Rapid Resolution of Non-Effusive Feline Infectious Peritonitis Uveitis with an Oral Adenosine Nucleoside Analogue and Feline Interferon Omega
Abstract
:1. Introduction
2. Case Report
3. Discussion
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Addie, D.D.; Toth, S.; Murray, G.D.; Jarrett, O. The risk of feline infectious peritonitis in cats naturally infected with feline coronavirus. Am. J. Vet. Res. 1995, 56, 429–434. [Google Scholar] [PubMed]
- Kipar, A.; May, H.; Menger, S.; Weber, M.; Leukert, W.; Reinacher, M. Morphologic features and development of granulomatous vasculitis in feline infectious peritonitis. Vet. Pathol. 2005, 42, 321–330. [Google Scholar] [CrossRef] [PubMed]
- De Groot, R.J.; Baker, S.C.; Baric, R.; Enjuanes, L.; Gorbalenya, A.E.; Holmes, K.V.; Perlman, S.; Poon, L.; Rottier, P.J.M.; Talbot, P.J.; et al. Family Coronaviridae. In Ninth Report International Committee on Taxonomy of Viruses; King AMQ; Adams, M.J., Carstens, E.B., Lefkowitz, E.J., Eds.; Elsevier: Amsterdam, The Netherlands, 2012; pp. 806–828. [Google Scholar]
- Pedersen, N.C.; Perron, M.; Bannasch, M.; Montgomery, E.; Murakami, E.; Liepnieks, M.; Liu, H. Efficacy and safety of the nucleoside analog GS-441524 for treatment of cats with naturally occurring feline infectious peritonitis. J. Feline Med. Surg. 2019, 21, 271–281. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Addie, D.D.; Curran, S.; Bellini, F.; Crowe, B.; Sheehan, E.; Ukrainchuk, L.; Decaro, N. Oral Mutian® X stopped faecal feline coronavirus shedding by naturally infected cats. Res. Vet. Sci. 2020, 130, 222–229. [Google Scholar] [CrossRef] [PubMed]
- Chang, H.W.; Egberink, H.F.; Halpin, R.; Spiro, D.J.; Rottier, P.J. Spike protein fusion peptide and feline coronavirus virulence. Emerg. Infect. Dis. 2012, 18, 1089–1095. [Google Scholar] [CrossRef] [PubMed]
- Duthie, S.; Eckersall, P.D.; Addie, D.D.; Lawrence, C.E.; Jarrett, O. Value of alpha1-acid glycoprotein in the diagnosis of feline infectious peritonitis. Vet. Rec. 1997, 141, 299–303. [Google Scholar] [CrossRef] [PubMed]
