Fine Root Dynamics in Three Forest Types with Different Origins in a Subalpine Region of the Eastern Qinghai-Tibetan Plateau
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Site
2.2. Sampling Design
2.3. Sequential Soil Coring
2.4. Stand Characteristics and Aboveground Biomass
2.5. Soil Characteristics
2.6. Data Analysis
3. Results
3.1. Comparison of Fine Roots among Forest Types
3.2. Seasonal Dynamics of Fine Root Biomass and Necromass
3.3. Vertical Distribution of Fine Roots
3.4. Relationships between Fine Root Dynamics and Soil N Availability
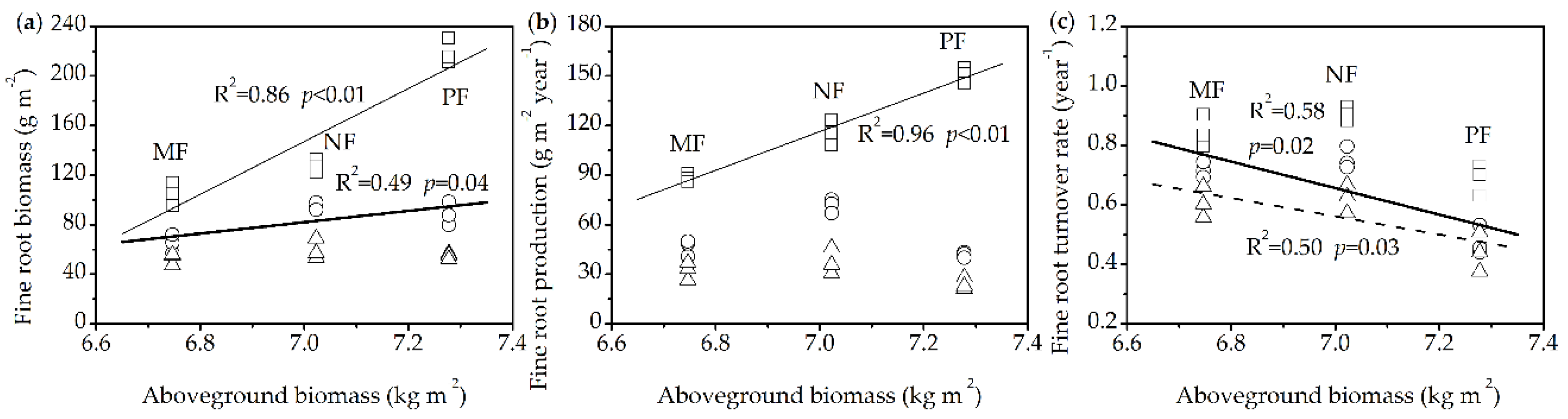
3.5. Relationships between Fine Root Dynamics and Aboveground Biomass
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Pransiska, Y.; Triadiati, T.; Tjitrosoedirjo, S.; Hertel, D.; Kotowska, M.M. Forest conversion impacts on the fine and coarse root system, and soil organic matter in tropical lowlands of Sumatera (Indonesia). For. Ecol. Manag. 2016, 379, 288–298. [Google Scholar] [CrossRef]
- Lindström, A.; Rune, G. Root deformation in plantations of container-grown Scots pine trees: Effects on root growth, tree stability and stem straightness. Plant Soil 1999, 217, 29–37. [Google Scholar] [CrossRef]
- Lukac, M.; Godbold, D.L. Fine root biomass and turnover in southern taiga estimated by root inclusion nets. Plant Soil 2010, 331, 505–513. [Google Scholar] [CrossRef]
- Hendrick, R.L.; Pregitzer, K.S. The Demography of Fine Roots in a Northern Hardwood Forest. Ecology 1992, 73, 1094–1104. [Google Scholar] [CrossRef]
- Barlow, P. Plant roots: Autopoietic and cognitive constructions. Plant Root 2010, 4, 40–52. [Google Scholar] [CrossRef]
- Montagnoli, A.; Terzaghi, M.; Di Iorio, A.; Scippa, G.S.; Chiatante, D. Fine-root morphological and growth traits in a Turkey-oak stand in relation to seasonal changes in soil moisture in the Southern Apennines, Italy. Ecol. Res. 2012, 27, 1015–1025. [Google Scholar] [CrossRef] [Green Version]
- Montagnoli, A.; Di Iorio, A.; Terzaghi, M.; Trupiano, D.; Scippa, G.S.; Chiatante, D. Influence of soil temperature and water content on fine-root seasonal growth of European beech natural forest in Southern Alps, Italy. Eur. J. For. Res. 2014, 133, 957–968. [Google Scholar] [CrossRef] [Green Version]
- Montagnoli, A.; Dumroese, R.K.; Terzaghi, M.; Pinto, J.R.; Fulgaro, N.; Scippa, G.S.; Chiatante, D. Tree seedling response to LED spectra: Implications for forest restoration. Plant Biosyst. 2018, 152, 515–523. [Google Scholar] [CrossRef]
- Eissenstat, D.M.; Wells, C.E.; Yanai, R.D.; Whitbeck, J.L.; Norby, R.; Fitter, A.; Jackson, R. Building roots in a changing environment: Implications for root longevity. New Phytol. 2000, 147, 33–42. [Google Scholar] [CrossRef]
