Effects of Disturbance on Tree Community Dynamics in Whitebark Pine (Pinus albicaulis Engelm.) Ecosystems
Abstract
:1. Introduction
- (1)
- How frequently were whitebark pine stands disturbed by mountain pine beetle, white pine blister rust, and wildfire?
- (2)
- Have there been changes in overall tree community composition or basal area of individual common tree species (whitebark pine, subalpine fir and Engelmann spruce) by size class? If so, are changes related to mountain pine beetle or white pine blister rust incidence?
- (3)
- Is whitebark pine regeneration (a) related to mountain pine beetle mortality, white pine blister rust incidence, total tree basal area or basal area of mature whitebark pine, or (b) higher than expected in microsites with specific characteristics (near rocks, stumps, logs, herbs or shrubs, or beneath tree canopies)?
2. Materials and Methods
2.1. Site Selection
2.2. Original Current Vegetation Survey Sampling Design
2.3. 2012 Field Sampling
2.3.1. Mature Trees and Poles
2.3.2. Saplings
2.3.3. Seedlings
2.3.4. Microsites
2.3.5. Wildfire
2.4. Statistical Analysis
2.4.1. Disturbance Frequency and Successional Dynamics (Questions 1 and 2)
2.4.2. Factors Influencing Whitebark Pine Regeneration (Q3)
3. Results
3.1. Disturbance Frequency and Successional Dynamics (Q1 and 2)
3.2. Factors Influencing Whitebark Pine Regeneration (Q3)
4. Discussion
4.1. Disturbance Frequency and Successional Dynamics
4.2. Factors Influencing Whitebark Pine Regeneration
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Paine, R.T. A note on trophic complexity and community stability. Am. Nat. 1969, 103, 91–93. [Google Scholar] [CrossRef]
- Arno, S.F.; Hoff, R.J. Pinus albicaulis Englm. Whitebark Pine. In Silvics of North America. Vol. 1. Conifers; Burns, R.M., Honkala, B.H., Eds.; United States Department of Agriculture: Washington, DC, USA, 1990; pp. 530–554. [Google Scholar]
- Keane, R.E.; Tomback, D.F.; Aubry, C.A.; Bower, A.D.; Campbell, E.M.; Cripps, C.L.; Jenkins, M.B.; Mahalovich, M.F.; Manning, M.; McKinney, S.T.; et al. A Range-Wide Restoration Strategy for Whitebark Pine (Pinus albicaulis); US Department of Agriculture, Forest Service, Rocky Mountain Research Station: Fort Collins, CO, USA, 2012.
- Logan, J.A.; Powell, J.A. Ghost forests, global warming, and the mountain pine beetle (Coleoptera: Scolytidae). Am. Entomol. 2001, 47, 160–173. [Google Scholar] [CrossRef]
- Tomback, D.F. Dispersal of whitebark pine seeds by Clark’s nutcracker—A mutualism hypothesis. J. Anim. Ecol. 1982, 51, 451–467. [Google Scholar] [CrossRef]
- Schwandt, J.W. Whitebark Pine in Peril: A Case for Restoration; Gen. Tech. Rep. R1-06-28; USDA Forest Service: Missoula, MT, USA, 2006.
- Keane, R.E.; Parsons, R.A. Restoring whitebark pine forests of the northern Rocky Mountains, USA. Ecol. Rest. 2010, 28, 56–70. [Google Scholar] [CrossRef]
- Maloney, P.E. The multivariate underpinnings of recruitment for three Pinus species in montane forests of the Sierra Nevada, USA. Plant Ecol. 2014, 215, 261–274. [Google Scholar] [CrossRef]
- Perkins, J.L. Fire enhances whitebark pine seedling establishment, survival, and growth. Fire Ecol. 2015, 11. [Google Scholar] [CrossRef]
- Keane, R.E. Can the fire-dependent whitebark pine be saved? Fire Mgmt. Today 2001, 61, 17–20. [Google Scholar]
- McKinney, S.T.; Fiedler, C.E.; Tomback, D.F. Invasive pathogen threatens bird-pine mutualism: Implications for sustaining a high-elevation ecosystem. Ecol. Appl. 2009, 19, 597–607. [Google Scholar] [CrossRef]
- Kegley, S.; Schwandt, J.W.; Gibson, K.; Perkins, D. Health of Whitebark Pine Forests after Mountain Pine Beetle Outbreaks; FHP Report 11-03; USDA Forest Service, Northern Region Forest Health Protection: Fort Collins, CO, USA, 2010.