- Shelly, S.M.; Scarlett-Kranz, J.; Blue, J.T. Protein electrophoresis on effusions from cats as a diagnostic test for feline infectious peritonitis. J. Am. Anim. Hosp. Assoc. 1988, 24, 495–500. [Google Scholar]
- Pedersen, N.C.; Kim, Y.; Liu, H.; Galasiti, K.A.C.; Eckstrand, C.; Groutas, W.C.; Bannasch, M.; Meadows, J.M.; Chang, K.O. Efficacy of a 3C-like protease inhibitor in treating various forms of acquired feline infectious peritonitis. J. Feline Med. Surg. 2018, 20, 378–392. [Google Scholar] [CrossRef]
- Dunbar, D.; Kwok, W.; Graham, E.; Armitage, A.; Irvine, R.; Johnston, P.; McDonald, M.; Montgomery, D.; Nicolson, L.; Robertson, E.; et al. Diagnosis of non-effusive feline infectious peritonitis by reverse transcriptase quantitative polymerase chain reaction from mesenteric lymph node fine needle aspirates. J. Feline Med. Surg. 2019, 21, 910–921. [Google Scholar] [CrossRef] [Green Version]
- Felten, S.; Leutenegger, C.M.; Balzer, H.J.; Pantchev, N.; Matiasek, K.; Wess, G.; Egberink, H.; Hartmann, K. Sensitivity and specificity of a real-time reverse transcriptase polymerase chain reaction detecting feline coronavirus mutations in effusion and serum/plasma of cats to diagnose feline infectious peritonitis. BMC Vet. Res. 2017, 13, 228. [Google Scholar] [CrossRef]
- Fish, E.J.; Diniz, P.P.V.; Juan, Y.C.; Bossong, F.; Collisson, E.W.; Drechsler, Y.; Kaltenboeck, B. Cross-sectional quantitative RT-PCR study of feline coronavirus viremia and replication in peripheral blood of healthy shelter cats in Southern California. J. Feline Med. Surg. 2018, 20, 295–301. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Simons, F.A.; Vennema, H.; Rofina, J.E.; Pol, J.M.; Horzinek, M.C.; Rottier, P.J.; Egberink, H.F. A mRNA PCR for the diagnosis of feline infectious peritonitis. J. Virol. Methods 2005, 124, 111–116. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Herrewegh, A.A.P.M.; de Groot, R.J.; Cepica, A.; Egberink, H.F.; Horzinek, M.C.; Rottier, P.J.M. Detection of feline coronavirus RNA in feces, tissue, and body fluids of naturally infected cats by reverse transcriptase PCR. J. Clin. Microbiol. 1995, 33, 684–689. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tasker, S. Diagnosis of feline infectious peritonitis: Update on evidence supporting available tests. J. Feline Med. Surg. 2018, 20, 228–243. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Porter, E.; Tasker, S.; Day, M.J.; Harley, R.; Kipar, A.; Siddell, S.G.; Helps, C.R. Amino acid changes in the spike protein of feline coronavirus correlate with systemic spread of virus from the intestine and not with feline infectious peritonitis. Vet. Res. 2014, 45, 49. [Google Scholar] [CrossRef] [Green Version]
- Giori, L.; Giordano, A.; Giudice, C.; Grieco, V.; Paltrinieri, S. Performances of different diagnostic tests for feline infectious peritonitis in challenging clinical cases. J. Small Anim. Pract. 2011, 52, 152–157. [Google Scholar] [CrossRef]
- Tsai, H.Y.; Chueh, L.L.; Lin, C.N.; Su, B.L. Clinicopathological findings and disease staging of feline infectious peritonitis: 51 cases from 2003 to 2009 in Taiwan. J. Feline Med. Surg. 2011, 13, 74–80. [Google Scholar] [CrossRef]
- Rohrer, C.; Suter, P.F.; Lutz, H. The diagnosis of feline infectious peritonitis (FIP): A retrospective and prospective study. Kleinterpraxis 1993, 38, 379–389. [Google Scholar]
- Riemer, F.; Kuehner, K.A.; Ritz, S.; Sauter-Louis, C.; Hartmann, K. Clinical and laboratory features of cats with feline infectious peritonitis–A retrospective study of 231 confirmed cases (2000–2010). J. Feline Med. Surg. 2016, 18, 348–356. [Google Scholar] [CrossRef] [Green Version]
- Addie, D.D.; Jarrett, J.O. Use of a reverse-transcriptase polymerase chain reaction for monitoring feline coronavirus shedding by healthy cats. Vet. Rec. 2001, 148, 649–653. [Google Scholar] [CrossRef]
