Genetic Variability and Evolution of Hepatitis E Virus
Abstract
:1. Introduction
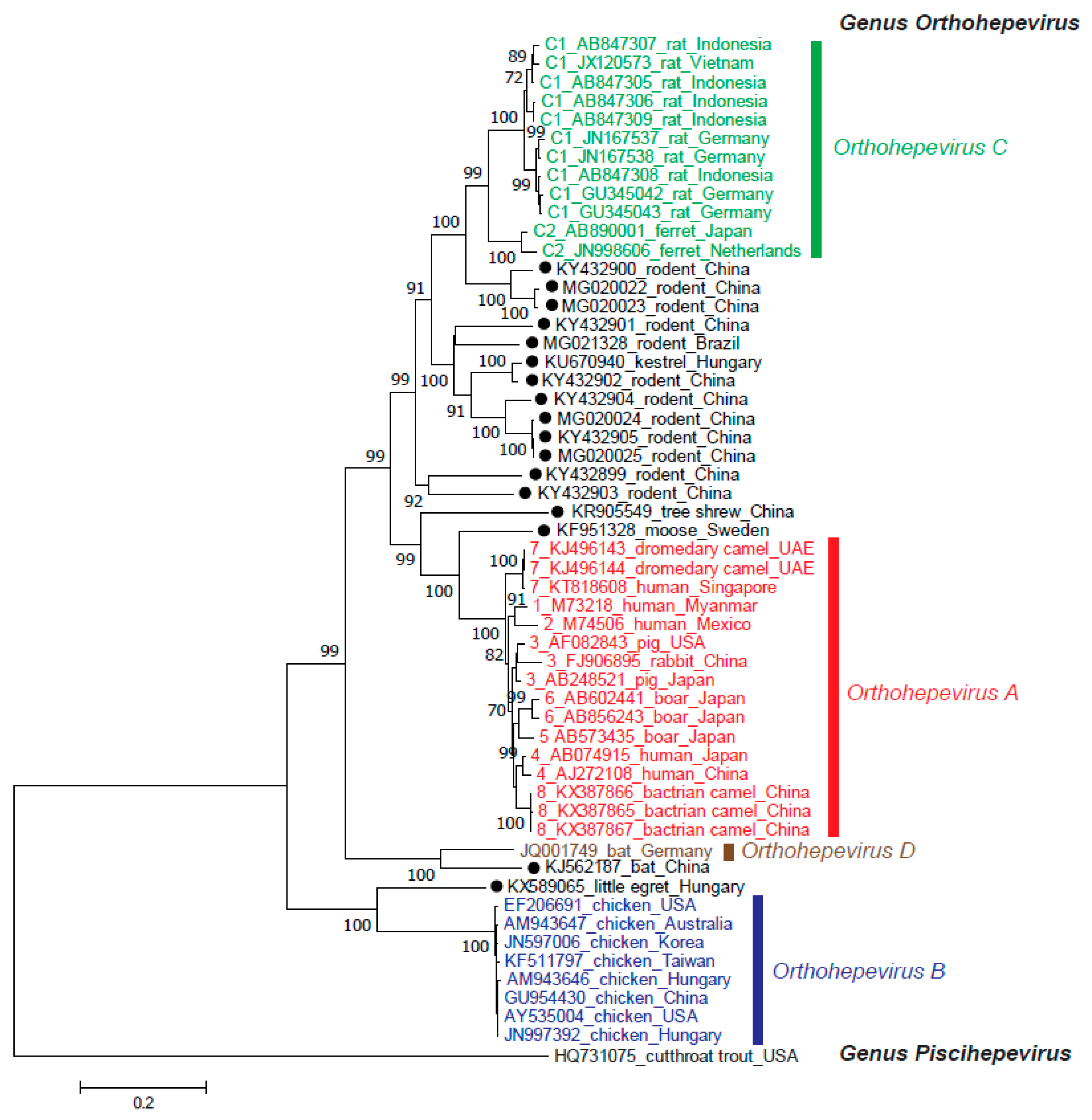
2. Taxonomy
2.1. Orthohepevirus A
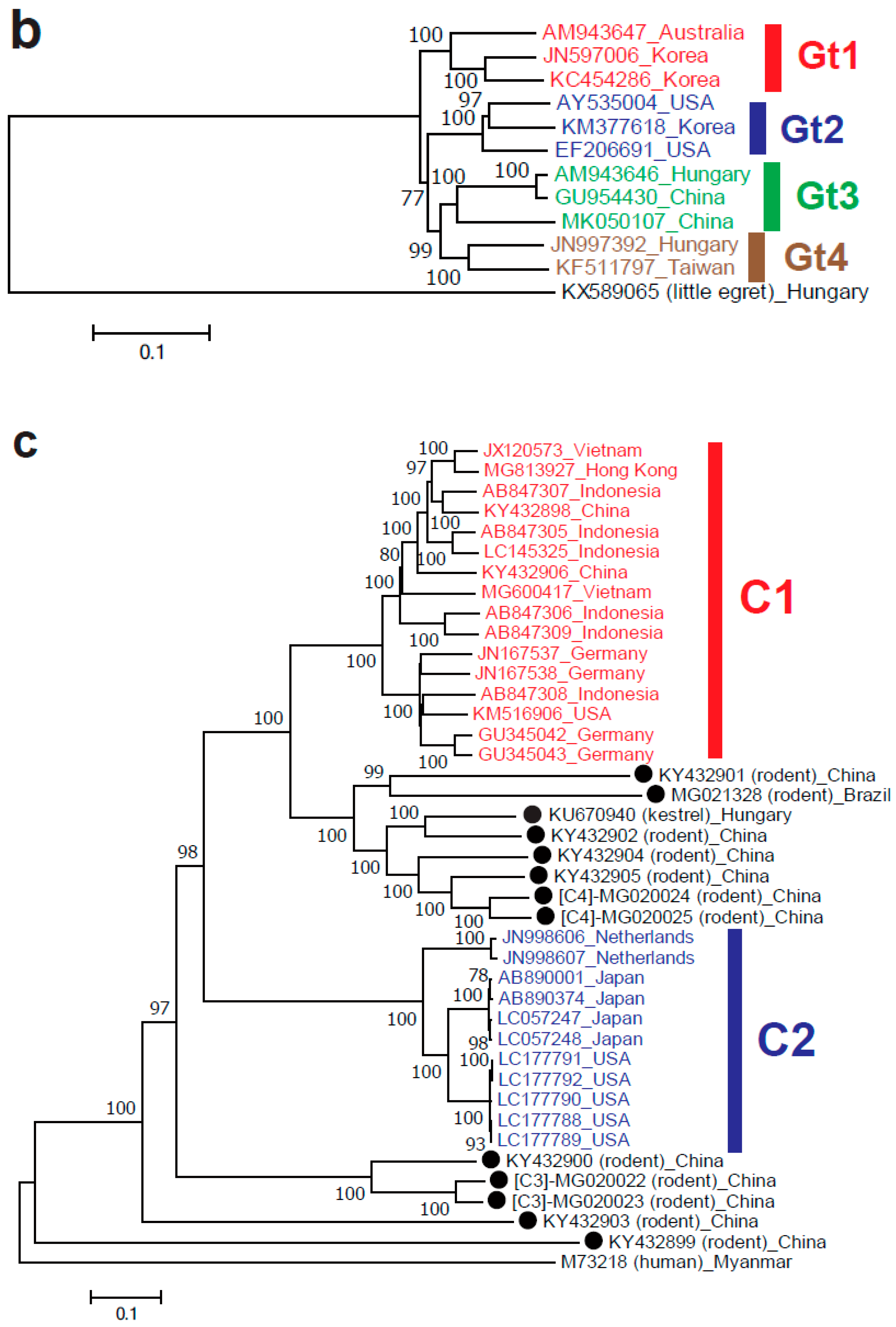
2.2. Orthohepevirus B
2.3. Orthohepevirus C
2.4. Orthohepevirus D
2.5. Other Unassigned Related Hepeviruses
3. HEV Genome
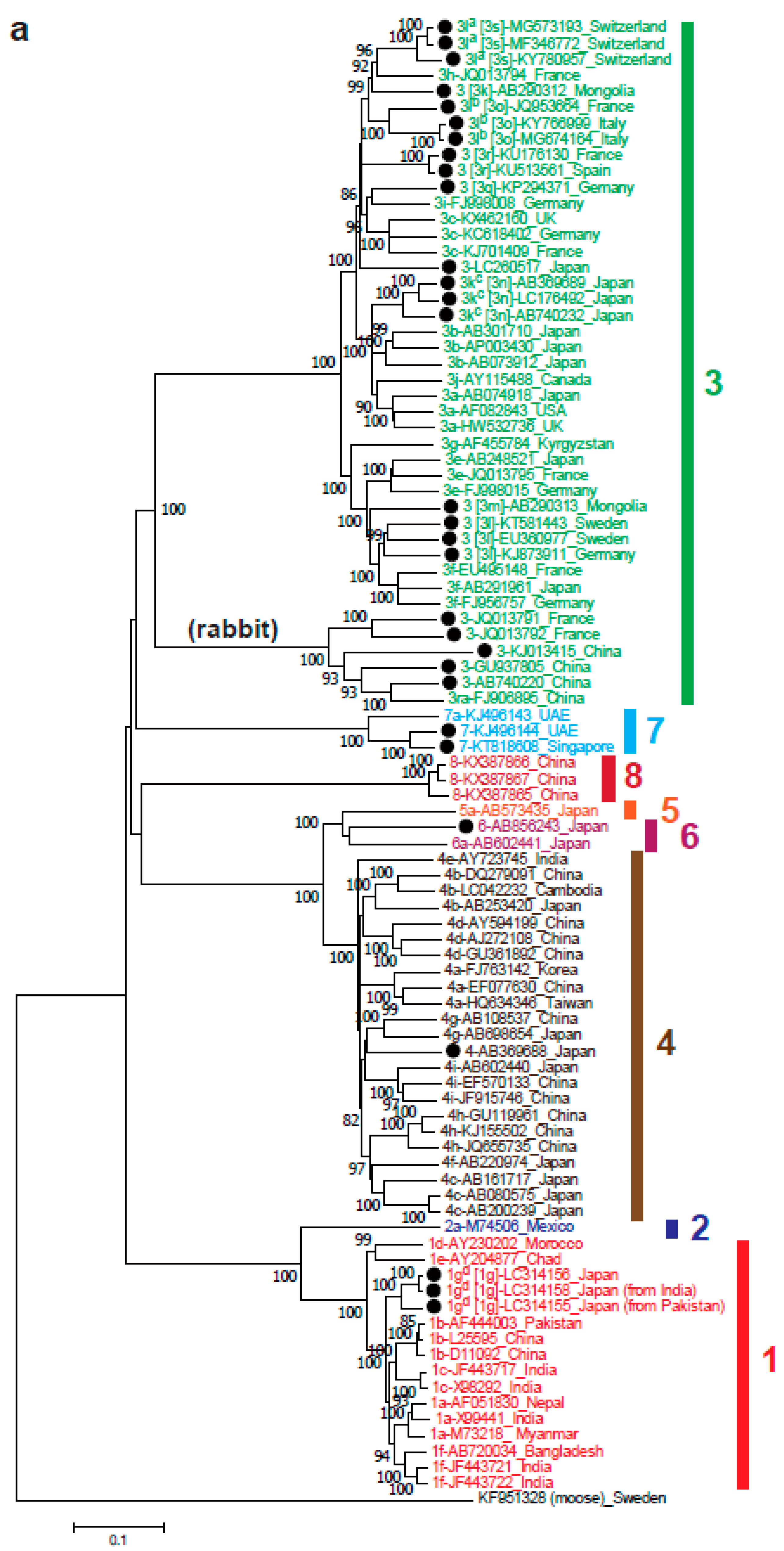
4. Distribution and Clinical Significance of HEV Genotype within Orthohepevirus A
5. Genomic Variability and Evolution
5.1. Nucleotide Mutations during Consecutive Passages in Cell Culture (Clinical Sample-Derived versus cDNA Clone-Derived)
5.2. Possible Clinical Implication of HEV Genomic Mutations
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Lhomme, S.; Marion, O.; Abravanel, F.; Chapuy-Regaud, S.; Kamar, N.; Izopet, J. Hepatitis E pathogenesis. Viruses 2016, 8, 212. [Google Scholar] [CrossRef]
- Kamar, N.; Izopet, J.; Pavio, N.; Aggarwal, R.; Labrique, A.; Wedemeyer, H.; Dalton, H.R. Hepatitis E virus infection. Nat. Rev. Dis. Primers 2017, 3, 17086. [Google Scholar] [CrossRef] [PubMed]
- Nimgaonkar, I.; Ding, Q.; Schwartz, R.E.; Ploss, A. Hepatitis E virus: Advances and challenges. Nat. Rev. Gastroenterol. Hepatol. 2018, 15, 96–110. [Google Scholar] [CrossRef] [PubMed]
- Hoofnagle, J.H.; Nelson, K.E.; Purcell, R.H. Hepatitis E. N. Engl. J. Med. 2012, 367, 1237–1244. [Google Scholar] [CrossRef]
- Kamar, N.; Marion, O.; Abravanel, F.; Izopet, J.; Dalton, H.R. Extrahepatic manifestations of hepatitis E virus. Liver Int. 2016, 36, 467–472. [Google Scholar] [CrossRef]
- World Health Organization. Hepatitis E. Available online: https://www.who.int/news-room/fact-sheets/detail/hepatitis-E. (accessed on 23 April 2019).