- Clason, A.J.; Macdonald, S.E.; Haeussler, S. Forest response to cumulative disturbance and stress: Two decades of change in whitebark pine ecosystems of west-central British Columbia. Ecoscience 2014, 21, 174–185. [Google Scholar] [CrossRef]
- Goeking, S.A.; Izlar, D.K. Pinus albicaulis Engelm. (whitebark pine) in mixed-species stands throughout its US Range: Broad-scale indicators of extent and recent decline. Forests 2018, 9, 131. [Google Scholar] [CrossRef]
- Brunelle, A.; Rehfeldt, G.E.; Bentz, B.; Munson, A.S. Holocene records of Dendroctonus bark beetles in high elevation pine forests of Idaho and Montana, USA. For. Ecol. Manag. 2008, 255, 836–846. [Google Scholar] [CrossRef]
- Negron, J.F.; Bentz, B.J.; Fettig, C.J.; Gillette, N.; Hansen, E.M.; Hayes, J.L.; Kelsey, R.G.; Lundquist, J.E.; Lynch, A.M.; Progar, R.A.; et al. US Forest Service bark beetle research in the western United States: Looking toward the future. J. For. 2008, 106, 325–331. [Google Scholar]
- Logan, J.A.; McFarlane, W.W.; Willcox, L. Whitebark pine vulnerability to climate-driven mountain pine beetle disturbance in the Greater Yellowstone Ecosystem. Ecol. Appl. 2010, 20, 895–902. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jamieson, M.A.; Trowbridge, A.M.; Raffa, K.F.; Lindroth, R.L. Consequences of climate warming and altered precipitation patterns for plant-insect and multitrophic interactions. Plant Physiol. 2012, 160, 1719–1727. [Google Scholar] [CrossRef] [PubMed]
- Shanahan, E.; Irvine, K.M.; Thoma, D.; Wilmoth, S.; Ray, A.; Legg, K.; Shovic, H. Whitebark pine mortality related to white pine blister rust, mountain pine beetle outbreak, and water availability. Ecosphere 2016, 7, e01610. [Google Scholar] [CrossRef]
- Amman, G.D. Mountain pine beetle brood production in relation to thickness of lodgepole pine phloem. J. Econ. Entomol. 1972, 65, 138–140. [Google Scholar] [CrossRef]
- Bartos, D.L.; Gibson, K.E. Insects of whitebark pine with emphasis on mountain pine beetle. In Proceedings of the Symposium on Whitebark Pine Ecosystems: Ecology and Management of a High-Mountain Resource, Bozeman, MT, USA, 29−30 March 1989; Schmidt, W.C., McDonald, K.J., Eds.; U.S. Department of Agriculture, Forest Service, Intermountain Research Station: Ogden, UT, USA, 1990; pp. 171–178. [Google Scholar]
- Perkins, D.L.; Roberts, D.W. Predictive models of whitebark pine mortality from mountain pine beetle. For. Ecol. Manag. 2003, 174, 495–510. [Google Scholar] [CrossRef]
- Keane, R.E.; Arno, S.F. Rapid decline of whitebark pine in western Montana: Evidence from 20-year re-measurements. West. J. Appl. For. 1993, 8, 44–47. [Google Scholar]
- Tomback, D.F.; Resler, L.M. Invasive pathogens at alpine treeline: Consequences for treeline dynamics. Phys. Geogr. 2007, 28, 397–418. [Google Scholar] [CrossRef]
- Tomback, D.F.; Sund, S.K.; Hoffmann, L.A. Post-fire regeneration of Pinus albicaulis: Height-age relationships, age structure, and microsite characteristics. Can. J. For. Res. 1993, 23, 113–119. [Google Scholar] [CrossRef]
- Tomback, D.F.; Achuff, P. Blister rust and western forest biodiversity: Ecology, values and outlook for white pines. For. Pathol. 2010, 40, 186–225. [Google Scholar] [CrossRef]
- Leirfallom, S.B.; Keane, R.E.; Tomback, D.F.; Dobrowski, S.Z. The effects of seed source health on whitebark pine (Pinus albicaulis) regeneration density after fire. Can. J. For. Res. 2015, 45, 1597–1606. [Google Scholar] [CrossRef]
- Hutchins, H.E.; Lanner, R.M. The central role of Clark’s nutcracker in the dispersal and establishment of whitebark pine. Oecologia 1982, 55, 192–201. [Google Scholar] [CrossRef] [PubMed]
- Lorenz, T.J.; Sullivan, K.A.; Bakian, A.V.; Aubry, C.A. Cache-site selection in Clark’s nutcracker. Auk 2011, 128, 237–247. [Google Scholar] [CrossRef]
- Larson, E.R. Status and Dynamics of Whitebark Pine (Pinus albicaulis Engelm.) Forests in Southwest Montana, Central Idaho, and Oregon, USA. Ph.D. Thesis, University of Minnesota, Minneapolis, MN, USA, 2009. [Google Scholar]
- Larson, E.R.; Kipfmueller, K.F. Patterns in whitebark pine regeneration and their relationships to biophysical site characteristics in southwest Montana, central Idaho, and Oregon, USA. Can. J. For. Res. 2010, 40, 476–487. [Google Scholar] [CrossRef]
- Meyer, M.D.; Bulaon, B.; MacKenzie, M.; Safford, H.D. Mortality, structure, and regeneration in whitebark pine stands impacted by mountain pine beetle in southern Sierra Nevada. Can. J. For. Res. 2016, 46, 572–581. [Google Scholar] [CrossRef]
- Keane, R.E.; Gray, K.L.; Dickinson, L.J. Whitebark Pine Diameter Growth Response to Removal of Competition; Research Note RMRS-RN-32; US Department of Agriculture, Forest Service, Rocky Mountain Research Station: Fort Collins, CO, USA, 2007.
- Baker, F.S. Principles of Silviculture; McGraw-Hill Book Co., Inc.: New York, NY, USA, 1950. [Google Scholar]
- McCaughey, W.W.; Scott, G.L.; Izlar, K.L. Whitebark pine planting guidelines. West. J. Appl. For. 2009, 24, 163–166. [Google Scholar]
- Perkins, J.L. Pinus albicaulis Seedling Regeneration after Fire. Ph.D. Thesis, University of Montana, Missoula, MU, USA, 2004. [Google Scholar]
- Campbell, E.M.; Antos, J.A. Postfire succession in Pinus albicaulis—Abies lasiocarpa forests of southern British Columbia. Can. J. Bot. 2003, 81, 383–397. [Google Scholar] [CrossRef]
- Izlar, D.K. Assessment of Whitebark Pine Seedling Survival for Rocky Mountain Planting. Ph.D. Thesis, University of Montana, Missoula, MT, USA, 2007. [Google Scholar]
- Lonergan, E.R.; Cripps, C.L.; Smith, C.M. Influence of site conditions, shelter objects, and ectomycorrhizal inoculation on the early survival of whitebark pine seedlings planted in Waterton Lakes National Park. For. Sci. 2014, 60, 603–612. [Google Scholar] [CrossRef]
- Maher, E.L.; Germino, M.J.; Hasselquist, N.J. Interactive effects of tree and herb cover on survivorship, physiology, and microclimate of conifer seedlings at the alpine tree-line ecotone. Can. J. For. Res. 2005, 35, 567–574. [Google Scholar] [CrossRef]
- Maher, E.L.; Germino, M.J. Microsite differentiation among conifer species during seedling establishment at alpine treeline. Ecoscience 2006, 13, 334–341. [Google Scholar] [CrossRef] [Green Version]
- Gelderman, M.S.; Macdonald, S.E.; Gould, A.J. Regeneration niche of whitebark pine in the Canadian Rocky Mountains: The basis to restoring an endangered species. Arct. Antarct. Alp. Res. 2016, 48, 279–292. [Google Scholar] [CrossRef]
- Pyatt, J.C.; Tomback, D.F.; Blakeslee, S.C.; Wunder, M.B.; Resler, L.M.; Boggs, L.A.; Bevency, H.D. The importance of conifers for facilitation at treeline: Comparing biophysical characteristics of leeward microsites in whitebark pine communities. Arct. Antarct. Alp. Res. 2016, 48, 427–444. [Google Scholar] [CrossRef]
- Pansing, E.R.; Tomback, D.F.; Wunder, M.B.; French, J.P.; Wagner, A.C. Microsite and elevation zone effects on seed pilferage, germination, and seedling survival during early whitebark pine recruitment. Ecol. Evol. 2017, 7, 9027–9040. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Callaway, R.M. Competition and facilitation on elevational gradients in subalpine forests of the northern Rocky Mountains, USA. Oikos 1998, 82, 561–573. [Google Scholar] [CrossRef]
- Kipfmueller, K.F.; Kupfer, J.A. Complexity of successional pathways in subalpine forests of the Selway-Bitterroot Wilderness Area. Ann. Assoc. Am. Geogr. 2005, 95, 495–510. [Google Scholar] [CrossRef]
- Wong, C.M.; Daniels, L.D. Novel forest decline triggered by multiple interactions among climate, an introduced pathogen and bark beetles. Glob. Chang. Biol. 2017, 23, 1926–1941. [Google Scholar] [CrossRef] [PubMed]
- Loehman, R.A.; Keane, R.E.; Holsinger, L.M.; Wu, Z. Interactions of landscape disturbances and climate change dictate ecological pattern and process: Spatial modeling of wildfire, insect, and disease dynamics under future climates. Landsc. Ecol. 2017, 32, 1447–1459. [Google Scholar] [CrossRef]
- Lillybridge, T.R.; Kovalchik, B.L.; Williams, C.K.; Smith, B.G. Field Guide for Forested Plant Associations of the Wenatchee National Forest; Gen. Tech. Rep. PNW-GTR-359; U.S. Department of Agriculture, Forest Service: Portland, OR, USA, 1995.
