The Sequence of Two Bacteriophages with Hypermodified Bases Reveals Novel Phage-Host Interactions
Abstract
:1. Introduction
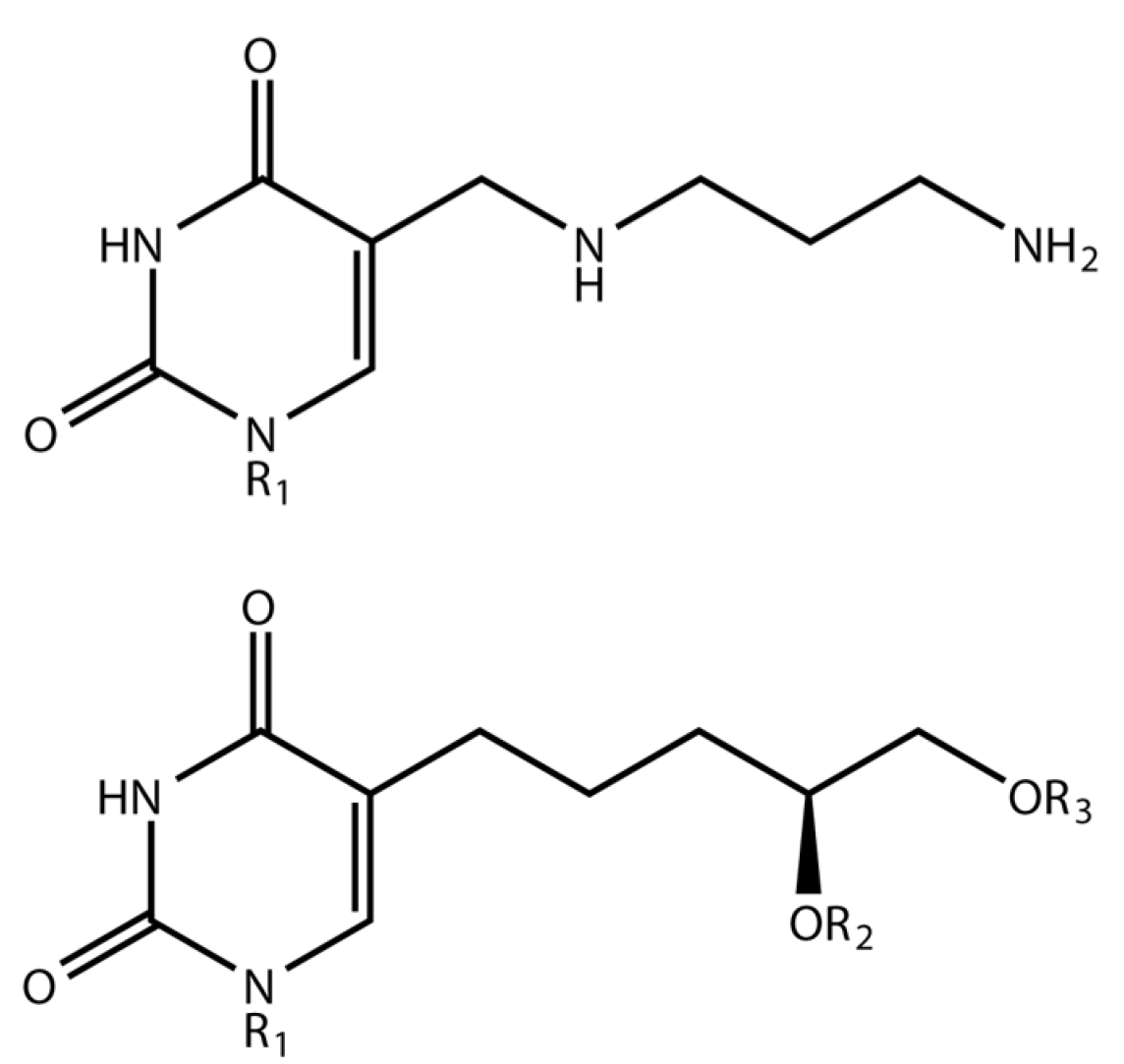
1.1. Delftia Phage ΦW-14
1.2. Bacillus Phage SP-15
2. Materials and Methods
2.1. Host and Phages
2.2. Propagation
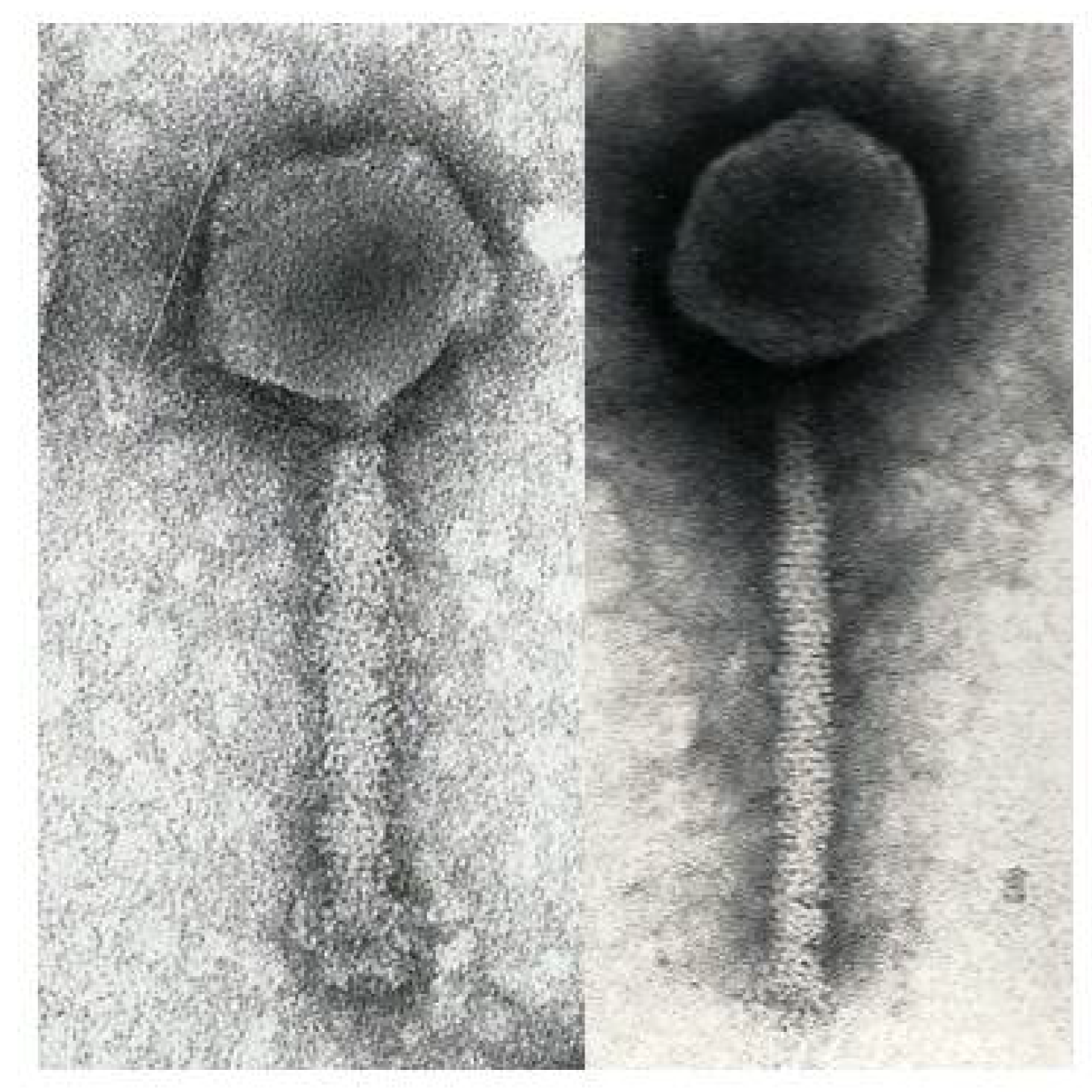
2.3. Electron Microscopy
2.4. DNA Sequencing, Sequence Assembly and Annotation
2.5. GenBank
2.6. Diagrams
3. Results
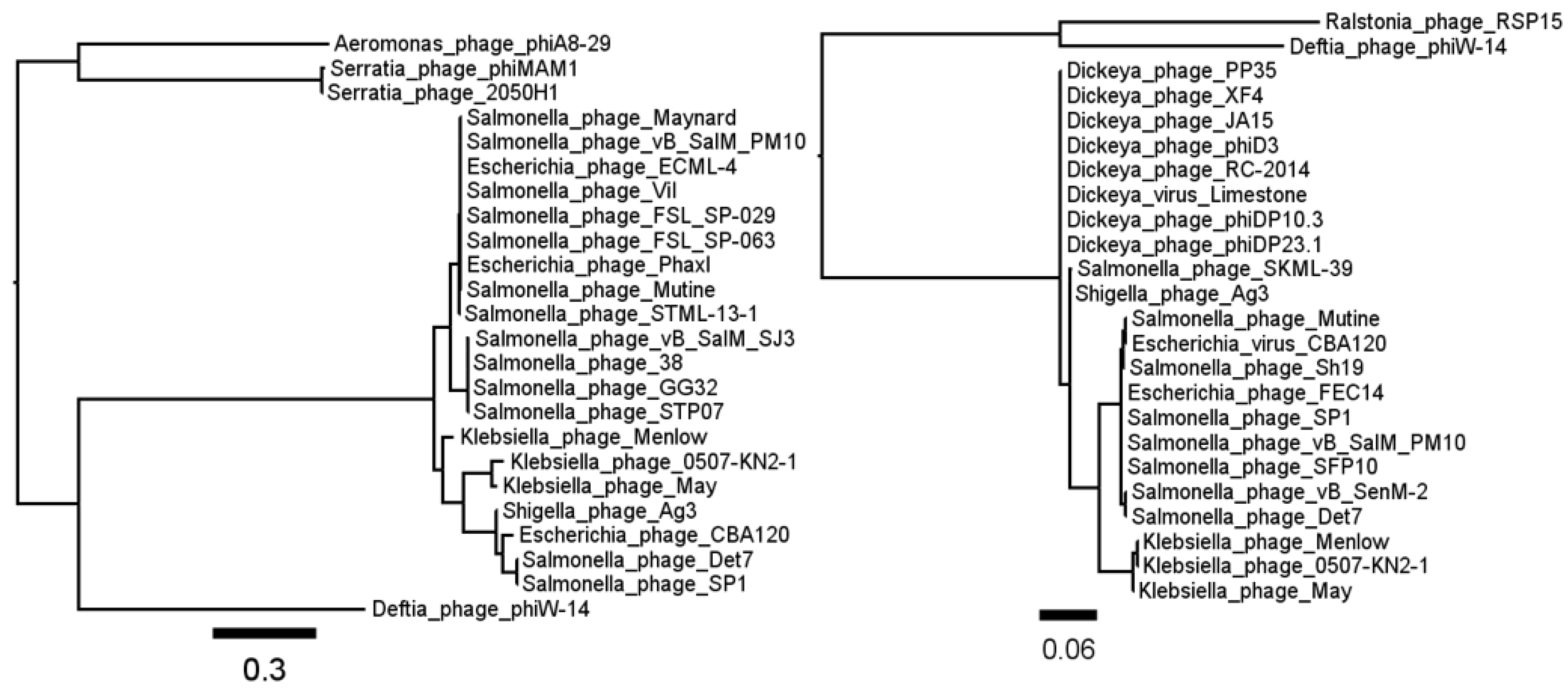
3.1. Phage ΦW-14
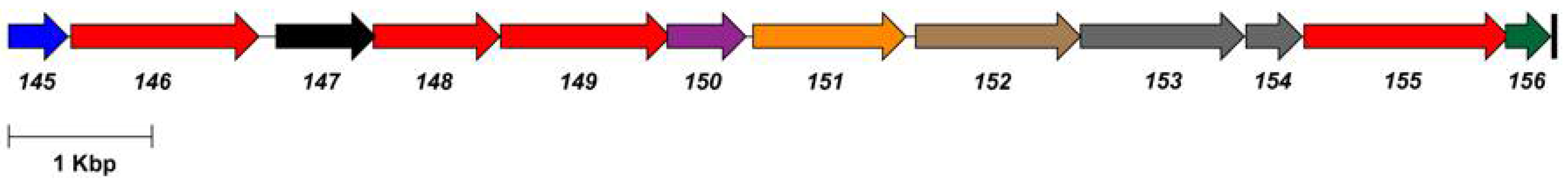
3.2. Phage SP-15
Supplementary Materials
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Mandel, M.; Igambi, L.; Bergendahl, J.; Dodson, M.L., Jr.; Scheltgen, E. Correlation of melting temperature and cesium chloride buoyant density of bacterial deoxyribonucleic acid. J. Bacteriol. 1970, 101, 333–338. [Google Scholar] [PubMed]
- Mandel, M.; Marmur, J. Use of ultraviolet absorbance-temperature profile for determining the guanine plus cytosine content of DNA. Methods Enzymol. 1968, 12, 195–206. [Google Scholar]
- Marmur, J.; Doty, P. Determination of the base composition of deoxyribonucleic acid from its thermal denaturation temperature. J. Mol. Biol. 1962, 5, 109–118. [Google Scholar] [CrossRef]
- Kiljunen, S.; Hakala, K.; Pinta, E.; Huttunen, S.; Pluta, P.; Gador, A.; Lonnberg, H.; Skurnik, M. Yersiniophage phiR1-37 is a tailed bacteriophage having a 270 kb DNA genome with thymidine replaced by deoxyuridine. Microbiology 2005, 151, 4093–4102. [Google Scholar] [CrossRef] [PubMed]
- Lopez, P.; Espinosa, M.; Piechowska, M.; Shugar, D. Influence of bacteriophage PBS1 and ΦW-14 deoxyribonucleic acids on homologous deoxyribonucleic acid uptake and transformation in competent Bacillus subtilis. J. Bacteriol. 1980, 143, 50–58. [Google Scholar] [PubMed]
- Parker, M.L.; Ralston, E.J.; Eiserling, F.A. Bacteriophage SPO1 structure and morphogenesis. II. Head structure and DNA size. J. Virol. 1983, 46, 250–259. [Google Scholar] [PubMed]
- Okubo, S.; Strauss, B.; Stodolsky, M. The possible role of recombination in the infection of competent Bacillus subtilis by bacteriophage deoxyribonucleic acid. Virology 1964, 24, 552–562. [Google Scholar] [CrossRef]
- Hoet, P.; Coene, M.; Cocito, C. Comparison of the physical maps and redundant ends of the chromosomes of phages 2C, SP01, SP82 and phi e. Eur. J. Biochem. 1983, 132, 63–67. [Google Scholar] [CrossRef] [PubMed]
- Yee, L.M.; Matsumoto, T.; Yano, K.; Matsuoka, S.; Sadaie, Y.; Yoshikawa, H.; Asai, K. The genome of Bacillus subtilis phage SP10: A comparative analysis with phage SPO1. Biosci. Biotechnol. Biochem. 2011, 75, 944–952. [Google Scholar] [CrossRef] [PubMed]