- Boccia, D.; Guthmann, J.P.; Klovstad, H.; Hamid, N.; Tatay, M.; Ciglenecki, I.; Nizou, J.Y.; Nicand, E.; Guerin, P.J. High mortality associated with an outbreak of hepatitis E among displaced persons in Darfur, Sudan. Clin. Infect. Dis. 2006, 42, 1679–1684. [Google Scholar] [CrossRef] [PubMed]
- Bhatnagar, G.; Sharma, S.; Kumar, A.; Prasad, S.; Agarwal, S.; Kar, P. Reduced glutathione in hepatitis E infection and pregnancy outcome. J. Obstet. Gynaecol. Res. 2016, 42, 789–795. [Google Scholar] [CrossRef] [PubMed]
- Perez-Gracia, M.T.; Suay-Garcia, B.; Mateos-Lindemann, M.L. Hepatitis E and pregnancy: Current state. Rev. Med. Virol. 2017, 27, e1929. [Google Scholar] [CrossRef] [PubMed]
- Galiana, C.; Fernandez-Barredo, S.; Garcia, A.; Gomez, M.T.; Perez-Gracia, M.T. Occupational exposure to hepatitis E virus (HEV) in swine workers. Am. J. Trop. Med. Hyg. 2008, 78, 1012–1015. [Google Scholar] [CrossRef] [PubMed]
- Teixeira, J.; Mesquita, J.R.; Pereira, S.S.; Oliveira, R.M.S.; Abreu-Silva, J.; Rodrigues, A.; Myrmel, M.; Stene-Johansen, K.; Overbo, J.; Goncalves, G.; et al. Prevalence of hepatitis E virus antibodies in workers occupationally exposed to swine in Portugal. Med. Microbiol. Immunol. 2017, 206, 77–81. [Google Scholar] [CrossRef] [PubMed]
- Matsubayashi, K.; Nagaoka, Y.; Sakata, H.; Sato, S.; Fukai, K.; Kato, T.; Takahashi, K.; Mishiro, S.; Imai, M.; Takeda, N.; et al. Transfusion-transmitted hepatitis E caused by apparently indigenous hepatitis E virus strain in Hokkaido, Japan. Transfusion 2004, 44, 934–940. [Google Scholar] [CrossRef]
- Mitsui, T.; Tsukamoto, Y.; Yamazaki, C.; Masuko, K.; Tsuda, F.; Takahashi, M.; Nishizawa, T.; Okamoto, H. Prevalence of hepatitis E virus infection among hemodialysis patients in Japan: Evidence for infection with a genotype 3 HEV by blood transfusion. J. Med. Virol. 2004, 74, 563–572. [Google Scholar] [CrossRef]
- Boxall, E.; Herborn, A.; Kochethu, G.; Pratt, G.; Adams, D.; Ijaz, S.; Teo, C.G. Transfusion-transmitted hepatitis E in a ‘nonhyperendemic’ country. Transfus. Med. 2006, 16, 79–83. [Google Scholar] [CrossRef]
- Satake, M.; Matsubayashi, K.; Hoshi, Y.; Taira, R.; Furui, Y.; Kokudo, N.; Akamatsu, N.; Yoshizumi, T.; Ohkohchi, N.; Okamoto, H.; et al. Unique clinical courses of transfusion-transmitted hepatitis E in patients with immunosuppression. Transfusion 2017, 57, 280–288. [Google Scholar] [CrossRef]
- Kamar, N.; Selves, J.; Mansuy, J.M.; Ouezzani, L.; Peron, J.M.; Guitard, J.; Cointault, O.; Esposito, L.; Abravanel, F.; Danjoux, M.; et al. Hepatitis E virus and chronic hepatitis in organ-transplant recipients. N. Engl. J. Med. 2008, 358, 811–817. [Google Scholar] [CrossRef]
- Shrestha, A.; Adhikari, A.; Bhattarai, M.; Rauniyar, R.; Debes, J.D.; Boonstra, A.; Lama, T.K.; Al Mahtab, M.; Butt, A.S.; Akbar, S.M.F.; et al. Prevalence and risk of hepatitis E virus infection in the HIV population of Nepal. Virol. J. 2017, 14, 228. [Google Scholar] [CrossRef]
- Tavitian, S.; Peron, J.M.; Huguet, F.; Kamar, N.; Abravanel, F.; Beyne-Rauzy, O.; Oberic, L.; Faguer, S.; Alric, L.; Roussel, M.; et al. Ribavirin for chronic hepatitis prevention among patients with hematologic malignancies. Emerg. Infect. Dis. 2015, 21, 1466–1469. [Google Scholar] [CrossRef]
- Dalton, H.R.; Kamar, N.; van Eijk, J.J.J.; McLean, B.N.; Cintas, P.; Bendall, R.P.; Jacobs, B.C. Hepatitis E virus and neurological injury. Nat. Rev. Neurol. 2016, 12, 77–85. [Google Scholar] [CrossRef]
- Bazerbachi, F.; Haffar, S.; Garg, S.K.; Lake, J.R. Extra-hepatic manifestations associated with hepatitis E virus infection: A comprehensive review of the literature. Gastroenterol. Rep. (Oxf). 2016, 4, 1–15. [Google Scholar] [CrossRef]
- Balayan, M.S.; Andjaparidze, A.G.; Savinskaya, S.S.; Ketiladze, E.S.; Braginsky, D.M.; Savinov, A.P.; Poleschuk, V.F. Evidence for a virus in non-A, non-B hepatitis transmitted via the fecal–oral route. Intervirology 1983, 20, 23–31. [Google Scholar]
- Tam, A.W.; Smith, M.M.; Guerra, M.E.; Huang, C.C.; Bradley, D.W.; Fry, K.E.; Reyes, G.R. Hepatitis E virus (HEV): Molecular cloning and sequencing of the full-length viral genome. Virology 1991, 185, 120–131. [Google Scholar] [CrossRef]
- Purdy, M.A.; Harrison, T.J.; Jameel, S.; Meng, X.J.; Okamoto, H.; Van der Poel, W.H.M.; Smith, D.B.; ICTV Report Consortium. ICTV virus taxonomy profile: Hepeviridae. J. Gen. Virol. 2017, 98, 2645–2646. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Stecher, G.; Tamura, K. MEGA7: Molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol. Biol. Evol. 2016, 33, 1870–1874. [Google Scholar] [CrossRef]
- Di Martino, B.; Di Profio, F.; Melegari, I.; Sarchese, V.; Robetto, S.; Marsilio, F.; Martella, V. Detection of hepatitis E virus (HEV) in goats. Virus Res. 2016, 225, 69–72. [Google Scholar] [CrossRef] [PubMed]
- Saad, M.D.; Hussein, H.A.; Bashandy, M.M.; Kamel, H.H.; Earhart, K.C.; Fryauff, D.J.; Younan, M.; Mohamed, A.H. Hepatitis E virus infection in work horses in Egypt. Infect. Genet. Evol. 2007, 7, 368–373. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Shen, Q.; Mou, J.; Gong, G.; Yang, Z.; Cui, L.; Zhu, J.; Ju, G.; Hua, X. Hepatitis E virus infection among domestic animals in eastern China. Zoonoses Public Health 2008, 55, 291–298. [Google Scholar] [CrossRef]
- Geng, J.B.; Fu, H.W.; Wang, L.; Wang, X.J.; Guan, J.M.; Chang, Y.B.; Li, L.J.; Zhu, Y.H.; Zhuang, H.; Liu, Q.H.; et al. Hepatitis E virus (HEV) genotype and the prevalence of anti-HEV in 8 species of animals in the suburbs of Beijing. Zhonghua Liu Xing Bing Xue Za Zhi 2010, 31, 47–50. [Google Scholar]
- Garcia-Bocanegra, I.; Rivero, A.; Caballero-Gomez, J.; Lopez-Lopez, P.; Cano-Terriza, D.; Frias, M.; Jimenez-Ruiz, S.; Risalde, M.A.; Gomez-Villamandos, J.C.; Rivero-Juarez, A. Hepatitis E virus infection in equines in Spain. Transbound. Emerg. Dis. 2019, 66, 66–71. [Google Scholar] [CrossRef]
- Montalvo Villalba, M.C.; Cruz Martinez, D.; Ahmad, I.; Rodriguez Lay, L.A.; Bello Corredor, M.; Guevara March, C.; Martinez, L.S.; Martinez-Campo, L.S.; Jameel, S. Hepatitis E virus in bottlenose dolphins Tursiops truncatus. Dis. Aquat. Organ. 2017, 123, 13–18. [Google Scholar] [CrossRef]
- Sarchese, V.; Di Profio, F.; Melegari, I.; Palombieri, A.; Sanchez, S.B.; Arbuatti, A.; Ciuffetelli, M.; Marsilio, F.; Martella, V.; Di Martino, B. Hepatitis E virus in sheep in Italy. Transbound. Emerg. Dis. 2019. [Google Scholar] [CrossRef]
- Hu, G.D.; Ma, X. Detection and sequences analysis of bovine hepatitis E virus RNA in Xinjiang Autonomous Region. Bing Du Xue Bao 2010, 26, 27–32. [Google Scholar]
- Vitral, C.L.; Pinto, M.A.; Lewis-Ximenez, L.L.; Khudyakov, Y.E.; dos Santos, D.R.; Gaspar, A.M.C. Serological evidence of hepatitis E virus infection in different animal species from the southeast of Brazil. Mem. Inst. Oswaldo Cruz 2005, 100, 117–122. [Google Scholar] [CrossRef]
- Huang, F.; Li, Y.; Yu, W.; Jing, S.; Wang, J.; Long, F.; He, Z.; Yang, C.; Bi, Y.; Cao, W.; et al. Excretion of infectious hepatitis E virus into milk in cows imposes high risks of zoonosis. Hepatology 2016, 64, 350–359. [Google Scholar] [CrossRef]
- Wu, J.; Si, F.; Jiang, C.; Li, T.; Jin, M. Molecular detection of hepatitis E virus in sheep from southern Xinjiang, China. Virus Genes 2015, 50, 410–417. [Google Scholar] [CrossRef]
- Li, S.; Liu, M.; Cong, J.; Zhou, Y.; Miao, Z. Detection and characterization of hepatitis E virus in goats at slaughterhouse in Tai’an region, China. Biomed Res. Int. 2017, 2017, 3723650. [Google Scholar] [CrossRef]
- Xu, F.; Pan, Y.; Baloch, A.R.; Tian, L.; Wang, M.; Na, W.; Ding, L.; Zeng, Q. Hepatitis E virus genotype 4 in yak, northwestern China. Emerg. Infect. Dis. 2014, 20, 2182–2184. [Google Scholar] [CrossRef]