- Johnson, M.D. Field Procedures for the Current Vegetation Survey; Natural Resource Inventory, US Department of Agriculture, Forest Service, Pacific Northwest Research Station: Portland, OR, USA, 2003.
- Safranyik, L.; Shrimpton, D.M.; Whitney, H.S. Management of Lodgepole Pine to Reduce Losses from the Mountain Pine Beetle; Tech. Rep.; Forestry Service, Environment Canada: Laurentian Hills, ON, Canada, 1974.
- Hoff, R.J. How to Recognize Blister Rust Infection on Whitebark Pine; No. INT-406; US Department of Agriculture, Forest Service, Intermountain Research Station: Ogden, UT, USA, 1992.
- Cole, W.E.; Amman, G.D. Mountain Pine Beetle Infestations in Relation to Lodgepole Pine Diameters; US Dept. of Agriculture, Forest Service, Intermountain Forest & Range Experiment Station: Ogden, UT, USA, 1969.
- Joliffe, I.T. Principal Component Analysis; Springer: New York, NY, USA, 2002. [Google Scholar]
- Biondini, M.E.; Mielke, P.W.; Berry, K.J. Data-dependent permutation techniques for the analysis of ecological data. Vegetatio 1988, 75, 161–168. [Google Scholar]
- Mielke, P.W. The application of multivariate permutation methods based on distance functions in the earth sciences. Earth Sci. Rev. 1991, 31, 55–71. [Google Scholar] [CrossRef]
- Faith, D.P.; Minchin, P.R.; Belbin, L. Compositional dissimilarity as a robust measure of ecological distance. Vegetatio 1987, 69, 57–68. [Google Scholar] [CrossRef]
- Demsar, J. Statistical comparisons of classifiers over multiple data sets. J. Mach. Learn. Res. 2006, 7, 1–30. [Google Scholar]
- Wilcoxon, F. Individual comparisons by ranking methods. Biometr. Bull. 1945, 1, 80–83. [Google Scholar] [CrossRef]
- Kendall, M. A new measure of rank correlation. Biometrika 1938, 30, 81–89. [Google Scholar] [CrossRef]
- Ott, L.R.; Longnecker, M. An Introduction to Statistical Methods and Data Analysis; Brooks/Cole: Pacific Grove, CA, USA, 2010. [Google Scholar]
- PC-ORD. Multivariate Analysis of Ecological Data, version 5 for windows edition; MjM Software Design: Gleneden Beach, OR, USA, 2005. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2017; Available online: http://www.R-project.org/ (accessed on 13 June 2018).