- Ehrlich, M.; Ehrlich, K.; Mayo, J.A. Unusual properties of the DNA from Xanthomonas phage XP-12 in which 5-methylcytosine completely replaces cytosine. Biochim. Biophys. Acta 1975, 395, 109–119. [Google Scholar] [CrossRef]
- Kuo, T.T.; Huang, T.C.; Wu, R.Y.; Chen, C.P. Phage Xp12 of Xanthomonas oryzae (Uyeda et Ishiyama) Dowson. Can. J. Microbiol. 1968, 14, 1139–1142. [Google Scholar] [CrossRef] [PubMed]
- Wyatt, G.R.; Cohen, S.S. The bases of the nucleic acids of some bacterial and animal viruses: The occurrence of 5-hydroxymethylcytosine. Biochem. J. 1953, 55, 774–782. [Google Scholar] [CrossRef] [PubMed]
- Wyatt, G.R.; Cohen, S.S. The base of the desoxyribonucleic acids of T2, T4, and T6 bacteriophages. Annales de l’Institut Pasteur 1953, 84, 143–146. [Google Scholar] [PubMed]
- Swinton, D.; Hattman, S.; Benzinger, R.; Buchanan-Wollaston, V.; Beringer, J. Replacement of the deoxycytidine residues in Rhizobium bacteriophage RL38JI DNA. FEBS Lett. 1985, 184, 294–298. [Google Scholar] [CrossRef]
- Khudyakov, I.Y.; Kirnos, M.D.; Alexandrushkina, N.I.; Vanyushin, B.F. Cyanophage S-2L contains DNA with 2,6-diaminopurine substituted for adenine. Virology 1978, 88, 8–18. [Google Scholar] [CrossRef]
- Miller, P.B.; Wakarchuk, W.W.; Warren, R.A. alpha-Putrescinylthymine and the sensitivity of bacteriophage ΦW-14 DNA to restriction endonucleases. Nucleic Acids Res. 1985, 13, 2559–2568. [Google Scholar] [CrossRef] [PubMed]
- Warren, R.A.J. Modified bases in bacteriophage DNA. Annu. Rev. Microbiol. 1980, 34, 137–158. [Google Scholar] [CrossRef] [PubMed]
- Tsai, R.; Correa, I.R.; Xu, M.Y.; Xu, S.Y. Restriction and modification of deoxyarchaeosine (dG(+))-containing phage 9 g DNA. Sci. Rep. 2017, 7, 8348. [Google Scholar] [CrossRef] [PubMed]
- Cao, X.M.; Huang, L.H.; Farnet, C.M.; Ehrlich, M. Ligation of highly modified bacteriophage DNA. Biochim. Biophys. Acta 1983, 741, 237–243. [Google Scholar] [CrossRef]
- Weigele, P.; Raleigh, E.A. Biosynthesis and Function of Modified Bases in Bacteria and Their Viruses. Chem. Rev. 2016, 116, 12655–12687. [Google Scholar] [CrossRef] [PubMed]
- Gommers-Ampt, J.H.; Borst, P. Hypermodified bases in DNA. FASEB J. 1995, 9, 1034–1042. [Google Scholar] [CrossRef] [PubMed]
- Stanier, R.Y.; Palleroni, N.J.; Doudoroff, M. The aerobic pseudomonads: A taxonomic study. J. Gen. Microbiol. 1966, 43, 159–271. [Google Scholar] [CrossRef] [PubMed]
- Kropinski, A.M.B. Isolation, and Characterization of a Bacteriophage Against Pseudomonas Acidovorans. Master’s Thesis, University of British Columbia, Vancouver, BC, Canada, 1969. [Google Scholar]