- Li, W.; Guan, D.; Su, J.; Takeda, N.; Wakita, T.; Li, T.C.; Ke, C.W. High prevalence of rat hepatitis E virus in wild rats in China. Vet. Microbiol. 2013, 165, 275–280. [Google Scholar] [CrossRef]
- Guan, D.; Li, W.; Su, J.; Fang, L.; Takeda, N.; Wakita, T.; Li, T.C.; Ke, C. Asian musk shrew as a reservoir of rat hepatitis E virus, China. Emerg. Infect. Dis. 2013, 19, 1341–1343. [Google Scholar] [CrossRef]
- Krog, J.S.; Breum, S.O.; Jensen, T.H.; Larsen, L.E. Hepatitis E virus variant in farmed mink, Denmark. Emerg. Infect. Dis. 2013, 19, 2028–2030. [Google Scholar] [CrossRef]
- Woo, P.C.Y.; Lau, S.K.P.; Teng, J.L.L.; Cao, K.Y.; Wernery, U.; Schountz, T.; Chiu, T.H.; Tsang, A.K.L.; Wong, P.C.; Wong, E.Y.M.; et al. New hepatitis E virus genotype in Bactrian camels, Xinjiang, China, 2013. Emerg. Infect. Dis. 2016, 22, 2219–2221. [Google Scholar] [CrossRef]
- Tei, S.; Kitajima, N.; Takahashi, K.; Mishiro, S. Zoonotic transmission of hepatitis E virus from deer to human beings. Lancet 2003, 362, 371–373. [Google Scholar] [CrossRef]
- Takahashi, K.; Kitajima, N.; Abe, N.; Mishiro, S. Complete or near-complete nucleotide sequences of hepatitis E virus genome recovered from a wild boar, a deer, and four patients who ate the deer. Virology 2004, 330, 501–505. [Google Scholar] [CrossRef]
- Nakamura, M.; Takahashi, K.; Taira, K.; Taira, M.; Ohno, A.; Sakugawa, H.; Arai, M.; Mishiro, S. Hepatitis E virus infection in wild mongooses of Okinawa, Japan: Demonstration of anti-HEV antibodies and a full-genome nucleotide sequence. Hepatol. Res. 2006, 34, 137–140. [Google Scholar] [CrossRef]
- Nidaira, M.; Takahashi, K.; Ogura, G.; Taira, K.; Okano, S.; Kudaka, J.; Itokazu, K.; Mishiro, S.; Nakamura, M. Detection and phylogenetic analysis of hepatitis E viruses from mongooses in Okinawa, Japan. J. Vet. Med. Sci. 2012, 74, 1665–1668. [Google Scholar] [CrossRef]
- Zhao, C.; Ma, Z.; Harrison, T.J.; Feng, R.; Zhang, C.; Qiao, Z.; Fan, J.; Ma, H.; Li, M.; Song, A.; et al. A novel genotype of hepatitis E virus prevalent among farmed rabbits in China. J. Med. Virol. 2009, 81, 1371–1379. [Google Scholar] [CrossRef]
- Cossaboom, C.M.; Cordoba, L.; Dryman, B.A.; Meng, X.J. Hepatitis E virus in rabbits, Virginia, USA. Emerg. Infect. Dis. 2011, 17, 2047–2049. [Google Scholar] [CrossRef]
- Wang, L.; Liu, L.; Wang, L. An overview: Rabbit hepatitis E virus (HEV) and rabbit providing an animal model for HEV study. Rev. Med. Virol. 2018, 28, e1961. [Google Scholar] [CrossRef]
- Woo, P.C.Y.; Lau, S.K.P.; Teng, J.L.L.; Tsang, A.K.L.; Joseph, M.; Wong, E.Y.M.; Tang, Y.; Sivakumar, S.; Xie, J.; Bai, R.; et al. New hepatitis E virus genotype in camels, the Middle East. Emerg. Infect. Dis. 2014, 20, 1044–1048. [Google Scholar] [CrossRef]
- Spahr, C.; Knauf-Witzens, T.; Vahlenkamp, T.; Ulrich, R.G.; Johne, R. Hepatitis E virus and related viruses in wild, domestic and zoo animals: A review. Zoonoses Public Health 2018, 65, 11–29. [Google Scholar] [CrossRef]
- Geng, Y.; Zhao, C.; Huang, W.; Wang, X.; Xu, Y.; Wu, D.; Du, Y.; Liu, H.; Wang, Y. Hepatitis E virus was not detected in feces and milk of cows in Hebei province of China: No evidence for HEV prevalence in cows. Int. J. Food Microbiol. 2019, 291, 5–9. [Google Scholar] [CrossRef]
- Baechlein, C.; Becher, P. No evidence for zoonotic hepatitis E virus infection through dairy milk in Germany. Hepatology 2017, 65, 394–395. [Google Scholar] [CrossRef]
- Vercouter, A.S.; Sayed, I.M.; Lipkens, Z.; De Bleecker, K.; De Vliegher, S.; Colman, R.; Koppelman, M.; Supre, K.; Meuleman, P. Absence of zoonotic hepatitis E virus infection in Flemish dairy cows. Int. J. Food Microbiol. 2018, 281, 54–59. [Google Scholar] [CrossRef]
- Yugo, D.M.; Cossaboom, C.M.; Heffron, C.L.; Huang, Y.W.; Kenney, S.P.; Woolums, A.R.; Hurley, D.J.; Opriessnig, T.; Li, L.; Delwart, E.; et al. Evidence for an unknown agent antigenically related to the hepatitis E virus in dairy cows in the United States. J. Med. Virol. 2019, 91, 677–686. [Google Scholar] [CrossRef]
- Sanford, B.J.; Emerson, S.U.; Purcell, R.H.; Engle, R.E.; Dryman, B.A.; Cecere, T.E.; Buechner-Maxwell, V.; Sponenberg, D.P.; Meng, X.J. Serological evidence for a hepatitis E virus (HEV)-related agent in goats in the United States. Transbound. Emerg. Dis. 2013, 60, 538–545. [Google Scholar] [CrossRef]
- Izopet, J.; Dubois, M.; Bertagnoli, S.; Lhomme, S.; Marchandeau, S.; Boucher, S.; Kamar, N.; Abravanel, F.; Guerin, J.L. Hepatitis E virus strains in rabbits and evidence of a closely related strain in humans, France. Emerg. Infect. Dis. 2012, 18, 1274–1281. [Google Scholar] [CrossRef]
- Takahashi, K.; Terada, S.; Kokuryu, H.; Arai, M.; Mishiro, S. A wild boar-derived hepatitis E virus isolate presumably representing so far unidentified “genotype 5”. Kanzo 2010, 51, 536–538. [Google Scholar] [CrossRef]
- Takahashi, M.; Nishizawa, T.; Sato, H.; Sato, Y.; Jirintai; Nagashima, S.; Okamoto, H. Analysis of the full-length genome of a hepatitis E virus isolate obtained from a wild boar in Japan that is classifiable into a novel genotype. J. Gen. Virol. 2011, 92, 902–908. [Google Scholar] [CrossRef]
- Rasche, A.; Saqib, M.; Liljander, A.M.; Bornstein, S.; Zohaib, A.; Renneker, S.; Steinhagen, K.; Wernery, R.; Younan, M.; Gluecks, I.; et al. Hepatitis E virus infection in dromedaries, North and East Africa, United Arab Emirates, and Pakistan, 1983–2015. Emerg. Infect. Dis. 2016, 22, 1249–1252. [Google Scholar] [CrossRef]
- Lee, G.H.; Tan, B.H.; Teo, E.C.Y.; Lim, S.G.; Dan, Y.Y.; Wee, A.; Aw, P.P.K.; Zhu, Y.; Hibberd, M.L.; Tan, C.K.; et al. Chronic infection with camelid hepatitis E virus in a liver transplant recipient who regularly consumes camel meat and milk. Gastroenterology 2016, 150, 355–357. [Google Scholar] [CrossRef]
- Li, T.C.; Bai, H.; Yoshizaki, S.; Ami, Y.; Suzaki, Y.; Doan, Y.H.; Takahashi, K.; Mishiro, S.; Takeda, N.; Wakita, T. Genotype 5 hepatitis E virus produced by a reverse genetics system has the potential for zoonotic infection. Hepatol. Commun. 2019, 3, 160–172. [Google Scholar] [CrossRef]
- Li, T.C.; Zhou, X.; Yoshizaki, S.; Ami, Y.; Suzaki, Y.; Nakamura, T.; Takeda, N.; Wakita, T. Production of infectious dromedary camel hepatitis E virus by a reverse genetic system: Potential for zoonotic infection. J. Hepatol. 2016, 65, 1104–1111. [Google Scholar] [CrossRef]
- Wang, L.; Teng, J.L.L.; Lau, S.K.P.; Sridhar, S.; Fu, H.; Gong, W.; Li, M.; Xu, Q.; He, Y.; Zhuang, H.; et al. Transmission of a novel genotype of hepatitis E virus from Bactrian camels to cynomolgus macaques. J. Virol. 2019, 93, e02014-18. [Google Scholar] [CrossRef]
- Smith, D.B.; Simmonds, P.; Izopet, J.; Oliveira-Filho, E.F.; Ulrich, R.G.; Johne, R.; Koenig, M.; Jameel, S.; Harrison, T.J.; Meng, X.J.; et al. Proposed reference sequences for hepatitis E virus subtypes. J. Gen. Virol. 2016, 97, 537–542. [Google Scholar] [CrossRef]
- Nishizawa, T.; Primadharsini, P.P.; Namikawa, M.; Yamazaki, Y.; Uraki, S.; Okano, H.; Horiike, S.; Nakano, T.; Takaki, S.; Kawakami, M.; et al. Full-length genomic sequences of new subtype 1g hepatitis E virus strains obtained from four patients with imported or autochthonous acute hepatitis E in Japan. Infect. Genet. Evol. 2017, 55, 343–349. [Google Scholar] [CrossRef]
- Okamoto, H. Genetic variability and evolution of hepatitis E virus. Virus Res. 2007, 127, 216–228. [Google Scholar] [CrossRef]
- Miura, M.; Inoue, J.; Tsuruoka, M.; Nishizawa, T.; Nagashima, S.; Takahashi, M.; Shimosegawa, T.; Okamoto, H. Full-length genomic sequence analysis of new subtype 3k hepatitis E virus isolates with 99.97% nucleotide identity obtained from two consecutive acute hepatitis patients in a city in northeast Japan. J. Med. Virol. 2017, 89, 1116–1120. [Google Scholar] [CrossRef]