- Jinks, M.R.; English, R.V.; Gilger, B.C. Causes of endogenous uveitis in cats presented to referral clinics in North Carolina. Vet. Ophthalmol. 2016, 19 (Suppl. S1), 30–37. [Google Scholar] [CrossRef] [PubMed]
- Legendre, A.M.; Kuritz, T.; Galyon, G.; Baylor, V.M.; Heidel, R.E. Polyprenyl Immunostimulant Treatment of Cats with Presumptive Non-Effusive Feline Infectious Peritonitis in a Field Study. Front. Vet. Sci. 2017, 4, 7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fischer, Y.; Ritz, S.; Weber, K.; Sauter-Louis, C.; Hartmann, K. Randomized, placebo controlled study of the effect of propentofylline on survival time and quality of life of cats with feline infectious peritonitis. J. Vet. Intern. Med. 2011, 25, 1270–1276. [Google Scholar] [CrossRef] [PubMed]
- Ritz, S.; Egberink, H.; Hartmann, K. Effect of feline interferon-omega on the survival time and quality of life of cats with feline infectious peritonitis. J. Vet. Intern. Med. 2007, 21, 1193–1197. [Google Scholar] [CrossRef]
- Jepson, R.E.; Syme, H.M.; Vallance, C.; Elliott, J. Plasma asymmetric dimethylarginine, symmetric dimethylarginine, l-arginine, and nitrite/nitrate concentrations in cats with chronic kidney disease and hypertension. J. Vet. Intern. Med. 2008, 22, 317–324. [Google Scholar] [CrossRef] [PubMed]
- Relford, R.; Robertson, J.; Clements, C. Symmetric Dimethylarginine: Improving the Diagnosis and Staging of Chronic Kidney Disease in Small Animals. Vet. Clin. N. Am. Small Anim. Pract. 2016, 46, 941–960. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Paltrinieri, S.; Giraldi, M.; Prolo, A.; Scarpa, P.; Piseddu, E.; Beccati, M.; Graziani, B.; Bo, S. Serum symmetric dimethylarginine and creatinine in Birman cats compared with cats of other breeds. J. Feline Med. Surg. 2018, 20, 905–912. [Google Scholar] [CrossRef]
- Gil, S.; Leal, R.O.; McGahie, D.; Sepúlveda, N.; Duarte, A.; Niza, M.M.; Tavares, L. Oral Recombinant Feline Interferon-Omega as an alternative immune modulation therapy in FIV positive cats, clinical and laboratory evaluation. Res. Vet. Sci. 2014, 96, 79–85. [Google Scholar] [CrossRef]