- Scraba, D.G.; Bradley, R.D.; Leyritz-Wills, M.; Warren, R.A. Bacteriophage ΦW-14: The contribution of covalently bound putrescine to DNA packing in the phage head. Virology 1983, 124, 152–160. [Google Scholar] [CrossRef]
- Abedon, S.T. Disambiguating bacteriophage pseudolysogeny: An historical analysis of lysogeny, pseudolysogeny, and the phage carrier state. In Contemporary Trends in Bacteriophage Research; Adams, H.T., Ed.; Nova Science Publishers, Inc.: New York, NY, USA, 2009; pp. 285–307. [Google Scholar]
- Kropinski, A.M.B. The Physico-Chemical Properties of Bacteriophage ΦW-14 Deoxyribonucleic Acid. Ph.D. Thesis, University of British Columbia, Vancouver, BC, Canada, 1973. [Google Scholar]
- Kropinski, A.M.; Bose, R.J.; Warren, R.A. 5-(4-Aminobutylaminomethyl)uracil, an unusual pyrimidine from the deoxyribonucleic acid of bacteriophage ΦW-14. Biochemistry 1973, 12, 151–157. [Google Scholar] [CrossRef] [PubMed]
- Maltman, K.L.; Neuhard, J.; Lewis, H.A.; Warren, R.A. Synthesis of thymine and alpha-putrescinylthymine in bacteriophage ΦW-14-infected Pseudomonas acidovorans. J. Virol. 1980, 34, 354–359. [Google Scholar] [PubMed]
- Kelln, R.A.; Warren, R.A. Studies on the biosynthesis of alpha-putrescinylthymine in bacteriophage ΦW-14-infected Pseudomonas acidovorans. J. Virol. 1973, 12, 1427–1433. [Google Scholar] [PubMed]
- Neuhard, J.; Maltman, K.L.; Warren, R.A. Bacteriophage ΦW-14-infected Pseudomonas acidovorans synthesizes hydroxymethyldeoxyuridine triphosphate. J. Virol. 1980, 34, 347–353. [Google Scholar] [PubMed]
- Quail, A.; Karrer, E.; Warren, R.A. Polyamines in bacteriophage ΦW-14 and in ΦW-14-infected Pseudomonas acidovorans. J. Gen. Virol. 1976, 33, 135–138. [Google Scholar] [CrossRef] [PubMed]
- Miller, P.B.; Maltman, K.L.; Warren, R.A. Isolation and preliminary characterization of amber mutants of bacteriophage ΦW-14 defective in DNA synthesis. J. Virol. 1982, 43, 67–72. [Google Scholar] [PubMed]
- Maltman, K.L.; Neuhard, J.; Warren, R.A. 5-[(Hydroxymethyl)-O-pyrophosphoryl]uracil, an intermediate in the biosynthesis of alpha-putrescinylthymine in deoxyribonucleic acid of bacteriophage ΦW-14. Biochemistry 1981, 20, 3586–3591. [Google Scholar] [CrossRef] [PubMed]
- Karrer, E.; Bose, R.J.; Warren, R.A. Polyamines of Pseudomonas acidovorans. J. Bacteriol. 1973, 114, 1365–1366. [Google Scholar] [PubMed]
- Bruce, D.L.; Warren, R.A.J. The pH-dependent uptake of putrescine by Pseudomonas acidovorans and its incorporation into bacteriophage ΦW-14 DNA. Can. J. Microbiol. 1983, 29, 827–829. [Google Scholar] [CrossRef]
- Lewis, H.A.; Miller, R.C., Jr.; Stone, J.C.; Warren, R.A. Alkali lability of bacteriophage ΦW-14 DNA. J. Virol. 1975, 16, 1375–1379. [Google Scholar] [PubMed]