- Wang, B.; Harms, D.; Hofmann, J.; Ciardo, D.; Kneubuhl, A.; Bock, C.T. Identification of a novel hepatitis E virus genotype 3 strain isolated from a chronic hepatitis E virus infection in a kidney transplant recipient in Switzerland. Genome Announc. 2017, 5, e00345-17. [Google Scholar] [CrossRef]
- De Sabato, L.; Lemey, P.; Vrancken, B.; Bonfanti, L.; Ceglie, L.; Vaccari, G.; Di Bartolo, I. Proposal for a new subtype of the zoonotic genotype 3 hepatitis E virus: HEV-3l. Virus Res. 2018, 248, 1–4. [Google Scholar] [CrossRef]
- Payne, C.J.; Ellis, T.M.; Plant, S.L.; Gregory, A.R.; Wilcox, G.E. Sequence data suggests big liver and spleen disease virus (BLSV) is genetically related to hepatitis E virus. Vet. Microbiol. 1999, 68, 119–125. [Google Scholar] [CrossRef]
- Haqshenas, G.; Shivaprasad, H.L.; Woolcock, P.R.; Read, D.H.; Meng, X.J. Genetic identification and characterization of a novel virus related to human hepatitis E virus from chickens with hepatitis-splenomegaly syndrome in the United States. J. Gen. Virol. 2001, 82, 2449–2462. [Google Scholar] [CrossRef]
- Bilic, I.; Jaskulska, B.; Basic, A.; Morrow, C.J.; Hess, M. Sequence analysis and comparison of avian hepatitis E viruses from Australia and Europe indicate the existence of different genotypes. J. Gen. Virol. 2009, 90, 863–873. [Google Scholar] [CrossRef]
- Banyai, K.; Toth, A.G.; Ivanics, E.; Glavits, R.; Szentpali-Gavaller, K.; Dan, A. Putative novel genotype of avian hepatitis E virus, Hungary, 2010. Emerg. Infect. Dis. 2012, 18, 1365–1368. [Google Scholar] [CrossRef]
- Zhao, Q.; Zhou, E.M.; Dong, S.W.; Qiu, H.K.; Zhang, L.; Hu, S.B.; Zhao, F.F.; Jiang, S.J.; Sun, Y.N. Analysis of avian hepatitis E virus from chickens, China. Emerg. Infect. Dis. 2010, 16, 1469–1472. [Google Scholar] [CrossRef]
- Kwon, H.M.; Sung, H.W.; Meng, X.J. Serological prevalence, genetic identification, and characterization of the first strains of avian hepatitis E virus from chickens in Korea. Virus Genes 2012, 45, 237–245. [Google Scholar] [CrossRef]
- Hsu, I.W.Y.; Tsai, H.J. Avian hepatitis E virus in chickens, Taiwan, 2013. Emerg. Infect. Dis. 2014, 20, 149–151. [Google Scholar] [CrossRef]
- Matczuk, A.K.; Cwiek, K.; Wieliczko, A. Avian hepatitis E virus is widespread among chickens in Poland and belongs to genotype 2. Arch. Virol. 2019, 164, 595–599. [Google Scholar] [CrossRef]
- Wang, B.; Li, W.; Zhou, J.H.; Li, B.; Zhang, W.; Yang, W.H.; Pan, H.; Wang, L.X.; Bock, C.T.; Shi, Z.L.; et al. Chevrier’s field mouse (Apodemus chevrieri) and Pere David’s vole (Eothenomys melanogaster) in China carry Orthohepeviruses that form two putative novel genotypes within the species Orthohepevirus C. Virol. Sin. 2018, 33, 44–58. [Google Scholar] [CrossRef]
- Johne, R.; Heckel, G.; Plenge-Bonig, A.; Kindler, E.; Maresch, C.; Reetz, J.; Schielke, A.; Ulrich, R.G. Novel hepatitis E virus genotype in Norway rats, Germany. Emerg. Infect. Dis. 2010, 16, 1452–1455. [Google Scholar] [CrossRef]
- Purcell, R.H.; Engle, R.E.; Rood, M.P.; Kabrane-Lazizi, Y.; Nguyen, H.T.; Govindarajan, S.; St Claire, M.; Emerson, S.U. Hepatitis E virus in rats, Los Angeles, California, USA. Emerg. Infect. Dis. 2011, 17, 2216–2222. [Google Scholar] [CrossRef]
- Lack, J.B.; Volk, K.; Van Den Bussche, R.A. Hepatitis E virus genotype 3 in wild rats, United States. Emerg. Infect. Dis. 2012, 18, 1268–1273. [Google Scholar] [CrossRef]
- Johne, R.; Dremsek, P.; Kindler, E.; Schielke, A.; Plenge-Bonig, A.; Gregersen, H.; Wessels, U.; Schmidt, K.; Rietschel, W.; Groschup, M.H.; et al. Rat hepatitis E virus: Geographical clustering within Germany and serological detection in wild Norway rats (Rattus norvegicus). Infect. Genet. Evol. 2012, 12, 947–956. [Google Scholar] [CrossRef]
- Mulyanto; Depamede, S.N.; Sriasih, M.; Takahashi, M.; Nagashima, S.; Jirintai, S.; Nishizawa, T.; Okamoto, H. Frequent detection and characterization of hepatitis E virus variants in wild rats (Rattus rattus) in Indonesia. Arch. Virol. 2013, 158, 87–96. [Google Scholar] [CrossRef]
- Mulyanto; Suparyatmo, J.B.; Andayani, I.G.; Khalid; Takahashi, M.; Ohnishi, H.; Jirintai, S.; Nagashima, S.; Nishizawa, T.; Okamoto, H. Marked genomic heterogeneity of rat hepatitis E virus strains in Indonesia demonstrated on a full-length genome analysis. Virus Res. 2014, 179, 102–112. [Google Scholar] [CrossRef]
- Li, T.C.; Ami, Y.; Suzaki, Y.; Yasuda, S.P.; Yoshimatsu, K.; Arikawa, J.; Takeda, N.; Takaji, W. Characterization of full genome of rat hepatitis E virus strain from Vietnam. Emerg. Infect. Dis. 2013, 19, 115–118. [Google Scholar] [CrossRef]
- Ryll, R.; Bernstein, S.; Heuser, E.; Schlegel, M.; Dremsek, P.; Zumpe, M.; Wolf, S.; Pepin, M.; Bajomi, D.; Muller, G.; et al. Detection of rat hepatitis E virus in wild Norway rats (Rattus norvegicus) and Black rats (Rattus rattus) from 11 European countries. Vet. Microbiol. 2017, 208, 58–68. [Google Scholar] [CrossRef]
- Primadharsini, P.P.; Mulyanto; Wibawa, I.D.N.; Anggoro, J.; Nishizawa, T.; Takahashi, M.; Jirintai, S.; Okamoto, H. The identification and characterization of novel rat hepatitis E virus strains in Bali and Sumbawa, Indonesia. Arch. Virol. 2018, 163, 1345–1349. [Google Scholar] [CrossRef]
- Raj, V.S.; Smits, S.L.; Pas, S.D.; Provacia, L.B.V.; Moorman-Roest, H.; Osterhaus, A.D.M.E.; Haagmans, B.L. Novel hepatitis E virus in ferrets, the Netherlands. Emerg. Infect. Dis. 2012, 18, 1369–1370. [Google Scholar] [CrossRef]
- Li, T.C.; Yonemitsu, K.; Terada, Y.; Takeda, N.; Takaji, W.; Maeda, K. Ferret hepatitis E virus infection in Japan. Jpn. J. Infect. Dis. 2015, 68, 60–62. [Google Scholar] [CrossRef]
- Wu, Z.; Lu, L.; Du, J.; Yang, L.; Ren, X.; Liu, B.; Jiang, J.; Yang, J.; Dong, J.; Sun, L.; et al. Comparative analysis of rodent and small mammal viromes to better understand the wildlife origin of emerging infectious diseases. Microbiome 2018, 6, 178. [Google Scholar] [CrossRef]
- de Souza, W.M.; Romeiro, M.F.; Sabino-Santos, G., Jr.; Maia, F.G.M.; Fumagalli, M.J.; Modha, S.; Nunes, M.R.T.; Murcia, P.R.; Figueiredo, L.T.M. Novel orthohepeviruses in wild rodents from São Paulo State, Brazil. Virology 2018, 519, 12–16. [Google Scholar] [CrossRef]
- Reuter, G.; Boros, A.; Matics, R.; Kapusinszky, B.; Delwart, E.; Pankovics, P. Divergent hepatitis E virus in birds of prey, common kestrel (Falco tinnunculus) and red-footed falcon (F. vespertinus), Hungary. Infect. Genet. Evol. 2016, 43, 343–346. [Google Scholar] [CrossRef]
- Dremsek, P.; Wenzel, J.J.; Johne, R.; Ziller, M.; Hofmann, J.; Groschup, M.H.; Werdermann, S.; Mohn, U.; Dorn, S.; Motz, M.; et al. Seroprevalence study in forestry workers from eastern Germany using novel genotype 3- and rat hepatitis E virus-specific immunoglobulin G ELISAs. Med. Microbiol. Immunol. 2012, 201, 189–200. [Google Scholar] [CrossRef]
- Shimizu, K.; Hamaguchi, S.; Ngo, C.C.; Li, T.C.; Ando, S.; Yoshimatsu, K.; Yasuda, S.P.; Koma, T.; Isozumi, R.; Tsuda, Y.; et al. Serological evidence of infection with rodent-borne hepatitis E virus HEV-C1 or antigenically related virus in humans. J. Vet. Med. Sci. 2016, 78, 1677–1681. [Google Scholar] [CrossRef]
- Sridhar, S.; Yip, C.C.Y.; Wu, S.; Cai, J.; Zhang, A.J.; Leung, K.H.; Chung, T.W.H.; Chan, J.F.W.; Chan, W.M.; Teng, J.L.L.; et al. Rat hepatitis E virus as cause of persistent hepatitis after liver transplant. Emerg. Infect. Dis. 2018, 24, 2241–2250. [Google Scholar] [CrossRef]
- Andonov, A.; Robbins, M.; Borlang, J.; Cao, J.; Hattchete, T.; Stueck, A.; Deschaumbault, Y.; Murnaghan, K.; Varga, J.; Johnston, B. Rat hepatitis E virus linked to severe acute hepatitis in an immunocompetent patient. J. Infect. Dis. 2019. [Google Scholar] [CrossRef]
