.I.; Auer, M.; Comolli, L.R.; Banfield, J.F. Correlative Microscopy for Phylogenetic and Ultrastructural Characterization of Microbial Communities: Correlative TEM and CARD-FISH Imaging. Environ. Microbiol. Rep. 2012, 4, 36–41. [Google Scholar] [CrossRef] [PubMed]
- Amann, R.; Fuchs, B.M. Single-Cell Identification in Microbial Communities by Improved Fluorescence in Situ Hybridization Techniques. Nat. Rev. Microbiol. 2008, 6, 339–348. [Google Scholar] [CrossRef] [PubMed]
- Kenzaka, T.; Tamaki, S.; Yamaguchi, N.; Tani, K.; Nasu, M. Recognition of Individual Genes in Diverse Microorganisms by Cycling Primed In Situ Amplification. Appl. Environ. Microbiol. 2005, 71, 7236–7244. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moraru, C.; Lam, P.; Fuchs, B.M.; Kuypers, M.M.M.; Amann, R. GeneFISH—An in Situ Technique for Linking Gene Presence and Cell Identity in Environmental Microorganisms: GeneFISH in Environmental Microorganisms. Environ. Microbiol. 2010, 12, 3057–3073. [Google Scholar] [CrossRef] [PubMed]
- Vincent, F.; Sheyn, U.; Porat, Z.; Vardi, A. Visualizing Active Viral Infection Reveals Diverse Cell Fates in Synchronized Algal Bloom Demise. Proc. Natl. Acad. Sci. USA 2021, 118, e2021586118. [Google Scholar] [CrossRef] [PubMed]
- Wagner, C.; Reddy, V.; Asturias, F.; Khoshouei, M.; Johnson, J.E.; Manrique, P.; Munson-McGee, J.; Baumeister, W.; Lawrence, C.M.; Young, M.J. Isolation and Characterization of Metallosphaera Turreted Icosahedral Virus, a Founding Member of a New Family of Archaeal Viruses. J. Virol. 2017, 91, e00925-17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roux, S.; Enault, F.; Hurwitz, B.L.; Sullivan, M.B. VirSorter: Mining Viral Signal from Microbial Genomic Data. PeerJ 2015, 3, e985. [Google Scholar] [CrossRef] [PubMed]
- Ren, J.; Ahlgren, N.A.; Lu, Y.Y.; Fuhrman, J.A.; Sun, F. VirFinder: A Novel k-Mer Based Tool for Identifying Viral Sequences from Assembled Metagenomic Data. Microbiome 2017, 5, 69. [Google Scholar] [CrossRef] [PubMed]
- Kieft, K.; Zhou, Z.; Anantharaman, K. VIBRANT: Automated Recovery, Annotation and Curation of Microbial Viruses, and Evaluation of Virome Function from Genomic Sequences. bioRxiv 2019, 855387. [Google Scholar] [CrossRef] [Green Version]
- Hills, G.J.; Plaskitt, K.A.; Young, N.D.; Dunigan, D.D.; Watts, J.W.; Wilson, T.M.A.; Zaitlin, M. Immunogold Localization of the Intracellular Sites of Structural and Nonstructural Tobacco Mosaic Virus Proteins. Virology 1987, 161, 488–496. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Turzynski, V.; Monsees, I.; Moraru, C.; Probst, A.J. Imaging Techniques for Detecting Prokaryotic Viruses in Environmental Samples. Viruses 2021, 13, 2126. https://doi.org/10.3390/v13112126
Turzynski V, Monsees I, Moraru C, Probst AJ. Imaging Techniques for Detecting Prokaryotic Viruses in Environmental Samples. Viruses. 2021; 13(11):2126. https://doi.org/10.3390/v13112126
Chicago/Turabian StyleTurzynski, Victoria, Indra Monsees, Cristina Moraru, and Alexander J. Probst. 2021. "Imaging Techniques for Detecting Prokaryotic Viruses in Environmental Samples" Viruses 13, no. 11: 2126. https://doi.org/10.3390/v13112126