- Gerhard, B.; Warren, R.A. Reactivity of the alpha-putrescinylthymine amino groups in ΦW-14 deoxyribonucleic acid. Biochemistry 1982, 21, 5458–5622. [Google Scholar] [CrossRef] [PubMed]
- Taylor, M.J.; Thorne, C.B. Transduction of Bacillus licheniformis and Bacillus subtilis by each of two phages. J. Bacteriol. 1963, 86, 452–461. [Google Scholar] [PubMed]
- Marmur, J.; Brandon, C.; Neubort, S.; Ehrlich, M.; Mandel, M.; Konvicka, J. Unique properties of nucleic acid from Bacillus subtilis phage SP-15. Nat. New Biol. 1972, 239, 68–70. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, H.; Nakanishi, K.; Brandon, C.; Marmur, J. Structure and synthesis of dihydroxypentyluracil from bacteriophage SP-15 deoxyribonucleic acid. J. Am. Chem. Soc. 1973, 95, 8749–8757. [Google Scholar] [CrossRef] [PubMed]
- Brandon, C.; Gallop, P.M.; Marmur, J.; Hayashi, H.; Nakanishi, K. Structure of a new pyrimidine from Bacillus subtilis phage SP-15 nucleic acid. Nat. New Biol. 1972, 239, 70–71. [Google Scholar] [CrossRef] [PubMed]
- Walker, M.S.; Mandel, M. Incorporation of label from ribose into 5-(4′,5′-dihydroxypentyl) uracil of bacteriophage SP15 DNA. J. Virol. 1978, 25, 695–697. [Google Scholar] [PubMed]
- Walker, M.S.; Mandel, M. Biosynthesis of 5-(4′5′-dihydroxypentyl) uracil as a nucleoside triphosphate in bacteriophage SP15-infected Bacillus subtilis. J. Virol. 1978, 25, 500–509. [Google Scholar] [PubMed]
- Neubort, S.; Marmur, J. Synthesis of the unusual DNA of Bacillus subtilis bacteriophage SP-15. J. Virol. 1973, 12, 1078–1084. [Google Scholar] [PubMed]
- Ehrlich, M.; Ehrlich, K.C. A novel, highly modified, bacteriophage DNA in which thymine is partly replaced by a phosphoglucuronate moiety covalently bound to 5-(4′,5′-dihydroxypentyl)uracil. J. Biol. Chem. 1981, 256, 9966–9972. [Google Scholar] [PubMed]
- Heather, J.M.; Chain, B. The sequence of sequencers: The history of sequencing DNA. Genomics 2016, 107, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Siqueira, J.F., Jr.; Fouad, A.F.; Rocas, I.N. Pyrosequencing as a tool for better understanding of human microbiomes. J. Oral Microbiol. 2012, 4, 10743. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, K.R.; Alberts, B.M.; Benzinger, R.; Lawhorne, L.; Treiber, G. Rapid bacteriophage sedimentation in the presence of polyethylene glycol and its application to large-scale virus purification. Virology 1970, 40, 734–744. [Google Scholar] [CrossRef]
- Sambrook, J.; Russell, D.W. Molecular Cloning: A Laboratory Manual, 3rd ed.; Cold Spring Harbor Press: Cold Spring Harbor, NY, USA, 2001; Volme 1. [Google Scholar]