- Addie, D. Feline coronavirus infections. In Infectious Diseases of the Dog and Cat; Green, C., Ed.; Elsevier: Maryland Heights, MO, USA, 2012; pp. 92–108. [Google Scholar]
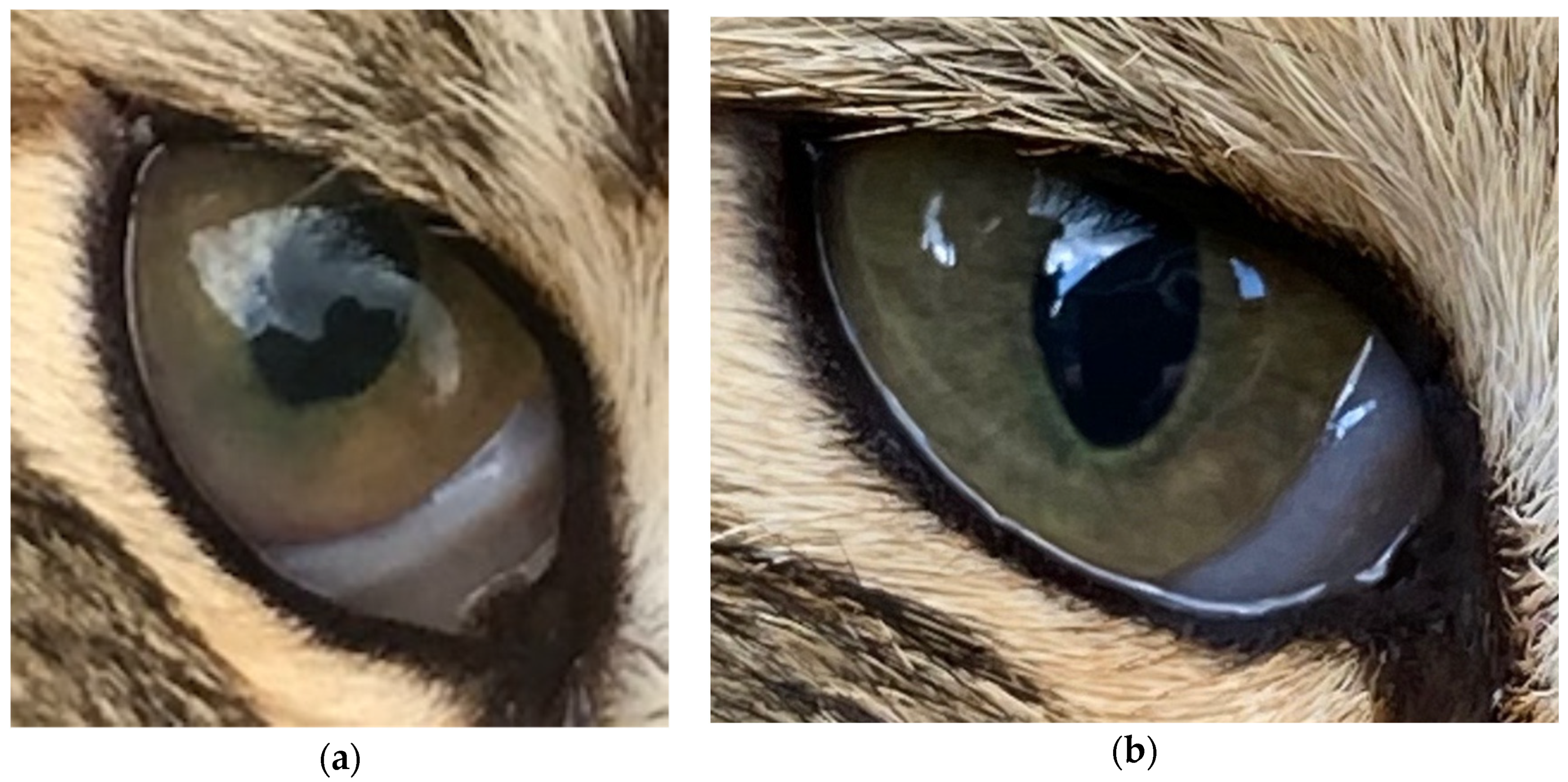
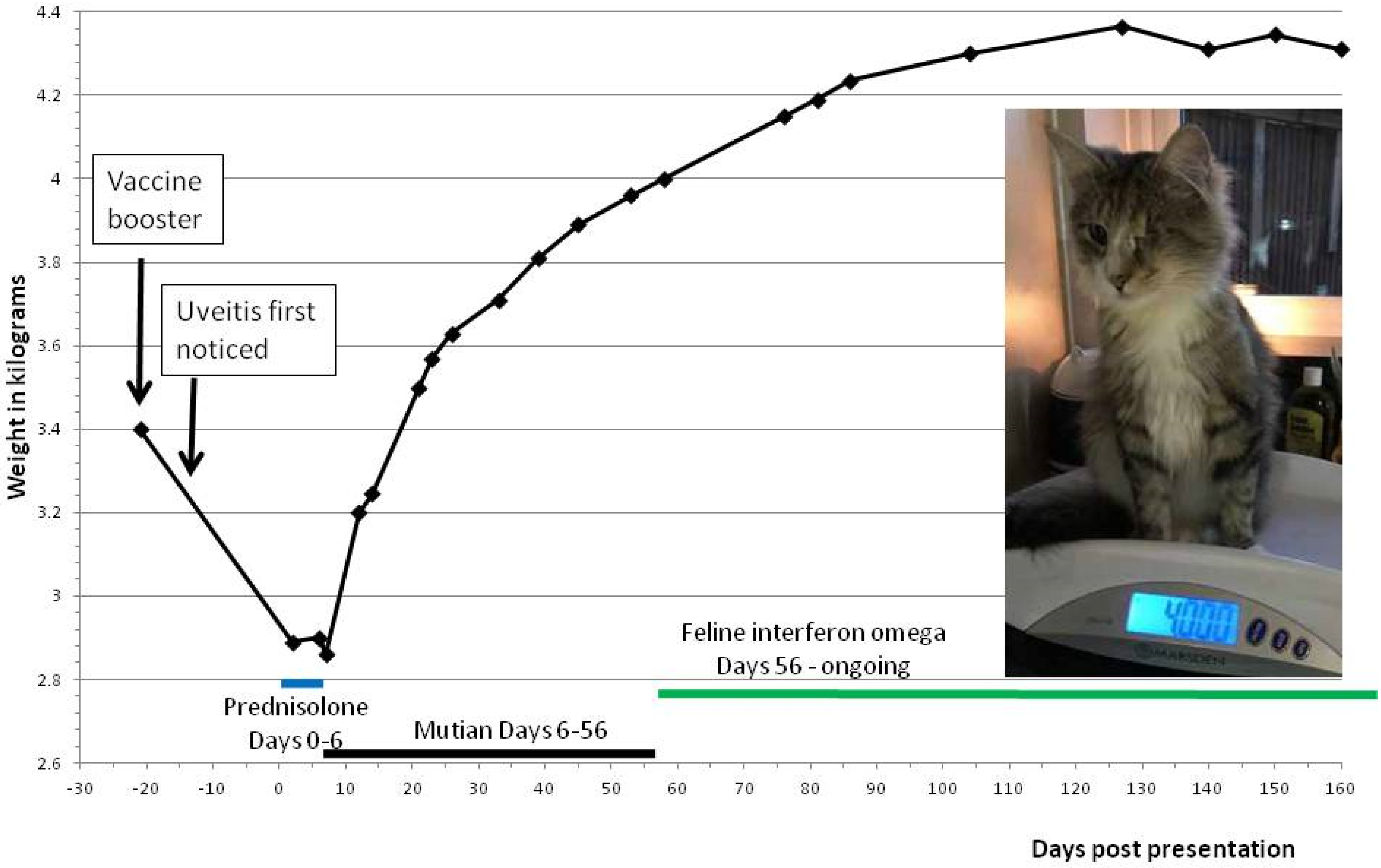
| Day −29 | Day −19 | Day 7 | Day 41 | ||
|---|---|---|---|---|---|
| Skywise | 2yo Norwegian forest cat (NFC) FIP | >10,240 | CT 30 | Neg | |
| Paddy | 2yo NFC persistent pyrexia, lethargy | >10,240 | CT 18 | Neg | |
| Oliver | 8 yo Domestic shorthair cat | >10,240 | CT 20 | Neg | |
| Link | 1 yo NFC | >10,240 | CT 20 | Neg | |
| Zelda | 1 yo NFC | 640 | Neg | Neg |
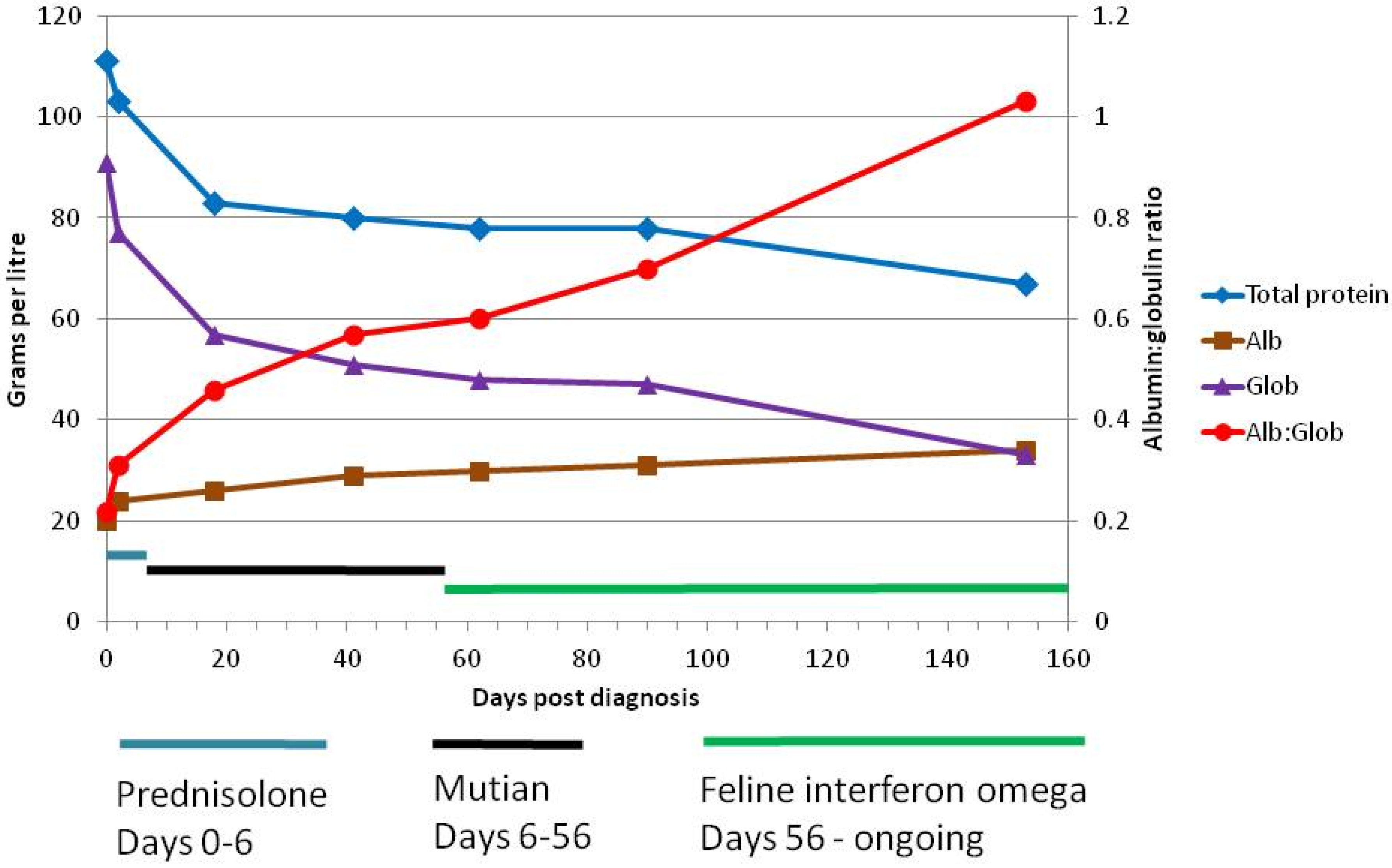
| AGP | TP | Alb | Glob | A:G | Bilirubin | Hct | Lymph. Count | ALT | AP | GGT | SDMA | Creat | Urea | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ref. Range | <500 μg/mL | 57–89 g/L | 22–40 g/L | 28–51 g/L | >0.8 * | 0–15 μmol/L | 30–52% | 0.92–6.88 × 109 | 12–130 U/L | 14–111 U/L | 0–4 U/L | 0–14 μg/dL | 71–212 μmol/L | 5.7–12.9 mmol/L |
| Day 0 | ND | 111 | 20 | 91 | 0.22 | 11 | 25.7 | 0.95 | 64 | ND | ND | ND | ND | ND |
| Day 2 | 3100 | 103 | 24 | 77 | 0.31 | ND | 22.0 | 2.07 | ND | ND | ND | ND | ND | ND |
| Day 18 | 700 | 83 | 26 | 57 | 0.46 | 6 | 34.4 | 3.61 | 27 | 32 | 0 | ND | 88 | 9.6 |
| Day 41 | 400 | 80 | 29 | 51 | 0.57 | 5 | 35.1 | 3.85 | 67 | 40 | 0 | 28 | 93 | 8.3 |
| Day 62 | 400 | 78 | 30 | 48 | 0.60 | 5 | 35.2 | 2.64 | 61 | 40 | 0 | 19 | 106 | 6.5 |
| Day 90 | 300 | 78 | 31 | 47 | 0.70 | 6 | 40.0 | 4.15 | 81 | 40 | 0 | 14 | 113 | 10.0 |
| Day 153 | 400 | 67 | 34 | 33 | 1.03 | 1 | 35.2 | 4.49 | 81 | 46 | 2 | ND ** | 98 | 7.8 |
| Treatment | Day | Ophthalmoscopic Findings | ||
|---|---|---|---|---|
| Systemic prednisolone | 0 | Day of presentation to primary veterinary surgeon. | ||
| 1 | Eye examined using a standard ophthalmoscope. Cornea unremarkable, anterior chamber slightly cloudy, but sedation would have been required for detailed examination. Cat not visual and was distressed. | |||