- Drexler, J.F.; Seelen, A.; Corman, V.M.; Tateno, A.F.; Cottontail, V.; Melim Zerbinati, R.; Gloza-Rausch, F.; Klose, S.M.; Adu-Sarkodie, Y.; Oppong, S.K.; et al. Bats worldwide carry hepatitis E virus-related viruses that form a putative novel genus within the family Hepeviridae. J. Virol. 2012, 86, 9134–9147. [Google Scholar] [CrossRef]
- Wu, Z.; Yang, L.; Ren, X.; He, G.; Zhang, J.; Yang, J.; Qian, Z.; Dong, J.; Sun, L.; Zhu, Y.; et al. Deciphering the bat virome catalog to better understand the ecological diversity of bat viruses and the bat origin of emerging infectious diseases. ISME J. 2016, 10, 609–620. [Google Scholar] [CrossRef]
- Lin, J.; Norder, H.; Uhlhorn, H.; Belak, S.; Widen, F. Novel hepatitis E like virus found in Swedish moose. J. Gen. Virol. 2014, 95, 557–570. [Google Scholar] [CrossRef]
- Lin, J.; Karlsson, M.; Olofson, A.S.; Belak, S.; Malmsten, J.; Dalin, A.M.; Widen, F.; Norder, H. High prevalence of hepatitis E virus in Swedish moose--a phylogenetic characterization and comparison of the virus from different regions. PLoS ONE 2015, 10, e0122102. [Google Scholar] [CrossRef]
- Bodewes, R.; van der Giessen, J.; Haagmans, B.L.; Osterhaus, A.D.M.E.; Smits, S.L. Identification of multiple novel viruses, including a parvovirus and a hepevirus, in feces of red foxes. J. Virol. 2013, 87, 7758–7764. [Google Scholar] [CrossRef]
- Reuter, G.; Boros, A.; Matics, R.; Kapusinszky, B.; Delwart, E.; Pankovics, P. A novel avian-like hepatitis E virus in wild aquatic bird, little egret (Egretta garzetta), in Hungary. Infect. Genet. Evol. 2016, 46, 74–77. [Google Scholar] [CrossRef]
- Yang, C.; Wang, L.; Shen, H.; Zheng, Y.; Gauger, P.C.; Chen, Q.; Zhang, J.; Yoon, K.J.; Harmon, K.M.; Main, R.G.; et al. Detection and genomic characterization of new avian-like hepatitis virus in a sparrow in the United States. Arch. Virol. 2018, 163, 2861–2864. [Google Scholar] [CrossRef]
- Reuter, G.; Boros, A.; Toth, Z.; Kapusinszky, B.; Delwart, E.; Pankovics, P. Detection of a novel RNA virus with hepatitis E virus-like non-structural genome organization in amphibian, agile frog (Rana dalmatina) tadpoles. Infect. Genet. Evol. 2018, 65, 112–116. [Google Scholar] [CrossRef]
- Kabrane-Lazizi, Y.; Meng, X.J.; Purcell, R.H.; Emerson, S.U. Evidence that the genomic RNA of hepatitis E virus is capped. J. Virol. 1999, 73, 8848–8850. [Google Scholar]
- Koonin, E.V.; Gorbalenya, A.E.; Purdy, M.A.; Rozanov, M.N.; Reyes, G.R.; Bradley, D.W. Computer-assisted assignment of functional domains in the nonstructural polyprotein of hepatitis E virus: Delineation of an additional group of positive-strand RNA plant and animal viruses. Proc. Natl. Acad. Sci. USA 1992, 89, 8259–8263. [Google Scholar] [CrossRef]
- Nan, Y.; Zhang, Y.J. Molecular biology and infection of hepatitis E virus. Front. Microbiol. 2016, 7, 1419. [Google Scholar] [CrossRef]
- Sridhar, S.; Teng, J.L.L.; Chiu, T.H.; Lau, S.K.P.; Woo, P.C.Y. Hepatitis E virus genotypes and evolution: Emergence of camel hepatitis E variants. Int. J. Mol. Sci. 2017, 18, 869. [Google Scholar] [CrossRef]
- Reyes, G.R.; Huang, C.C.; Tam, A.W.; Purdy, M.A. Molecular organization and replication of hepatitis E virus (HEV). Arch. Virol. Suppl. 1993, 7, 15–25. [Google Scholar]
- Kalia, M.; Chandra, V.; Rahman, S.A.; Sehgal, D.; Jameel, S. Heparan sulfate proteoglycans are required for cellular binding of the hepatitis E virus ORF2 capsid protein and for viral infection. J. Virol. 2009, 83, 12714–12724. [Google Scholar] [CrossRef]
- Xing, L.; Wang, J.C.; Li, T.C.; Yasutomi, Y.; Lara, J.; Khudyakov, Y.; Schofield, D.; Emerson, S.U.; Purcell, R.H.; Takeda, N.; et al. Spatial configuration of hepatitis E virus antigenic domain. J. Virol. 2011, 85, 1117–1124. [Google Scholar] [CrossRef]
- Yamada, K.; Takahashi, M.; Hoshino, Y.; Takahashi, H.; Ichiyama, K.; Nagashima, S.; Tanaka, T.; Okamoto, H. ORF3 protein of hepatitis E virus is essential for virion release from infected cells. J. Gen. Virol. 2009, 90, 1880–1891. [Google Scholar] [CrossRef]
- Emerson, S.U.; Nguyen, H.T.; Torian, U.; Burke, D.; Engle, R.; Purcell, R.H. Release of genotype 1 hepatitis E virus from cultured hepatoma and polarized intestinal cells depends on open reading frame 3 protein and requires an intact PXXP motif. J. Virol. 2010, 84, 9059–9069. [Google Scholar] [CrossRef]
- Nagashima, S.; Takahashi, M.; Jirintai; Tanaka, T.; Yamada, K.; Nishizawa, T.; Okamoto, H. A PSAP motif in the ORF3 protein of hepatitis E virus is necessary for virion release from infected cells. J. Gen. Virol. 2011, 92, 269–278. [Google Scholar] [CrossRef]
- Ding, Q.; Heller, B.; Capuccino, J.M.V.; Song, B.; Nimgaonkar, I.; Hrebikova, G.; Contreras, J.E.; Ploss, A. Hepatitis E virus ORF3 is a functional ion channel required for release of infectious particles. Proc. Natl. Acad. Sci. USA 2017, 114, 1147–1152. [Google Scholar] [CrossRef]
- Gouttenoire, J.; Pollan, A.; Abrami, L.; Oechslin, N.; Mauron, J.; Matter, M.; Oppliger, J.; Szkolnicka, D.; Dao Thi, V.L.; van der Goot, F.G.; et al. Palmitoylation mediates membrane association of hepatitis E virus ORF3 protein and is required for infectious particle secretion. PLoS Pathog. 2018, 14, e1007471. [Google Scholar] [CrossRef]
- Graff, J.; Torian, U.; Nguyen, H.; Emerson, S.U. A bicistronic subgenomic mRNA encodes both the ORF2 and ORF3 proteins of hepatitis E virus. J. Virol. 2006, 80, 5919–5926. [Google Scholar] [CrossRef]
- Ichiyama, K.; Yamada, K.; Tanaka, T.; Nagashima, S.; Jirintai; Takahashi, M.; Okamoto, H. Determination of the 5’-terminal sequence of subgenomic RNA of hepatitis E virus strains in cultured cells. Arch. Virol. 2009, 154, 1945–1951. [Google Scholar] [CrossRef]
- Batts, W.; Yun, S.; Hedrick, R.; Winton, J. A novel member of the family Hepeviridae from cutthroat trout (Oncorhynchus clarkii). Virus Res. 2011, 158, 116–123. [Google Scholar] [CrossRef]
- Takahashi, M.; Hoshino, Y.; Tanaka, T.; Takahashi, H.; Nishizawa, T.; Okamoto, H. Production of monoclonal antibodies against hepatitis E virus capsid protein and evaluation of their neutralizing activity in a cell culture system. Arch. Virol. 2008, 153, 657–666. [Google Scholar] [CrossRef]
- Takahashi, M.; Tanaka, T.; Takahashi, H.; Hoshino, Y.; Nagashima, S.; Jirintai; Mizuo, H.; Yazaki, Y.; Takagi, T.; Azuma, M.; et al. Hepatitis E Virus (HEV) strains in serum samples can replicate efficiently in cultured cells despite the coexistence of HEV antibodies: characterization of HEV virions in blood circulation. J. Clin. Microbiol. 2010, 48, 1112–1125. [Google Scholar] [CrossRef]
- Nagashima, S.; Takahashi, M.; Kobayashi, T.; Tanggis; Nishizawa, T.; Nishiyama, T.; Primadharsini, P.P.; Okamoto, H. Characterization of the quasi-enveloped hepatitis E virus particles released by the cellular exosomal pathway. J. Virol. 2017, 91, e00822-17. [Google Scholar] [CrossRef]
- Yin, X.; Ambardekar, C.; Lu, Y.; Feng, Z. Distinct entry mechanisms for nonenveloped and quasi-enveloped hepatitis E viruses. J. Virol. 2016, 90, 4232–4242. [Google Scholar] [CrossRef]
- Nair, V.P.; Anang, S.; Subramani, C.; Madhvi, A.; Bakshi, K.; Srivastava, A.; Shalimar; Nayak, B.; Ranjith Kumar, C.T.; Surjit, M. Endoplasmic reticulum stress induced synthesis of a novel viral factor mediates efficient replication of genotype-1 hepatitis E virus. PLoS Pathog. 2016, 12, e1005521. [Google Scholar] [CrossRef]
- Tanggis; Kobayashi, T.; Takahashi, M.; Jirintai, S.; Nishizawa, T.; Nagashima, S.; Nishiyama, T.; Kunita, S.; Hayama, E.; Tanaka, T.; et al. An analysis of two open reading frames (ORF3 and ORF4) of rat hepatitis E virus genome using its infectious cDNA clones with mutations in ORF3 or ORF4. Virus Res. 2018, 249, 16–30. [Google Scholar] [CrossRef]