- Ackermann, H.W. Basic phage electron microscopy. Methods Mol. Biol. 2009, 501, 113–126. [Google Scholar] [PubMed]
- Aziz, R.K.; Devoid, S.; Disz, T.; Edwards, R.A.; Henry, C.S.; Olsen, G.J.; Olson, R.; Overbeek, R.; Parrello, B.; Pusch, G.D.; et al. SEED servers: High-performance access to the SEED genomes, annotations, and metabolic models. PLoS ONE 2012, 7, e48053. [Google Scholar] [CrossRef] [PubMed]
- Aziz, R.K.; Ackermann, H.W.; Petty, N.K.; Kropinski, A.M. Essential Steps in Characterizing Bacteriophages: Biology, Taxonomy, and Genome Analysis. Methods Mol. Biol. 2018, 1681, 197–215. [Google Scholar] [PubMed]
- Altschul, S.F.; Madden, T.L.; Schaffer, A.A.; Zhang, J.; Zhang, Z.; Miller, W.; Lipman, D.J. Gapped BLAST and PSI-BLAST: A new generation of protein database search programs. Nucleic Acids Res. 1997, 25, 3389–4022. [Google Scholar] [CrossRef] [PubMed]
- Jones, P.; Binns, D.; Chang, H.Y.; Fraser, M.; Li, W.; McAnulla, C.; McWilliam, H.; Maslen, J.; Mitchell, A.; Nuka, G.; et al. InterProScan 5: Genome-scale protein function classification. Bioinformatics 2014, 30, 1236–1240. [Google Scholar] [CrossRef] [PubMed]
- Hildebrand, A.; Remmert, M.; Biegert, A.; Soding, J. Fast and accurate automatic structure prediction with HHpred. Proteins 2009, 77 (Suppl. S9), 128–132. [Google Scholar] [CrossRef] [PubMed]
- Kropinski, A.M.; Borodovsky, M.; Carver, T.J.; Cerdeno-Tarraga, A.M.; Darling, A.; Lomsadze, A.; Mahadevan, P.; Stothard, P.; Seto, D.; Van, D.G.; et al. In silico identification of genes in bacteriophage DNA. Methods Mol. Biol. 2009, 502, 57–89. [Google Scholar] [PubMed]
- Turner, D.; Reynolds, D.; Seto, D.; Mahadevan, P. CoreGenes3.5: A webserver for the determination of core genes from sets of viral and small bacterial genomes. BMC Res. Notes 2013, 6, 140–146. [Google Scholar] [CrossRef] [PubMed]
- Dereeper, A.; Guignon, V.; Blanc, G.; Audic, S.; Buffet, S.; Chevenet, F.; Dufayard, J.F.; Guindon, S.; Lefort, V.; Lescot, M.; et al. Phylogeny.fr: Robust phylogenetic analysis for the non-specialist. Nucleic Acids Res. 2008, 36, W465–W469. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Drummond, A.J.; Suchard, M.A.; Xie, D.; Rambaut, A. Bayesian phylogenetics with BEAUti and the BEAST 1.7. Mol. Biol. Evolut. 2012, 29, 1969–1973. [Google Scholar] [CrossRef] [PubMed]
- Grant, J.R.; Arantes, A.S.; Stothard, P. Comparing thousands of circular genomes using the CGView Comparison Tool. BMC Genom. 2012, 13, 202–213. [Google Scholar] [CrossRef] [PubMed]
- Stothard, P.; Grant, J.R.; Van Domselaar, G. Visualizing and comparing circular genomes using the CGView family of tools. Brief. Bioinform. 2017. [Google Scholar] [CrossRef] [PubMed]