- Larson, E.R.; van De Gevel, S.L.; Grissino-Mayer, H.D. Variability in Fire Regimes of High-Elevation Whitebark Pine Communities, Western Montana, USA. Ecoscience 2009, 16, 282–298. [Google Scholar] [CrossRef]
- Westerling, A.L.; Hidalgo, H.G.; Cayan, D.R.; Swetnam, T.W. Warming and earlier spring increase western U.S. forest wildfire activity. Science 2006, 313, 940–943. [Google Scholar] [CrossRef] [PubMed]
- Larson, E.R. Influences of the biophysical environment on blister rust and mountain pine beetle, and their interactions, in whitebark pine forests. J. Biogeogr. 2011, 38, 453–470. [Google Scholar] [CrossRef]
- Six, D.; Adams, J. White pine blister rust severity and selection of individual whitebark pine by the mountain pine beetle (Coleoptera: Curculionidae, Scolytinae). J. Entomol. Sci. 2006, 42, 345–353. [Google Scholar] [CrossRef]
- Van Mantgem, P.J.; Stephenson, N.L.; Byrne, J.C.; Daniels, L.D.; Franklin, J.F.; Fule, P.Z.; Harmon, M.E.; Larson, A.J.; Smith, J.M.; Taylor, A.H.; et al. Widespread increase of tree mortality rates in the western United States. Science 2009, 323, 521–524. [Google Scholar] [CrossRef] [PubMed]
- Breshears, D.D.; Cobb, N.S.; Rich, P.M.; Kevin, P.P.; Allen, C.D.; Balice, R.G.; Romme, W.H.; Kastens, J.H.; Floyd, L.; Belnap, J.; et al. Regional vegetation die-off in response to global-change-type drought. Proc. Nat. Acad. Sci. USA 2005, 102, 15144–15148. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bigler, C.; Gavin, D.G.; Gunning, C.; Veblen, T.T. Drought induces lagged tree mortality in a subalpine forest in the Rocky Mountains. Oikos 2007, 116, 1983–1994. [Google Scholar] [CrossRef]