- Tsatsralt-Od, B.; Baasanjav, N.; Nyamkhuu, D.; Ohnishi, H.; Takahashi, M.; Okamoto, H. Prevalence of hepatitis viruses in patients with acute hepatitis and characterization of the detected genotype 4 hepatitis E virus sequences in Mongolia. J. Med. Virol. 2016, 88, 282–291. [Google Scholar] [CrossRef]
- Lapa, D.; Capobianchi, M.R.; Garbuglia, A.R. Epidemiology of hepatitis E virus in European countries. Int. J. Mol. Sci. 2015, 16, 25711–25743. [Google Scholar] [CrossRef]
- Takahashi, M.; Nishizawa, T.; Yoshikawa, A.; Sato, S.; Isoda, N.; Ido, K.; Sugano, K.; Okamoto, H. Identification of two distinct genotypes of hepatitis E virus in a Japanese patient with acute hepatitis who had not travelled abroad. J. Gen. Virol. 2002, 83, 1931–1940. [Google Scholar] [CrossRef]
- Takahashi, K.; Kang, J.H.; Ohnishi, S.; Hino, K.; Mishiro, S. Genetic heterogeneity of hepatitis E virus recovered from Japanese patients with acute sporadic hepatitis. J. Infect. Dis. 2002, 185, 1342–1345. [Google Scholar] [CrossRef]
- Okamoto, H.; Takahashi, M.; Nishizawa, T. Features of hepatitis E virus infection in Japan. Intern. Med. 2003, 42, 1065–1071. [Google Scholar] [CrossRef]
- Takahashi, M.; Nishizawa, T.; Miyajima, H.; Gotanda, Y.; Iita, T.; Tsuda, F.; Okamoto, H. Swine hepatitis E virus strains in Japan form four phylogenetic clusters comparable with those of Japanese isolates of human hepatitis E virus. J. Gen. Virol. 2003, 84, 851–862. [Google Scholar] [CrossRef]
- Takahashi, K.; Kang, J.H.; Ohnishi, S.; Hino, K.; Miyakawa, H.; Miyakawa, Y.; Maekubo, H.; Mishiro, S. Full-length sequences of six hepatitis E virus isolates of genotypes III and IV from patients with sporadic acute or fulminant hepatitis in Japan. Intervirology 2003, 46, 308–318. [Google Scholar] [CrossRef]
- Takahashi, K.; Okada, K.; Kang, J.H.; Karino, Y.; Ichida, T.; Matsuda, H.; Ohnishi, S.; Toyota, J.; Yamagiwa, S.; Maekubo, H.; et al. A lineage of hepatitis E virus within genotype IV, associated with severe forms of hepatitis. Kanzo 2005, 46, 389–390. [Google Scholar] [CrossRef]
- Urayama, T.; Sapsutthipas, S.; Tsujikawa, M.; Yamashita, A.; Nishigaki, H.; Ibrahim, M.S.; Hagiwara, K.; Yunoki, M.; Yasunaga, T.; Yamaguchi, T.; et al. Full-length sequences of one genotype 4 and three genotype 3 hepatitis E viruses in fecal samples from domestic swine in Japan. Open Vet. Sci. J. 2010, 4, 11–19. [Google Scholar] [CrossRef]
- Sato, Y.; Sato, H.; Naka, K.; Furuya, S.; Tsukiji, H.; Kitagawa, K.; Sonoda, Y.; Usui, T.; Sakamoto, H.; Yoshino, S.; et al. A nationwide survey of hepatitis E virus (HEV) infection in wild boars in Japan: Identification of boar HEV strains of genotypes 3 and 4 and unrecognized genotypes. Arch. Virol. 2011, 156, 1345–1358. [Google Scholar] [CrossRef]
- Takahashi, M.; Okamoto, H. Features of hepatitis E virus infection in humans and animals in Japan. Hepatol. Res. 2014, 44, 43–58. [Google Scholar] [CrossRef]
- Hara, Y.; Terada, Y.; Yonemitsu, K.; Shimoda, H.; Noguchi, K.; Suzuki, K.; Maeda, K. High prevalence of hepatitis E virus in wild boar (Sus scrofa) in Yamaguchi Prefecture, Japan. J. Wildl. Dis. 2014, 50, 378–383. [Google Scholar] [CrossRef]
- Motoya, T.; Nagata, N.; Komori, H.; Doi, I.; Kurosawa, M.; Keta, T.; Sasaki, N.; Ishii, K. The high prevalence of hepatitis E virus infection in wild boars in Ibaraki Prefecture, Japan. J. Vet. Med. Sci. 2016, 77, 1705–1709. [Google Scholar] [CrossRef]
- Sasaki, Y.; Haruna, M.; Uema, M.; Noda, M.; Yamada, Y. Prevalence and phylogenetic analysis of hepatitis E virus among pigs in Japan. Jpn. J. Infect. Dis. 2018, 71, 75–78. [Google Scholar] [CrossRef]
- Zehender, G.; Ebranati, E.; Lai, A.; Luzzago, C.; Paladini, S.; Tagliacarne, C.; Galli, C.; Galli, M.; Ciccozzi, M.; Zanetti, A.R.; et al. Phylogeography and phylodynamics of European genotype 3 hepatitis E virus. Infect. Genet. Evol. 2014, 25, 138–143. [Google Scholar] [CrossRef]
- Pavio, N.; Meng, X.J.; Renou, C. Zoonotic hepatitis E: Animal reservoirs and emerging risks. Vet. Res. 2010, 41, 46. [Google Scholar] [CrossRef]
- Bouwknegt, M.; Frankena, K.; Rutjes, S.A.; Wellenberg, G.J.; de Roda Husman, A.M.; van der Poel, W.H.; de Jong, M.C.M. Estimation of hepatitis E virus transmission among pigs due to contact-exposure. Vet. Res. 2008, 39, 40. [Google Scholar] [CrossRef]
- Primadharsini, P.P.; Miyake, M.; Kunita, S.; Nishizawa, T.; Takahashi, M.; Nagashima, S.; Tanggis; Ohnishi, H.; Kobayashi, T.; Nishiyama, T.; et al. Full-length genome of a novel genotype 3 hepatitis E virus strain obtained from domestic pigs in Japan. Virus Res. 2017, 240, 147–153. [Google Scholar] [CrossRef]
- Nakano, T.; Takahashi, M.; Takahashi, K.; Nagashima, S.; Suzuki, Y.; Nishigaki, Y.; Tomita, E.; Okano, H.; Oya, Y.; Shiraki, K.; et al. Hepatitis E virus subtype 3f strains isolated from Japanese hepatitis patients with no history of travel to endemic areas - The origin analyzed by molecular evolution. Virology 2018, 513, 146–152. [Google Scholar] [CrossRef]
- Ren, X.; Wu, P.; Wang, L.; Geng, M.; Zeng, L.; Zhang, J.; Xia, N.; Lai, S.; Dalton, H.R.; Cowling, B.J.; et al. Changing epidemiology of hepatitis A and hepatitis E viruses in China, 1990–2014. Emerg. Infect. Dis. 2017, 23, 276–279. [Google Scholar] [CrossRef]
- Tsatsralt-Od, B.; Primadharsini, P.P.; Nishizawa, T.; Ohnishi, H.; Nagashima, S.; Takahashi, M.; Jirintai, S.; Nyamkhuu, D.; Okamoto, H. Distinct changing profiles of hepatitis A and E virus infection among patients with acute hepatitis in Mongolia: The first report of the full genome sequence of a novel genotype 1 hepatitis E virus strain. J. Med. Virol. 2018, 90, 84–92. [Google Scholar] [CrossRef]
- Intharasongkroh, D.; Thongmee, T.; Sa-Nguanmoo, P.; Klinfueng, S.; Duang-In, A.; Wasitthankasem, R.; Theamboonlers, A.; Charoonruangrit, U.; Oota, S.; Payungporn, S.; et al. Hepatitis E virus infection in Thai blood donors. Transfusion 2019, 59, 1035–1043. [Google Scholar] [CrossRef]
- Nouhin, J.; Prak, S.; Madec, Y.; Barennes, H.; Weissel, R.; Hok, K.; Pavio, N.; Rouet, F. Hepatitis E virus antibody prevalence, RNA frequency, and genotype among blood donors in Cambodia (Southeast Asia). Transfusion 2016, 56, 2597–2601. [Google Scholar] [CrossRef]
- Nouhin, J.; Madec, Y.; Prak, S.; Ork, M.; Kerleguer, A.; Froehlich, Y.; Pavio, N.; Rouet, F. Declining hepatitis E virus antibody prevalence in Phnom Penh, Cambodia during 1996–2017. Epidemiol. Infect. 2019, 147, e26. [Google Scholar] [CrossRef]
- Teo, E.C.Y.; Tan, B.H.; Purdy, M.A.; Wong, P.S.; Ting, P.J.; Chang, P.E.J.; Oon, L.L.E.; Sue, A.; Teo, C.G.; Tan, C.K. Hepatitis E in Singapore: A case-series and viral phylodynamics study. Am. J. Trop. Med. Hyg. 2017, 96, 922–928. [Google Scholar] [CrossRef]
- Tan, L.T.; Tan, J.; Ang, L.W.; Chan, K.P.; Chiew, K.T.; Cutter, J.; Chew, S.K.; Goh, K.T. Epidemiology of acute hepatitis E in Singapore. J. Infect. 2013, 66, 453–459. [Google Scholar] [CrossRef]
- Zhu, Y.O.; Aw, P.; Aung, M.M.; Lee, H.K.; Hibberd, M.; Lee, G.H. Patterns of mutation within an emerging endemic lineage of HEV-3a. J. Viral. Hepat. 2019, 26, 191–198. [Google Scholar] [CrossRef]
- Yamada, H.; Takahashi, K.; Lim, O.; Svay, S.; Chuon, C.; Hok, S.; Do, S.H.; Fujimoto, M.; Akita, T.; Goto, N.; et al. Hepatitis E virus in Cambodia: Prevalence among the general population and complete genome sequence of genotype 4. PLoS ONE 2015, 10, e0136903. [Google Scholar] [CrossRef]
- Hudu, S.A.; Niazlin, M.T.; Nordin, S.A.; Harmal, N.S.; Tan, S.S.; Omar, H.; Shahar, H.; Mutalib, N.A.; Sekawi, Z. Hepatitis E virus isolated from chronic hepatitis B patients in Malaysia: Sequences analysis and genetic diversity suggest zoonotic origin. Alexandria J. Med. 2018, 54, 487–494. [Google Scholar] [CrossRef]
- Bouamra, Y.; Gerolami, R.; Arzouni, J.P.; Grimaud, J.C.; Lafforgue, P.; Nelli, M.; Tivoli, N.; Ferretti, A.; Motte, A.; Colson, P. Emergence of autochthonous infections with hepatitis E virus of genotype 4 in Europe. Intervirology 2014, 57, 43–48. [Google Scholar] [CrossRef]