- Evans, D.A. History of the Harvard ChemDraw project. Angew. Chem. Int. Ed. Engl. 2014, 53, 11140–11145. [Google Scholar] [CrossRef] [PubMed]
- Sullivan, M.J.; Petty, N.K.; Beatson, S.A. Easyfig: A genome comparison visualizer. Bioinformatics 2011, 27, 1009–1010. [Google Scholar] [CrossRef] [PubMed]
- Simoliunas, E.; Kaliniene, L.; Truncaite, L.; Zajanckauskaite, A.; Staniulis, J.; Kaupinis, A.; Ger, M.; Valius, M.; Meskys, R. Klebsiella phage vB_KleM-RaK2—A giant singleton virus of the family Myoviridae. PLoS ONE 2013, 8, e60717. [Google Scholar] [CrossRef] [PubMed]
- Petrov, V.M.; Ratnayaka, S.; Nolan, J.M.; Miller, E.S.; Karam, J.D. Genomes of the T4-related bacteriophages as windows on microbial genome evolution. Virol. J. 2010, 7, 292. [Google Scholar] [CrossRef] [PubMed]
- Adriaenssens, E.M.; Wittmann, J.; Kuhn, J.H.; Turner, D.; Sullivan, M.B.; Dutilh, B.E.; Jang, H.B.; van Zyl, L.J.; Klumpp, J.; Lobocka, M.; et al. Taxonomy of prokaryotic viruses: 2017 update from the ICTV Bacterial and Archaeal Viruses Subcommittee. Arch. Virol. 2018. [Google Scholar] [CrossRef] [PubMed]
- Adriaenssens, E.M.; Ackermann, H.W.; Anany, H.; Blasdel, B.; Connerton, I.F.; Goulding, D.; Griffiths, M.W.; Hooton, S.P.; Kutter, E.M.; Kropinski, A.M.; et al. A suggested new bacteriophage genus: “Viunalikevirus”. Arch. Virol. 2012, 157, 2035–2046. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.J.; Dai, N.; Walsh, S.E.; Muller, S.; Fraser, M.E.; Kauffman, K.M.; Guan, C.; Correa, I.R., Jr.; Weigele, P.R. Identification and biosynthesis of thymidine hypermodifications in the genomic DNA of widespread bacterial viruses. Proc. Natl. Acad. Sci. USA 2018, 115, E3116–E3125. [Google Scholar] [CrossRef] [PubMed]
- Magill, D.J.; Krylov, V.N.; Shaburova, O.V.; McGrath, J.W.; Allen, C.C.R.; Quinn, J.P.; Kulakov, L.A. Pf16 and phiPMW: Expanding the realm of Pseudomonas putida bacteriophages. PLoS ONE 2017, 12, e0184307. [Google Scholar] [CrossRef] [PubMed]
- Adriaenssens, E.M.; Mattheus, W.; Cornelissen, A.; Shaburova, O.; Krylov, V.N.; Kropinski, A.M.; Lavigne, R. Complete genome sequence of the giant Pseudomonas phage Lu11. J. Virol. 2012, 86, 6369–6370. [Google Scholar] [CrossRef] [PubMed]
- Shaburova, O.V.; Hertveldt, K.; de la Crus, D.M.; Krylov, S.V.; Pleteneva, E.A.; Burkaltseva, M.V.; Lavigne, R.; Volcaert, G.; Krylov, V.N. Comparison of new giant bacteriophages OBP and Lu11 of soil pseudomonads with bacteriophages of phiKZ-supergroup of Pseudomonas aeruginosa. Genetika 2006, 42, 1065–1074. [Google Scholar] [PubMed]
- Lavigne, R.; KU Leuven, Leuven, Belgium. Personal communication, 2014.