- Larson, E.R.; Kipfmueller, K.F. Ecological disaster or the limits of observation? Reconciling modern declines with the long-term dynamics of whitebark pine communities. Geogr. Compass 2012, 40, 476–487. [Google Scholar] [CrossRef]
- Frelich, L.E.; Reich, P.B. Neighborhood effects, disturbance, and succession in forests of the western Great Lakes Region. Ecoscience 1995, 2, 148–158. [Google Scholar] [CrossRef]
- Bergeron, Y. Species and stand dynamics in the mixed woods of Quebec’s southern boreal forest. Ecology 2000, 81, 1500–1516. [Google Scholar] [CrossRef]
- Arevalo, J.R.; DeCoster, J.K.; McAlister, S.D.; Palmer, M.W. Changes in two Minnesota forests during 14 years following catastrophic windthrow. J. Veg. Sci. 2000, 11, 833–840. [Google Scholar] [CrossRef]
- Harcombe, P.A.; Bill, C.J.; Fulton, M.; Glitzenstein, J.S.; Marks, P.L.; Elsik, I.S. Stand dynamics over 18 years in a southern mixed hardwood forest, Texas, USA. J. Ecol. 2002, 90, 947–957. [Google Scholar] [CrossRef] [Green Version]
- Meyers, R.K.; van Lear, D.H. Hurricane-fire interactions in coastal forests of the south: A review and hypothesis. For. Ecol. Manag. 1998, 103, 265–276. [Google Scholar] [CrossRef]
- Paine, R.T.; Tegner, M.J.; Johnson, E.A. Compounded perturbations yield ecological surprises. Ecosystems 1998, 1, 535–545. [Google Scholar] [CrossRef]
- Barringer, L.E.; Tomback, D.F.; Wunder, M.B.; McKinney, S.T. 2012. Whitebark pine stand condition, tree abundance, and cone production as predictors of visitation by Clark’s nutcracker. PLoS ONE 2012, 7, e37663. [Google Scholar] [CrossRef] [PubMed]