- Montagnoli, A.; Terzaghi, M.; Baesso, B.; Santamaria, R.; Scippa, G.S.; Chiatante, D. Drought and fire stress influence seedling competition in oak forests: Fine-root dynamics as indicator of adaptation strategies to climate change. Reforesta 2016, 22, 86–105. [Google Scholar] [CrossRef]
- Brunner, I.; Herzog, C.; Dawes, M.A.; Arend, M.; Sperisen, C. How tree roots respond to drought. Front. Plant Sci. 2015, 6, 547. [Google Scholar] [CrossRef] [PubMed]
- McCormack, M.L.; Dickie, I.A.; Eissenstat, D.M.; Fahey, T.J.; Fernandez, C.W.; Guo, D.; Helmisaari, H.-S.; Hobbie, E.A.; Iversen, C.M.; Jackson, R.B.; et al. Redefining fine roots improves understanding of below-ground contributions to terrestrial biosphere processes. New Phytol. 2015, 207, 505–518. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yuan, Z.Y.; Chen, H.Y.H. Fine root biomass, production, turnover rates, and nutrient contents in boreal forest ecosystems in relation to species, climate, fertility, and stand age: Literature review and meta-analyses. Crit. Rev. Plant Sci. 2010, 29, 204–221. [Google Scholar] [CrossRef]
- Brédoire, F.; Nikitich, P.; Barsukov, P.A.; Derrien, D.; Litvinov, A.; Rieckh, H.; Rusalimova, O.; Zeller, B.; Bakker, M.R. Distributions of fine root length and mass with soil depth in natural ecosystems of southwestern Siberia. Plant Soil 2016, 400, 315–335. [Google Scholar] [CrossRef]
- Matamala, R.; Gonzàlez-Meler, M.A.; Jastrow, J.D.; Norby, R.J.; Schlesinger, W.H. Impacts of fine root turnover on forest NPP and soil C sequestration potential. Science 2003, 302, 1385–1387. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Z.Y.; Chen, H.Y.H. Effects of disturbance on fine root dynamics in the boreal forests of Northern Ontario, Canada. Ecosystems 2013, 16, 467–477. [Google Scholar] [CrossRef]
- Jamro, G.M.; Chang, S.X.; Naeth, M.A.; Duan, M.; House, J. Fine root dynamics in lodgepole pine and white spruce stands along productivity gradients in reclaimed oil sands sites. Ecol. Evol. 2015, 5, 4655–4670. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hickler, T.; Prentice, I.C.; Smith, B.; Sykes, M.T.; Zaehle, S. Implementing plant hydraulic architecture within the LPJ Dynamic Global Vegetation Model. Glob. Ecol. Biogeogr. 2006, 15, 567–577. [Google Scholar] [CrossRef]
- Cienciala, E.; Tatarinov, F.A. Application of BIOME-BGC model to managed forests: 2. Comparison with long-term observations of stand production for major tree species. For. Ecol. Manag. 2006, 237, 252–266. [Google Scholar] [CrossRef]
- Børja, I.; De Wit, H.A.; Steffenrem, A.; Majdi, H. Stand age and fine root biomass, distribution and morphology in a Norway spruce chronosequence in southeast Norway. Tree Physiol. 2008, 28, 773–784. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, T.; Dong, L.; Mao, Z.; Li, Y. Fine root dynamics of trees and understorey vegetation in a chronosequence of Betula platyphylla stands. For. Ecol. Manag. 2015, 346, 1–9. [Google Scholar] [CrossRef]