- Tyeryar, F.J., Jr.; Taylor, M.J.; Lawton, W.D.; Goldberg, I.D. Cotransduction and cotransformation of genetic markers in Bacillus subtilis and Bacillus licheniformis. J. Bacteriol. 1969, 100, 1027–1036. [Google Scholar] [PubMed]
- EMBL-EBI. Family: Thy1 (PF02511) Summary: Thymidylate Synthase Complementing Protein. Available online: http://pfam.xfam.org/family/PF02511 (accessed on 21 April 2018).
- Finn, R.D.; Coggill, P.; Eberhardt, R.Y.; Eddy, S.R.; Mistry, J.; Mitchell, A.L.; Potter, S.C.; Punta, M.; Qureshi, M.; Sangrador-Vegas, A.; et al. The Pfam protein families database: Towards a more sustainable future. Nucleic Acids Res. 2016, 44, D279–D285. [Google Scholar] [CrossRef] [PubMed]
- Burley, S.K.; Berman, H.M.; Christie, C.; Duarte, J.M.; Feng, Z.; Westbrook, J.; Young, J.; Zardecki, C. RCSB Protein Data Bank: Sustaining a living digital data resource that enables breakthroughs in scientific research and biomedical education. Protein Sci. 2018, 27, 316–330. [Google Scholar] [CrossRef] [PubMed]
- RCSB PDB. 4GT9 T. Maritima FDTS with FAD, dUMP and Folate. Available online: https://www.rcsb.org/structure/4gt9 (accessed on 21 April 2018).
- Deng, L.; Anderson, J.S. Biosynthesis of teichuronic acid in the bacterial cell wall. Purification and characterization of the glucosyltransferase of Micrococcus luteus. J. Biol. Chem. 1997, 272, 479–485. [Google Scholar] [CrossRef] [PubMed]
- Deng, L.L.; Alexander, A.A.; Lei, S.; Anderson, J.S. The Cell Wall Teichuronic Acid Synthetase (TUAS) Is an Enzyme Complex Located in the Cytoplasmic Membrane of Micrococcus luteus. Biochem. Res. Int. 2010, 2010, 395758. [Google Scholar] [CrossRef] [PubMed]
- Hatfull, G.F.; Hendrix, R.W. Bacteriophages and their genomes. Curr. Opin. Virol. 2011, 1, 298–303. [Google Scholar] [CrossRef] [PubMed]
- Mann, N.H.; Cook, A.; Millard, A.; Bailey, S.; Clokie, M. Marine ecosystems: Bacterial photosynthesis genes in a virus. Nature 2003, 424, 741. [Google Scholar] [CrossRef] [PubMed]
- Hargreaves, K.R.; Kropinski, A.M.; Clokie, M.R. What does the talking?: Quorum sensing signalling genes discovered in a bacteriophage genome. PLoS ONE 2014, 9, e85131. [Google Scholar] [CrossRef] [PubMed]
- Casella, E.; Markewych, O.; Dosmar, M.; Witmer, H. Production and expression of dTMP-enriched DNA of bacteriophage SP15. J. Virol. 1978, 28, 753–766. [Google Scholar] [PubMed]