- Wichmann, O.; Schimanski, S.; Koch, J.; Kohler, M.; Rothe, C.; Plentz, A.; Jilg, W.; Stark, K. Phylogenetic and case-control study on hepatitis E virus infection in Germany. J. Infect. Dis. 2008, 198, 1732–1741. [Google Scholar] [CrossRef]
- Hakze-van der Honing, R.W.; van Coillie, E.; Antonis, A.F.; van der Poel, W.H. First isolation of hepatitis E virus genotype 4 in Europe through swine surveillance in the Netherlands and Belgium. PLoS ONE 2011, 6, e22673. [Google Scholar] [CrossRef]
- Tesse, S.; Lioure, B.; Fornecker, L.; Wendling, M.J.; Stoll-Keller, F.; Bigaillon, C.; Nicand, E. Circulation of genotype 4 hepatitis E virus in Europe: First autochthonous hepatitis E infection in France. J. Clin. Virol. 2012, 54, 197–200. [Google Scholar] [CrossRef]
- Colson, P.; Romanet, P.; Moal, V.; Borentain, P.; Purgus, R.; Benezech, A.; Motte, A.; Gerolami, R. Autochthonous infections with hepatitis E virus genotype 4, France. Emerg. Infect. Dis. 2012, 18, 1361–1364. [Google Scholar] [CrossRef]
- Jeblaoui, A.; Haim-Boukobza, S.; Marchadier, E.; Mokhtari, C.; Roque-Afonso, A.M. Genotype 4 hepatitis E virus in France: An autochthonous infection with a more severe presentation. Clin. Infect. Dis. 2013, 57, e122–e126. [Google Scholar] [CrossRef]
- Colson, P.; Brunet, P.; Lano, G.; Moal, V. Hepatitis E virus genotype 4 in Southeastern France: Still around. Liver Int. 2016, 36, 765–767. [Google Scholar] [CrossRef]
- Garbuglia, A.R.; Scognamiglio, P.; Petrosillo, N.; Mastroianni, C.M.; Sordillo, P.; Gentile, D.; La Scala, P.; Girardi, E.; Capobianchi, M.R. Hepatitis E virus genotype 4 outbreak, Italy, 2011. Emerg. Infect. Dis. 2013, 19, 110–114. [Google Scholar] [CrossRef]
- Midgley, S.; Vestergaard, H.T.; Dalgaard, C.; Enggaard, L.; Fischer, T.K. Hepatitis E virus genotype 4, Denmark, 2012. Emerg. Infect. Dis. 2014, 20, 156–157. [Google Scholar] [CrossRef]
- Mikhailov, M.L.; Malinnikova, E.Y.; Kyuregyan, K.K.; Isaeva, O.V. A case of import of genotype 4 hepatitis E virus into Russia. Zh. Mikrobiol. Epidemiol. Immunobiol. 2016, 3, 64–69. [Google Scholar]
- Rolfe, K.J.; Curran, M.D.; Mangrolia, N.; Gelson, W.; Alexander, G.J.; L’Estrange, M.; Vivek, R.; Tedder, R.; Ijaz, S. First case of genotype 4 human hepatitis E virus infection acquired in India. J. Clin. Virol. 2010, 48, 58–61. [Google Scholar] [CrossRef]
- Jin, H.; Zhao, Y.; Zhang, X.; Wang, B.; Liu, P. Case-fatality risk of pregnant women with acute viral hepatitis type E: A systematic review and meta-analysis. Epidemiol. Infect. 2016, 144, 2098–2106. [Google Scholar] [CrossRef]
- Tabatabai, J.; Wenzel, J.J.; Soboletzki, M.; Flux, C.; Navid, M.H.; Schnitzler, P. First case report of an acute hepatitis E subgenotype 3c infection during pregnancy in Germany. J. Clin. Virol. 2014, 61, 170–172. [Google Scholar] [CrossRef]
- Anty, R.; Ollier, L.; Peron, J.M.; Nicand, E.; Cannavo, I.; Bongain, A.; Giordanengo, V.; Tran, A. First case report of an acute genotype 3 hepatitis E infected pregnant woman living in South-Eastern France. J. Clin. Virol. 2012, 54, 76–78. [Google Scholar] [CrossRef]
- Charre, C.; Ramiere, C.; Dumortier, J.; Abravanel, F.; Lhomme, S.; Gincul, R.; Scholtes, C. Chronic genotype 3 hepatitis E in pregnant woman receiving infliximab and azathioprine. Emerg. Infect. Dis. 2018, 24, 941–943. [Google Scholar] [CrossRef]
- Aikawa, T.; Yamagata, K.; Miyamoto, K.; Tsuda, F.; Takahashi, M.; Okamoto, H. A first case of pregnant woman who contracted infection of indigenous genotype 3 hepatitis E virus in Japan. Kanzo 2009, 50, 163–165. [Google Scholar] [CrossRef]
- Brayne, A.B.; Dearlove, B.L.; Lester, J.S.; Kosakovsky Pond, S.L.; Frost, S.D. Genotype-specific evolution of hepatitis E virus. J. Virol. 2017, 91, e02241-02216. [Google Scholar] [CrossRef]
- Mizuo, H.; Yazaki, Y.; Sugawara, K.; Tsuda, F.; Takahashi, M.; Nishizawa, T.; Okamoto, H. Possible risk factors for the transmission of hepatitis E virus and for the severe form of hepatitis E acquired locally in Hokkaido, Japan. J. Med. Virol. 2005, 76, 341–349. [Google Scholar] [CrossRef]
- Ohnishi, S.; Kang, J.H.; Maekubo, H.; Arakawa, T.; Karino, Y.; Toyota, J.; Takahashi, K.; Mishiro, S. Comparison of clinical features of acute hepatitis caused by hepatitis E virus (HEV) genotypes 3 and 4 in Sapporo, Japan. Hepatol. Res. 2006, 36, 301–307. [Google Scholar] [CrossRef]
- Geng, Y.; Zhang, H.; Huang, W.; Harrison, T.J.; Geng, K.; Li, Z.; Wang, Y. Persistent hepatitis E virus genotype 4 infection in a child with acute lymphoblastic leukemia. Hepat. Mon. 2014, 14, e15618. [Google Scholar] [CrossRef]
- Perumpail, R.B.; Ahmed, A.; Higgins, J.P.; So, S.K.; Cochran, J.L.; Drobeniuc, J.; Mixson-Hayden, T.R.; Teo, C.G. Fatal accelerated cirrhosis after imported HEV genotype 4 infection. Emerg. Infect. Dis. 2015, 21, 1679–1681. [Google Scholar] [CrossRef]
- Sridhar, S.; Chan, J.F.W.; Yap, D.Y.H.; Teng, J.L.L.; Huang, C.; Yip, C.C.Y.; Hung, I.F.N.; Tang, S.C.W.; Lau, S.K.P.; Woo, P.C.Y.; et al. Genotype 4 hepatitis E virus is a cause of chronic hepatitis in renal transplant recipients in Hong Kong. J. Viral. Hepat. 2018, 25, 209–213. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, G.; Pan, Q.; Zhao, J. Chronic hepatitis E in a renal transplant recipient: The first report of genotype 4 hepatitis E virus caused chronic infection in organ recipient. Gastroenterology 2018, 154, 1199–1201. [Google Scholar] [CrossRef]
- Wu, C.H.; Ho, C.M.; Tsai, J.H.; Sun, H.Y.; Hu, R.H.; Lee, P.H. First case genotype 4 hepatitis E infection after a liver transplant. Exp. Clin. Transplant. 2017, 15, 228–230. [Google Scholar]
- Sridhar, S.; Cheng, V.C.C.; Wong, S.C.; Yip, C.C.Y.; Wu, S.; Lo, A.W.I.; Leung, K.H.; Mak, W.W.N.; Cai, J.; Li, X.; et al. Donor-derived genotype 4 hepatitis E virus infection, Hong Kong, China, 2018. Emerg. Infect. Dis. 2019, 25, 425–433. [Google Scholar] [CrossRef]
- Kamar, N.; Garrouste, C.; Haagsma, E.B.; Garrigue, V.; Pischke, S.; Chauvet, C.; Dumortier, J.; Cannesson, A.; Cassuto-Viguier, E.; Thervet, E.; et al. Factors associated with chronic hepatitis in patients with hepatitis E virus infection who have received solid organ transplants. Gastroenterology 2011, 140, 1481–1489. [Google Scholar] [CrossRef]
- Debing, Y.; Ramiere, C.; Dallmeier, K.; Piorkowski, G.; Trabaud, M.A.; Lebosse, F.; Scholtes, C.; Roche, M.; Legras-Lachuer, C.; de Lamballerie, X.; et al. Hepatitis E virus mutations associated with ribavirin treatment failure result in altered viral fitness and ribavirin sensitivity. J. Hepatol. 2016, 65, 499–508. [Google Scholar] [CrossRef]
- Neukam, K.; Barreiro, P.; Macias, J.; Avellon, A.; Cifuentes, C.; Martin-Carbonero, L.; Echevarria, J.M.; Vargas, J.; Soriano, V.; Pineda, J.A. Chronic hepatitis E in HIV patients: Rapid progression to cirrhosis and response to oral ribavirin. Clin. Infect. Dis. 2013, 57, 465–468. [Google Scholar] [CrossRef]
- Haffar, S.; Shalimar; Kaur, R.J.; Wang, Z.; Prokop, L.J.; Murad, M.H.; Bazerbachi, F. Acute liver failure caused by hepatitis E virus genotype 3 and 4: A systematic review and pooled analysis. Liver Int. 2018, 38, 1965–1973. [Google Scholar] [CrossRef]
- Lhomme, S.; Garrouste, C.; Kamar, N.; Saune, K.; Abravanel, F.; Mansuy, J.M.; Dubois, M.; Rostaing, L.; Izopet, J. Influence of polyproline region and macro domain genetic heterogeneity on HEV persistence in immunocompromised patients. J. Infect. Dis. 2014, 209, 300–303. [Google Scholar] [CrossRef]
- Lorenzo, F.R.; Tanaka, T.; Takahashi, H.; Ichiyama, K.; Hoshino, Y.; Yamada, K.; Inoue, J.; Takahashi, M.; Okamoto, H. Mutational events during the primary propagation and consecutive passages of hepatitis E virus strain JE03–1760F in cell culture. Virus Res. 2008, 137, 86–96. [Google Scholar] [CrossRef]