| 2 | Blood samples and mesenteric lymph node fine-needle aspirates taken under sedation. | |||
| Topical prednisolone acetate 1% tid | 4 | Keratic (protein) precipitates on lens present, partially obscuring fundus. Pupillary light reflex present but reduced. | ||
| Mutian at 8 mg/k for 19 days, then 6 mg/kg | 6 | Cat’s guardian reported that she thought the cat could see. | ||
| 17 | Cat’s guardian reported that the cat chased a bird. | |||
| 18 | Uveitis resolving, anterior chamber clear. Keratic precipitates as before. | |||
| 41 | Uveitis appeared largely resolved, keratic precipitates as before. Fundus seen this time, possible bulla seen, resolving chorioretinitis, but cat’s temperament precluded detailed examination. Excellent visual acuity. | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Addie, D.D.; Covell-Ritchie, J.; Jarrett, O.; Fosbery, M. Rapid Resolution of Non-Effusive Feline Infectious Peritonitis Uveitis with an Oral Adenosine Nucleoside Analogue and Feline Interferon Omega. Viruses 2020, 12, 1216. https://doi.org/10.3390/v12111216
Addie DD, Covell-Ritchie J, Jarrett O, Fosbery M. Rapid Resolution of Non-Effusive Feline Infectious Peritonitis Uveitis with an Oral Adenosine Nucleoside Analogue and Feline Interferon Omega. Viruses. 2020; 12(11):1216. https://doi.org/10.3390/v12111216
Chicago/Turabian StyleAddie, Diane D., Johanna Covell-Ritchie, Oswald Jarrett, and Mark Fosbery. 2020. "Rapid Resolution of Non-Effusive Feline Infectious Peritonitis Uveitis with an Oral Adenosine Nucleoside Analogue and Feline Interferon Omega" Viruses 12, no. 11: 1216. https://doi.org/10.3390/v12111216
APA StyleAddie, D. D., Covell-Ritchie, J., Jarrett, O., & Fosbery, M. (2020). Rapid Resolution of Non-Effusive Feline Infectious Peritonitis Uveitis with an Oral Adenosine Nucleoside Analogue and Feline Interferon Omega. Viruses, 12(11), 1216. https://doi.org/10.3390/v12111216