- Kelleher, P.; Murphy, J.; Mahony, J.; van Sinderen, D. Identification of DNA Base Modifications by Means of Pacific Biosciences RS Sequencing Technology. Methods Mol. Biol. 2018, 1681, 127–137. [Google Scholar] [PubMed]

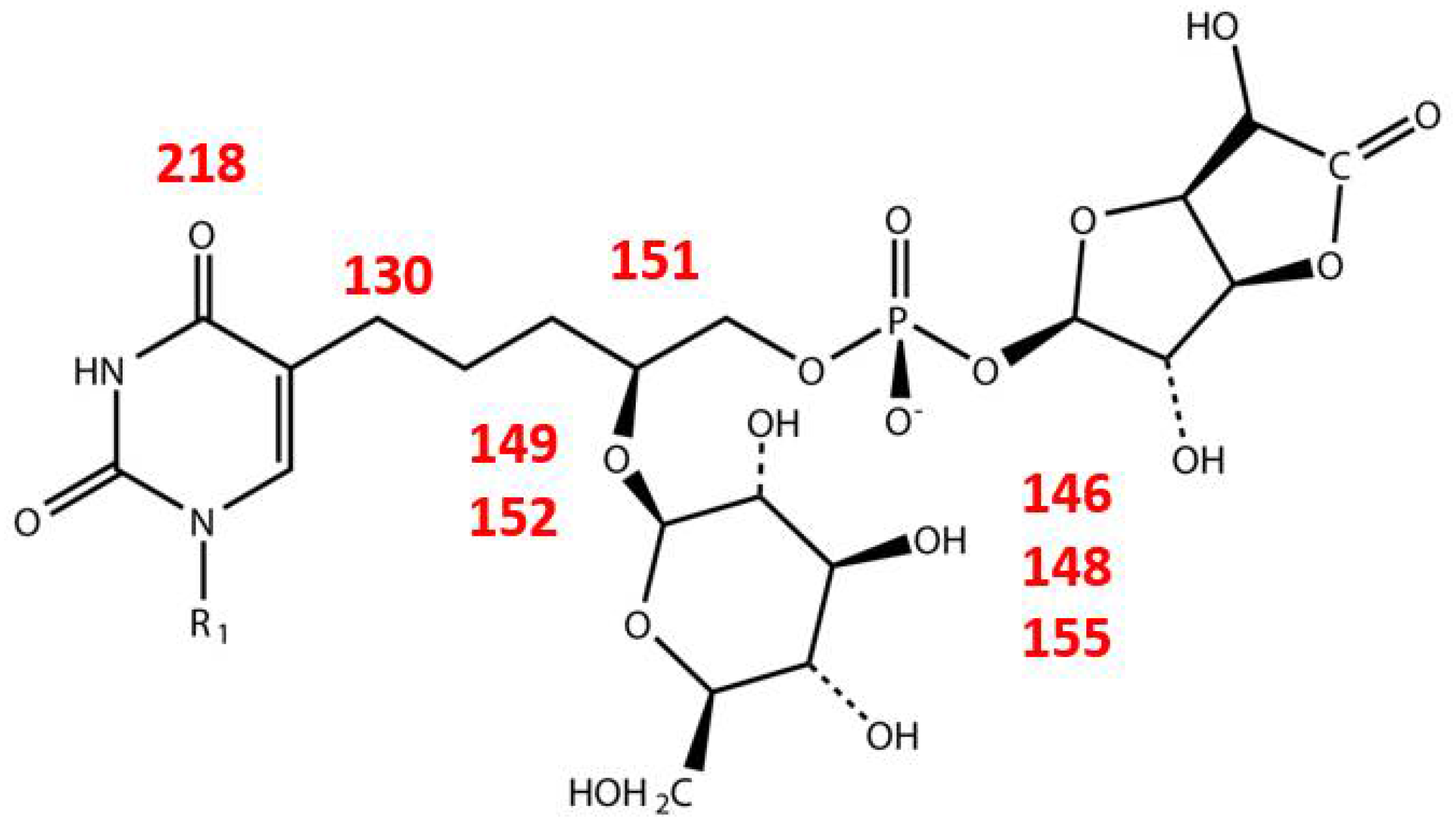
| Phage | Host | Substitution | Reference |
|---|---|---|---|
| ΦR1-37 | Yersinia | Thy → Ura | [4] |
| PBS1 | Bacillus | Thy → Ura | [5] |
| SPO1, SP8, SP10 | Bacillus | Thy → 5HmUra (a portion becomes α-glutamylthymine) | [6,7,8,9] |
| XP-12 | Xanthomonas | Cyt → 5MeCyt | [10,11] |
| Teven phages | Escherichia | Cyt → 5HmCyt (glycosylated) | [12,13] |
| RL38JI | Rhizobium | Cyt → 5HmCyt (variably glycosylated) | [14] |
| S-2L | Synechococcus | Ade → 2AminoAde | [15] |
| Gene | Product | Function |
|---|---|---|
| 11 | glucose-6-phosphate isomerase | Glc-6-P → Fru-6-P |
| 129 | acyl carrier protein reductase | |
| 146 | UDP-glucose dehydrogenase | UDP-Glc → UDP-GlcA |
| 148 | UTP-glucose-1-phosphate uridylyltransferase | Glc-1-P → UDP-Glc |
| 149 | glycosyl transferase | |
| 151 | CDP-glycerol:poly(glycero-phosphate) glycerophosphotransferase | |
| 152 | glycosyl transferase | |
| 155 | phosphomannomutase | Glc-6-P → Glc-1-P |
| 219 | dCMP deaminase | dCMP → dUMP |
| 130 | hydroxymethyldeoxyuridine synthase | dUMP → dHmdUMP |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kropinski, A.M.; Turner, D.; Nash, J.H.E.; Ackermann, H.-W.; Lingohr, E.J.; Warren, R.A.; Ehrlich, K.C.; Ehrlich, M. The Sequence of Two Bacteriophages with Hypermodified Bases Reveals Novel Phage-Host Interactions. Viruses 2018, 10, 217. https://doi.org/10.3390/v10050217
Kropinski AM, Turner D, Nash JHE, Ackermann H-W, Lingohr EJ, Warren RA, Ehrlich KC, Ehrlich M. The Sequence of Two Bacteriophages with Hypermodified Bases Reveals Novel Phage-Host Interactions. Viruses. 2018; 10(5):217. https://doi.org/10.3390/v10050217
Chicago/Turabian StyleKropinski, Andrew M., Dann Turner, John H. E. Nash, Hans-Wolfgang Ackermann, Erika J. Lingohr, Richard A. Warren, Kenneth C. Ehrlich, and Melanie Ehrlich. 2018. "The Sequence of Two Bacteriophages with Hypermodified Bases Reveals Novel Phage-Host Interactions" Viruses 10, no. 5: 217. https://doi.org/10.3390/v10050217