- Okamoto, H. Hepatitis E virus cell culture models. Virus Res. 2011, 161, 65–77. [Google Scholar] [CrossRef] [PubMed]
- Nagashima, S.; Kobayashi, T.; Tanaka, T.; Tanggis; Jirintai, S.; Takahashi, M.; Nishizawa, T.; Okamoto, H. Analysis of adaptive mutations selected during the consecutive passages of hepatitis E virus produced from an infectious cDNA clone. Virus Res. 2016, 223, 170–180. [Google Scholar] [CrossRef]
- Jansen, R.W.; Newbold, J.E.; Lemon, S.M. Complete nucleotide sequence of a cell culture-adapted variant of hepatitis A virus: Comparison with wild-type virus with restricted capacity for in vitro replication. Virology 1988, 163, 299–307. [Google Scholar] [CrossRef]
- Meister, T.L.; Bruening, J.; Todt, D.; Steinmann, E. Cell culture systems for the study of hepatitis E virus. Antiviral Res. 2019, 163, 34–49. [Google Scholar] [CrossRef] [PubMed]
- Lara, J.; Purdy, M.A.; Khudyakov, Y.E. Genetic host specificity of hepatitis E virus. Infect. Genet. Evol. 2014, 24, 127–139. [Google Scholar] [CrossRef]
- Takahashi, K.; Okamoto, H.; Abe, N.; Kawakami, M.; Matsuda, H.; Mochida, S.; Sakugawa, H.; Suginoshita, Y.; Watanabe, S.; Yamamoto, K.; et al. Virulent strain of hepatitis E virus genotype 3, Japan. Emerg. Infect. Dis. 2009, 15, 704–709. [Google Scholar] [CrossRef]
- Borkakoti, J.; Ahmed, G.; Kar, P. Report of a novel C1483W mutation in the hepatitis E virus polymerase in patients with acute liver failure. Infect. Genet. Evol. 2016, 44, 51–54. [Google Scholar] [CrossRef]
- Pudupakam, R.S.; Huang, Y.W.; Opriessnig, T.; Halbur, P.G.; Pierson, F.W.; Meng, X.J. Deletions of hypervariable region (HVR) in open reading frame 1 of hepatitis E virus do not abolish virus infectivity: Evidence for attenuation of HVR deletion mutants in vivo. J. Virol. 2009, 83, 384–395. [Google Scholar] [CrossRef]
- Chen, Z.; Zhou, X.; Lunney, J.K.; Lawson, S.; Sun, Z.; Brown, E.; Christopher-Hennings, J.; Knudsen, D.; Nelson, E.; Fang, Y. Immunodominant epitopes in nsp2 of porcine reproductive and respiratory syndrome virus are dispensable for replication, but play an important role in modulation of the host immune response. J. Gen. Virol. 2010, 91, 1047–1057. [Google Scholar] [CrossRef]
- Eriksson, K.K.; Cervantes-Barragan, L.; Ludewig, B.; Thiel, V. Mouse hepatitis virus liver pathology is dependent on ADP-ribose-1”-phosphatase, a viral function conserved in the alpha-like supergroup. J. Virol. 2008, 82, 12325–12334. [Google Scholar] [CrossRef]
- Lhomme, S.; Abravanel, F.; Dubois, M.; Sandres-Saune, K.; Rostaing, L.; Kamar, N.; Izopet, J. Hepatitis E virus quasispecies and the outcome of acute hepatitis E in solid-organ transplant patients. J. Virol. 2012, 86, 10006–10014. [Google Scholar] [CrossRef]
- van Tong, H.; Hoan, N.X.; Wang, B.; Wedemeyer, H.; Bock, C.T.; Velavan, T.P. Hepatitis E virus mutations: Functional and clinical relevance. EBioMedicine 2016, 11, 31–42. [Google Scholar] [CrossRef]
- Todt, D.; Walter, S.; Brown, R.J.; Steinmann, E. Mutagenic effects of ribavirin on hepatitis E virus-viral extinction versus selection of fitness-enhancing mutations. Viruses 2016, 8, 283. [Google Scholar] [CrossRef]
- Todt, D.; Gisa, A.; Radonic, A.; Nitsche, A.; Behrendt, P.; Suneetha, P.V.; Pischke, S.; Bremer, B.; Brown, R.J.; Manns, M.P.; et al. In vivo evidence for ribavirin-induced mutagenesis of the hepatitis E virus genome. Gut 2016, 65, 1733–1743. [Google Scholar] [CrossRef]
- Purdy, M.A.; Khudyakov, Y.E. Evolutionary history and population dynamics of hepatitis E virus. PLoS ONE 2010, 5, e14376. [Google Scholar] [CrossRef]
- Fenaux, H.; Chassaing, M.; Berger, S.; Gantzer, C.; Bertrand, I.; Schvoere, E. Transmission of hepatitis E virus by water: An issue still pending in industrialized countries. Water Res. 2019, 151, 144–157. [Google Scholar] [CrossRef]
- Mirazo, S.; Mir, D.; Bello, G.; Ramos, N.; Musto, H.; Arbiza, J. New insights into the hepatitis E virus genotype 3 phylodynamics and evolutionary history. Infect. Genet. Evol. 2016, 43, 267–273. [Google Scholar] [CrossRef]

| Family | Genus | Species | Genotype | Host |
|---|---|---|---|---|
| Hepeviridae | Orthohepevirus | Orthohepevirus A | 1 | human |
| 2 | human | |||
| 3 | human, pig, wild boar, deer, mongoose, rabbit, goat a, horse b, bottlenose dolphin c, sheep d | |||
| 4 | human, pig, wild boar, cattle e, cow f, sheep g, goat h, yak i | |||
| 5 | wild boar | |||
| 6 | wild boar | |||
| 7 | dromedary camel | |||
| 8 | Bactrian camel | |||
| Orthohepevirus B | chicken | |||
| Orthohepevirus C | C1 | rat, greater bandicoot rat j, Asian musk shrew k | ||
| C2 | ferret, mink l | |||
| Orthohepevirus D | bat | |||
| Piscihepevirus | Piscihepevirus A | cutthroat trout |
| Nucleotide Position | Region | Nucleotide | Amino Acid | ||||
|---|---|---|---|---|---|---|---|
| JE03-1760F/wt | p10f/A (Feces-Derived) | p10f/B (Feces-Derived) | p10c (cDNA-Derived) | Position | Substitution | ||
| 22 | 5’UTR | U | A | U | U | NA a | - |
| 61 | ORF1 (MeT) | U | U | C | U | 12 | - |
| 370 | ORF1 (MeT) | C | U | C | C | 115 | - |
| 445 | ORF1 (MeT) | U | U | C | U | 140 | - |
| 591 | ORF1 (MeT) | C | U | C | C | 189 | Ala to Val |
| 829 | ORF1 (Y) | C | C | U | C | 268 | - |
| 1213 | ORF1 (Y) | C | C | C | U | 396 | - |
| 1378 | ORF1 (PCP) | C | C | U | C | 451 | - |
| 1549 | ORF1 (PCP) | U | U | C | U | 508 | - |
| 2191 | ORF1 (HVR) | C | C | U | C | 722 | - |
| 2236 | ORF1 (HVR) | C | C | U | C | 737 | - |
| 2246 | ORF1 (HVR) | U | C | C | U | 741 | Trp to Arg |
| 2557 | ORF1 (X) | U | U | U | C | 844 | - |
| 2704 | ORF1 (X) | U | C | U | U | 893 | - |
| 2808 | ORF1 (X) | U | U | C | C | 928 | Val to Ala |
| 2913 | ORF1 (Hel) | A | A | G | A | 963 | Glu to Gly |
| 2915 | ORF1(Hel) | G | G | U | G | 964 | Val to Leu |
| 2938 | ORF1 (Hel) | C | U | C | C | 971 | - |
| 3106 | ORF1 (Hel) | A | G | A | A | 1027 | - |
| 3118 | ORF1 (Hel) | C | C | C | U | 1031 | - |
| 3223 | ORF1 (Hel) | U | U | C | U | 1066 | - |
| 3235 | ORF1 (Hel) | C | U | C | C | 1070 | - |
| 3453 | ORF1 (Hel) | C | U | C | C | 1143 | Ala to Val |
| 3475 | ORF1 (Hel) | C | C | U | C | 1150 | - |
| 3496 | ORF1 (Hel) | C | U | C | C | 1157 | - |
| 4015 | ORF1 (RdRp) | C | U | C | C | 1330 | - |
| 4309 | ORF1 (RdRp) | C | C | U | C | 1428 | - |
| 4435 | ORF1 (RdRp) | C | C | C | U | 1470 | - |
| 4462 | ORF1 (RdRp) | C | U | C | C | 1479 | - |
| 5054 | ORF1 (RdRp) | A | A | A | G | 1677 | Ile to Val |
| 5312 | ORF2 | U | U | C | U | 47 | - |
| ORF3 | 51 | Ile to Thr | |||||
| 5378 | ORF2 | A | G | G | A | 69 | - |
| ORF3 | 73 | Asn to Ser | |||||
| 5456 | ORF2 | C | U | U | C | 95 | - |
| ORF3 | 99 | Pro to Leu | |||||
| 6047 | ORF2 | U | U | C | U | 292 | - |
| 6470 | ORF2 | C | U | C | C | 433 | - |
| 6578 | ORF2 | C | U | C | C | 469 | - |
| 6611 | ORF2 | C | U | C | C | 480 | - |
| 6626 | ORF2 | U | C | U | U | 485 | - |
| 6652 | ORF2 | U | U | C | U | 494 | Val to Ala |
| 6855 | ORF2 | A | A | G | A | 562 | Asn to Asp |
| 6944 | ORF2 | U | U | C | U | 591 | - |
| 7186 | 3’UTR | C | C | U | C | NA a | - |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Primadharsini, P.P.; Nagashima, S.; Okamoto, H. Genetic Variability and Evolution of Hepatitis E Virus. Viruses 2019, 11, 456. https://doi.org/10.3390/v11050456
Primadharsini PP, Nagashima S, Okamoto H. Genetic Variability and Evolution of Hepatitis E Virus. Viruses. 2019; 11(5):456. https://doi.org/10.3390/v11050456
Chicago/Turabian StylePrimadharsini, Putu Prathiwi, Shigeo Nagashima, and Hiroaki Okamoto. 2019. "Genetic Variability and Evolution of Hepatitis E Virus" Viruses 11, no. 5: 456. https://doi.org/10.3390/v11050456