- McCaughey, W.W.; Weaver, T. Biotic and Microsite Factors Affecting Whitebark Pine Establishment. In Proceedings of the Symposium on Whitebark Pine Ecosystems: Ecology and Management of a High-Mountain Resource, Bozeman, MT, USA, 29–30 March 1989; Schmidt, W.C., McDonald, K.J., Eds.; U.S. Department of Agriculture, Forest Service, Intermountain Research Station: Ogden, UT, USA, 1990; pp. 140–150. [Google Scholar]
- Tomback, D.F.; Anderies, A.J.; Carsey, K.S.; Powell, M.L.; Mellman-Brown, S. Delayed seed germination in whitebark pine and regeneration patterns following the Yellowstone fires. Ecology 2001, 82, 2587–2600. [Google Scholar] [CrossRef]
- Hadley, J.L.; Smith, W.K. Wind effects on needles of timberline conifers: Seasonal influence on mortality. Ecology 1986, 67, 12–19. [Google Scholar] [CrossRef]
- Germino, M.J.; Smith, W.K. Sky exposure, crown architecture, and low-temperature photoinhibition in conifer seedlings at alpine treeline. Plant Cell Environ. 1999, 22, 407–415. [Google Scholar] [CrossRef] [Green Version]
- Maher, C.T.; Nelson, C.R.; Larson, A.J.; Sala, A. Ecological effects and effectiveness of silvicultural restoration treatments in whitebark pine. For. Ecol. Manag. 2018, 429, 534–548. [Google Scholar] [CrossRef]

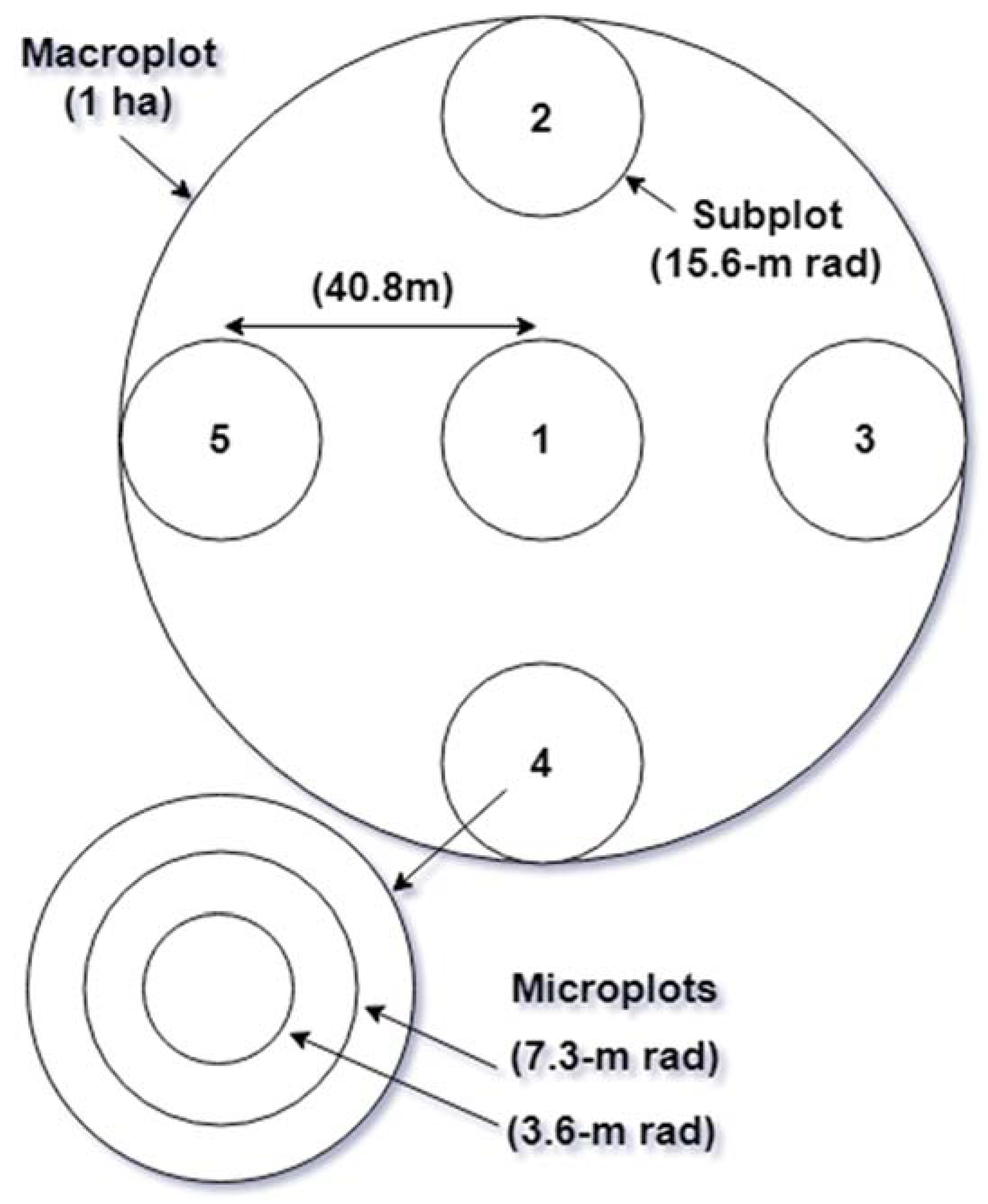
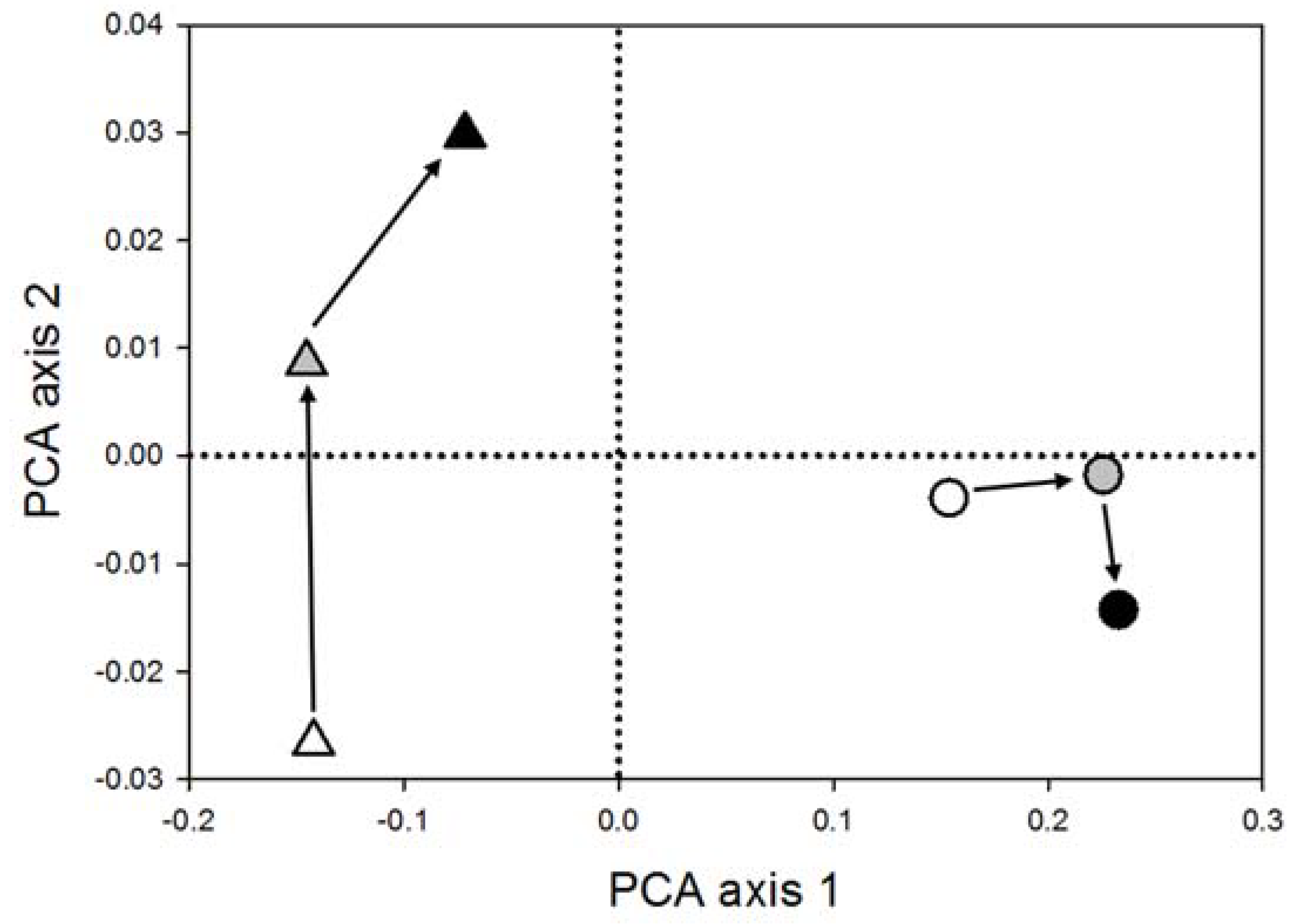
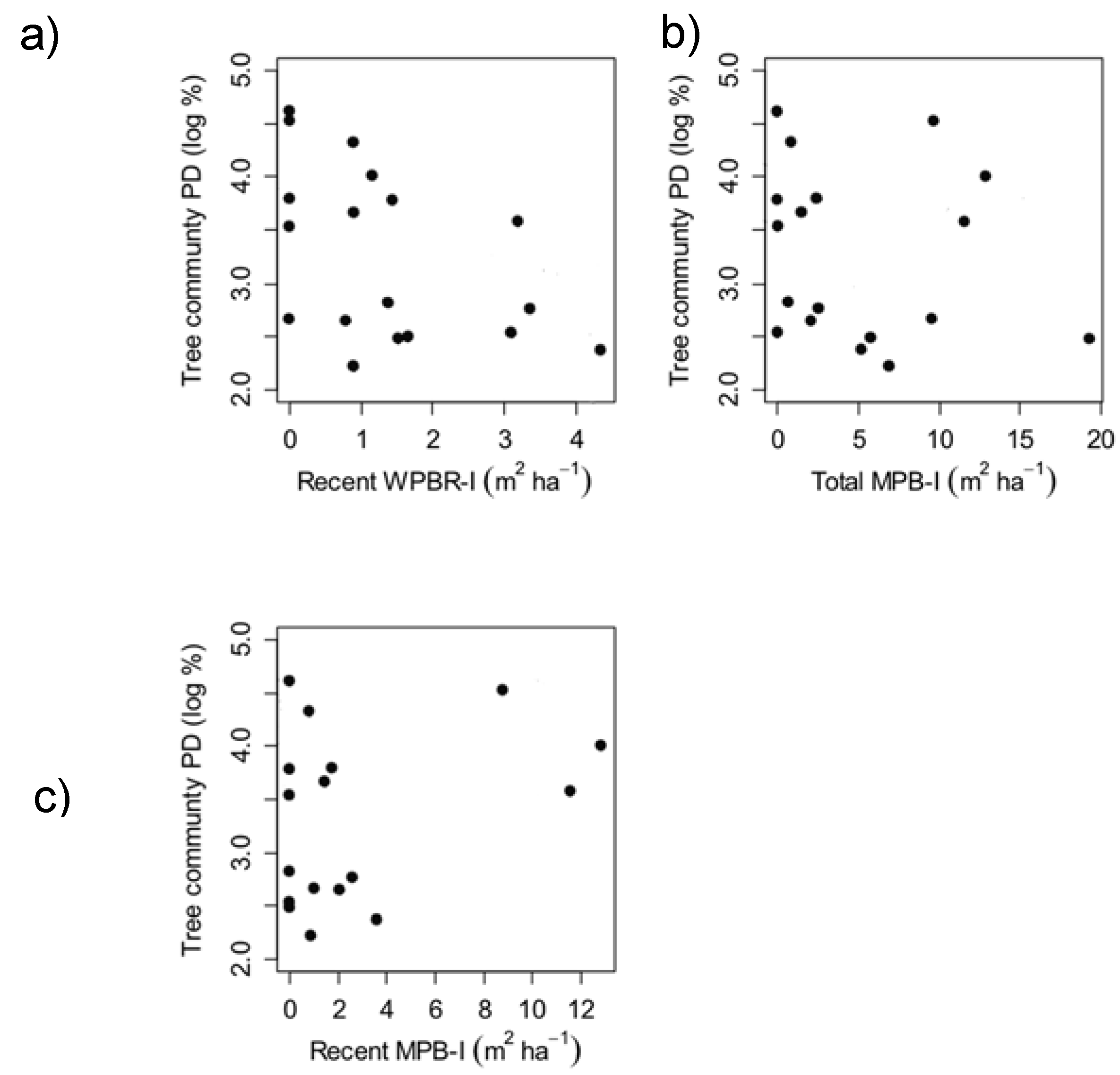
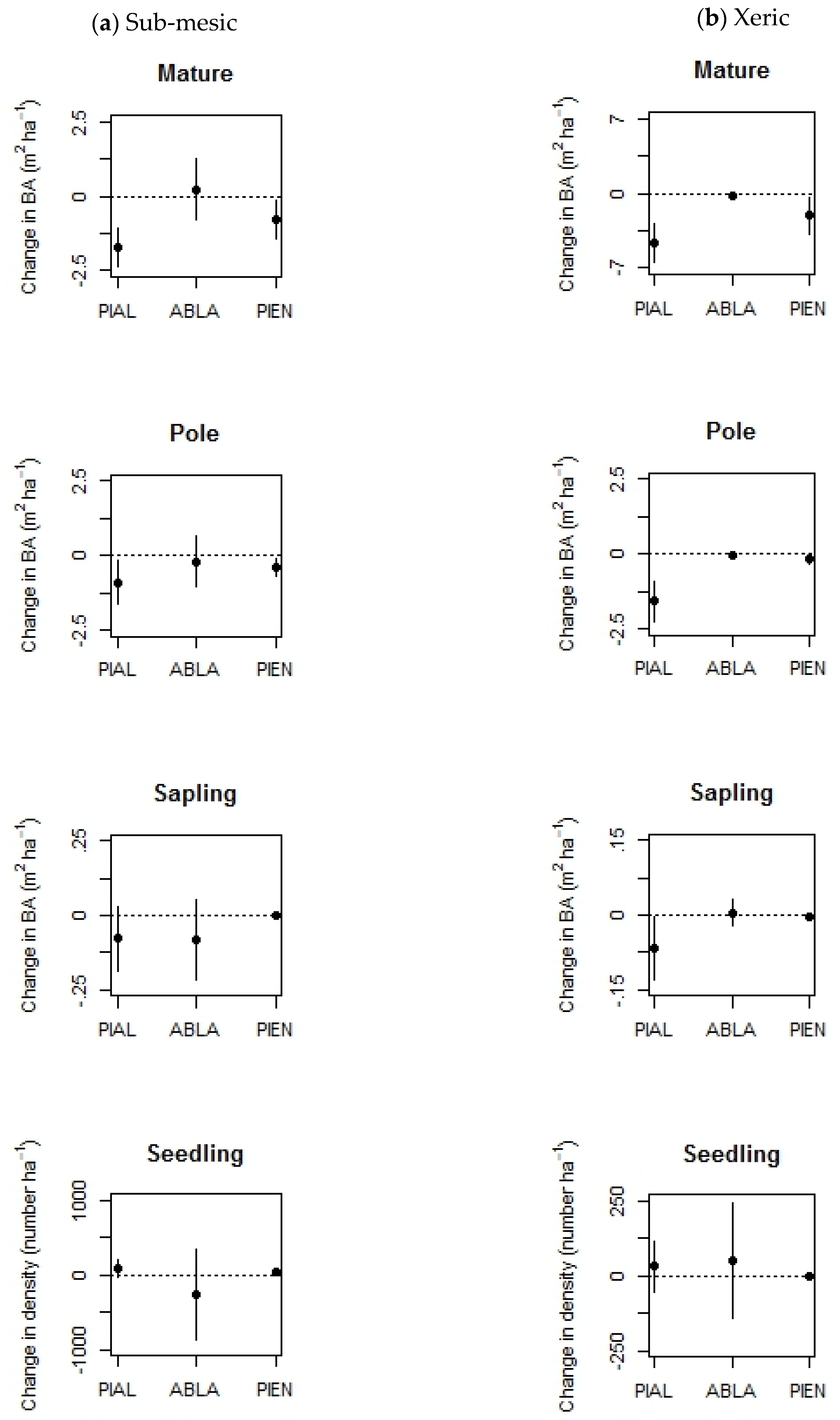
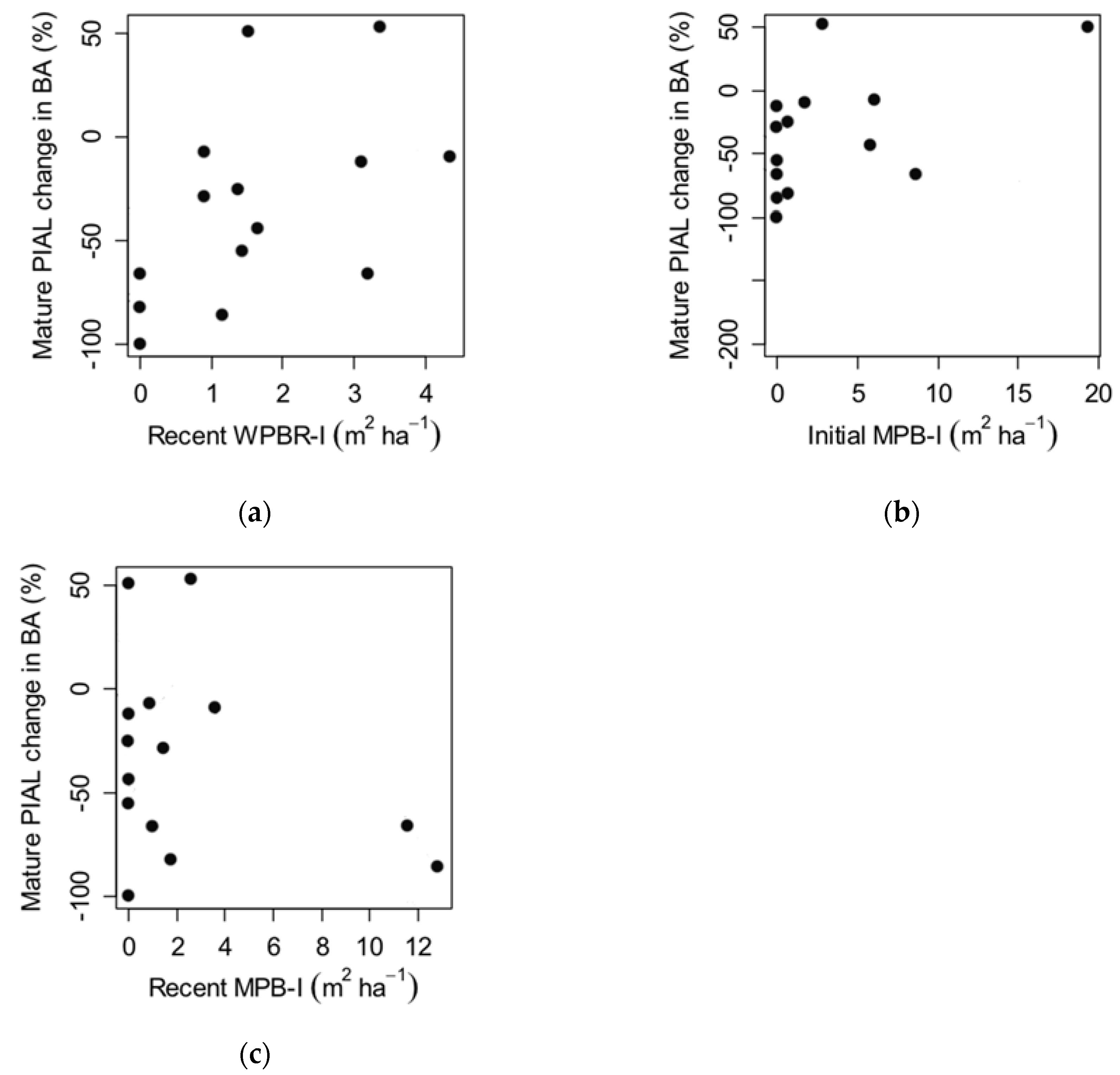
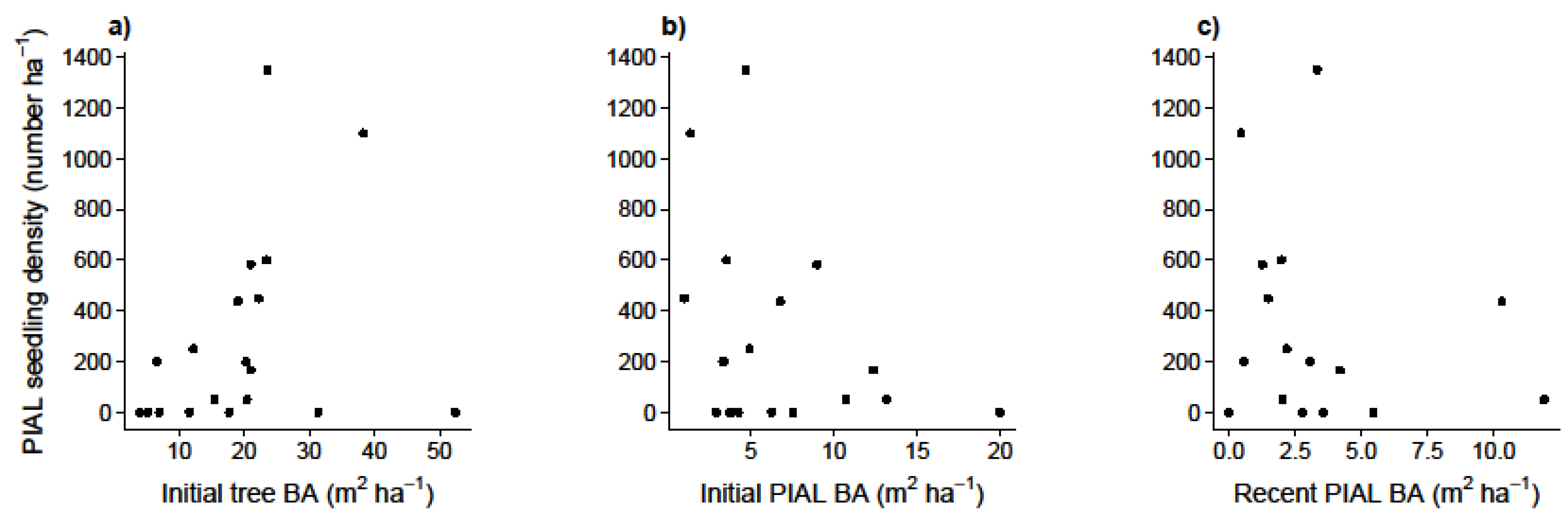
| Macro-Plot | Elev. (m) | Northing, Easting (m) | Initial Basal Area (m2 ha−1) | Initial PIAL Basal Area (m2 ha−1) | Initial PIAL MPB Basal Area (m2 ha−1) | Initial ABLA Basal Area (m2 ha−1) | Recent Fire History (Year. of Wildfire If Present) | Plant Association (s) |