- Montagnoli, A.; Terzaghi, M.; Di Iorio, A.; Scippa, G.S.; Chiatante, D. Fine-root seasonal pattern, production and turnover rate of European beech (Fagus sylvatica L.) stands in Italy Prealps: Possible implications of coppice conversion to high forest. Plant Biosyst. 2012, 146, 1012–1022. [Google Scholar] [CrossRef] [Green Version]
- Terzaghi, M.; Montagnoli, A.; Di Iorio, A.; Scippa, G.S.; Chiatante, D. Fine-root carbon and nitrogen concentration of European beech (Fagus sylvatica L.) in Italy Prealps: Possible implications of coppice conversion to high forest. Front. Plant Sci. 2013, 4, 192. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hertel, D.; Harteveld, M.A.; Leuschner, C. Conversion of a tropical forest into agroforest alters the fine root-related carbon flux to the soil. Soil Biol. Biochem. 2009, 41, 481–490. [Google Scholar] [CrossRef]
- Ma, C.; Zhang, W.; Wu, M.; Xue, Y.; Ma, L.; Zhou, J. Effect of aboveground intervention on fine root mass, production, and turnover rate in a Chinese cork oak (Quercus variabilis Blume) forest. Plant Soil 2013, 368, 201–214. [Google Scholar] [CrossRef]
- Ma, Z.; Chen, H.Y.H. Effects of species diversity on fine root productivity increase with stand development and associated mechanisms in a boreal forest. J. Ecol. 2017, 105, 237–245. [Google Scholar] [CrossRef]
- Liu, C.; Xiang, W.; Lei, P.; Deng, X.; Tian, D.; Fang, X.; Peng, C. Standing fine root mass and production in four Chinese subtropical forests along a succession and species diversity gradient. Plant Soil 2014, 376, 445–459. [Google Scholar] [CrossRef]
- Russell, E.A.; Kivlin, N.S.; Hawkes, V.C. Tropical tree species effects on soil pH and biotic factors and the consequences for macroaggregate dynamics. Forests 2018, 9, 184. [Google Scholar] [CrossRef]
- Shu, W.; Shen, X.; Lei, P.; Xiang, W.; Ouyang, S.; Yan, W. Temporal changes of fine root overyielding and foraging strategies in planted monoculture and mixed forests. BMC Ecol. 2018, 18, 9. [Google Scholar] [CrossRef] [PubMed]
- Xiang, W.; Fan, G.; Lei, P.; Zeng, Y.; Tong, J.; Fang, X.; Deng, X.; Peng, C. Fine root interactions in subtropical mixed forests in China depend on tree species composition. Plant Soil 2015, 395, 335–349. [Google Scholar] [CrossRef]
- Brassard, B.W.; Chen, H.Y.H.; Bergeron, Y.; Paré, D. Differences in fine root productivity between mixed- and single-species stands. Funct. Ecol. 2011, 25, 238–246. [Google Scholar] [CrossRef]
- Brassard, B.W.; Chen, H.Y.H.; Cavard, X.; Laganière, J.; Reich, P.B.; Bergeron, Y.; Paré, D.; Yuan, Z. Tree species diversity increases fine root productivity through increased soil volume filling. J. Ecol. 2013, 101, 210–219. [Google Scholar] [CrossRef]