|---|---|---|---|---|---|---|---|---|
| 1 | 1798 | 5,209,096, 650,589 | 7.02 | 7.02 | 0.00 | 0.00 | PIAL/CARU | |
| 2 | 1707 | 5,252,903, 661,536 | 11.63 | 4.24 | 0.00 | 4.20 | PSME/AGSP | |
| 3 | 1737 | 5,255,604, 650,579 | 4.00 | 4.00 | 0.66 | 0.00 | PIAL/JUCO6 | |
| 4 | 1829 | 5,269,249, 664,247 | 23.44 | 3.63 | 5.77 | 6.32 | ABLA2/RHAL/LUHI | |
| 5 | 1859 | 5,304,797, 688,821 | 20.33 | 5.43 | 6.00 | 7.11 | TSME/RHAL-VAME, ABLA2/RHAL/LUHI | |
| 6 | 1981 | 5,313,060, 691,632 | 22.23 | 2.45 | 19.34 | 11.74 | ABLA2/VASC/LUHI, ABLA2/RHAL/LUHI | |
| 7 | 1737 | 5,315,809, 661,506 | 12.21 | 6.81 | 0.00 | 3.61 | ABLA2/VAME | |
| 8 | 1951 | 5,318,556, 672,456 | 19.02 | 7.46 | 2.82 | 10.80 | PIAL/VASC/LUHI | |
| 9 | 2103 | 5,332,217, 694,367 | 15.45 | 14.69 | 1.62 | 0.76 | PIAL/FEVI | |
| 10 | 2073 | 5,337,692, 694,369 | 5.24 | 5.24 | 0.00 | 0.00 | 2001 | PIAL/FEVI |
| 11 | 1920 | 5,375,519, 286,651 | 31.40 | 8.59 | 0.00 | 10.65 | 2006 | ABLA2/VASC |
| 12 | 1798 | 5,381,529, 672,472 | 6.59 | 4.60 | 0.65 | 1.26 | PIAL/JUCO6 | |
| 13 | 1829 | 5,384,225, 672,345 | 21.03 | 14.30 | 0.00 | 4.56 | PIAL/CARU | |
| 14 | 2073 | 5,389,192, 285,021 | 20.42 | 16.53 | 0.00 | 0.00 | 2006 | PIAL/VASC/LUHI |
| 15 | 2073 | 5,394,648, 285,457 | 52.39 | 26.71 | 0.85 | 2.87 | 2003 | PIAL/VASC/LUHI |
| 16 | 2256 | 5,394,851, 282,732 | 17.76 | 9.65 | 0.00 | 0.00 | 2006 | PIAL/VASC/LUHI, PIAL/CARU |
| 17 | 1768 | 5,400,583, 705,281 | 23.58 | 8.23 | 0.00 | 0.00 | PIAL/CARU | |
| 18 | 1707 | 5,403,326, 702,607 | 38.31 | 2.51 | 8.61 | 24.19 | ABLA2/VAME | |
| 19 | 2134 | 5,408,845, 697,110 | 21.05 | 14.58 | 0.00 | 6.11 | PIAL/VASC/LUHI |
| Sample Cycle | Mature | PIAL Pole | Sapling | Mature | ABLA Pole | Sapling | Mature | PIEN Pole | Sapling |
|---|---|---|---|---|---|---|---|---|---|
| 1990 | 6.45 | 2.14 | 0.18 | 2.89 | 1.86 | 0.21 | 2.46 | 0.33 | 0.02 |
| 2000 | 4.74 | 1.55 | 0.18 | 2.83 | 1.87 | 0.23 | 1.28 | 0.16 | 0.01 |
| 2012 | 2.87 | 0.81 | 0.12 | 2.86 | 1.75 | 0.18 | 0.84 | 0.07 | 0.01 |
| Microsite Characteristic | Proportion of Microplot Area with Microsite Characteristic | Proportion of PIAL Seedlings in Areas with Microsite Characteristic | p-Value |
|---|---|---|---|
| Herb/Shrub | 0.392 | 0.563 | 0.033 |
| Rock | 0.031 | 0.221 | 0.062 |
| Log | 0.041 | 0.207 | 0.074 |
| Stump | 0.001 | 0.000 | 0.163 |
| Live Tree Canopy | 0.272 | 0.327 | 0.465 |
| PIAL-MPB Canopy | 0.099 | 0.085 | 0.700 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Amberson, J.T.; Keville, M.P.; Nelson, C.R. Effects of Disturbance on Tree Community Dynamics in Whitebark Pine (Pinus albicaulis Engelm.) Ecosystems. Forests 2018, 9, 566. https://doi.org/10.3390/f9090566
Amberson JT, Keville MP, Nelson CR. Effects of Disturbance on Tree Community Dynamics in Whitebark Pine (Pinus albicaulis Engelm.) Ecosystems. Forests. 2018; 9(9):566. https://doi.org/10.3390/f9090566
Chicago/Turabian StyleAmberson, Jeremy T., Megan P. Keville, and Cara R. Nelson. 2018. "Effects of Disturbance on Tree Community Dynamics in Whitebark Pine (Pinus albicaulis Engelm.) Ecosystems" Forests 9, no. 9: 566. https://doi.org/10.3390/f9090566