- Liu, B.; Li, H.; Zhu, B.; Koide, R.T.; Eissenstat, D.M.; Guo, D. Complementarity in nutrient foraging strategies of absorptive fine roots and arbuscular mycorrhizal fungi across 14 coexisting subtropical tree species. New Phytol. 2015, 208, 125–136. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jacob, A.; Hertel, D.; Leuschner, C. Diversity and species identity effects on fine root productivity and turnover in a species-rich temperate broad-leaved forest. Funct. Plant Biol. 2014, 41, 678–689. [Google Scholar] [CrossRef]
- Hooper, D.U.; Chapin, F.S.; Ewel, J.J.; Hector, A.; Inchausti, P.; Lavorel, S.; Lawton, J.H.; Lodge, D.M.; Loreau, M.; Naeem, S.; et al. Effects of biodiversity on ecosystem functioning: A consensus of current knowledge. Ecol. Monogr. 2005, 75, 3–35. [Google Scholar] [CrossRef] [Green Version]
- De Kroon, H.; Hendriks, M.; van Ruijven, J.; Ravenek, J.; Padilla, F.M.; Jongejans, E.; Visser, E.J.W.; Mommer, L. Root responses to nutrients and soil biota: Drivers of species coexistence and ecosystem productivity. J. Ecol. 2012, 100, 6–15. [Google Scholar] [CrossRef]
- Richards, A.E.; Forrester, D.I.; Bauhus, J.; Schererlorenzen, M. The influence of mixed tree plantations on the nutrition of individual species: A review. Tree Physiol. 2010, 30, 1192–1208. [Google Scholar] [CrossRef] [PubMed]
- Bolte, A.; Villanueva, I. Interspecific competition impacts on the morphology and distribution of fine roots in European beech (Fagus sylvatica L.) and Norway spruce (Picea abies (L.) Karst.). Eur. J. For. Res. 2006, 125, 15–26. [Google Scholar] [CrossRef]
- Beyer, F.; Hertel, D.; Jung, K.; Fender, A.-C.; Leuschner, C. Competition effects on fine root survival of Fagus sylvatica and Fraxinus excelsior. For. Ecol. Manag. 2013, 302, 14–22. [Google Scholar] [CrossRef]
- Lei, P.; Scherer-Lorenzen, M.; Bauhus, J. The effect of tree species diversity on fine-root production in a young temperate forest. Oecologia 2012, 169, 1105–1115. [Google Scholar] [CrossRef] [PubMed]
- Ma, Z.; Chen, H.Y.H. Positive species mixture effects on fine root turnover and mortality in natural boreal forests. Soil Biol. Biochem. 2018, 121, 130–137. [Google Scholar] [CrossRef]
- Zadworny, M.; McCormack, M.L.; Żytkowiak, R.; Karolewski, P.; Mucha, J.; Oleksyn, J. Patterns of structural and defense investments in fine roots of Scots pine (Pinus sylvestris L.) across a strong temperature and latitudinal gradient in Europe. Glob. Chang. Biol. 2017, 23, 1218–1231. [Google Scholar] [CrossRef] [PubMed]
- Di Iorio, A.; Montagnoli, A.; Terzaghi, M.; Scippa, G.S.; Chiatante, D. Effect of tree density on root distribution in Fagus sylvatica stands: A semi-automatic digitising device approach to trench wall method. Trees 2013, 27, 1503–1513. [Google Scholar] [CrossRef] [Green Version]
- Chen, W.; Zhang, Q.; Cihlar, J.; Bauhus, J.; Price, D.T. Estimating fine-root biomass and production of boreal and cool temperate forests using aboveground measurements: A new approach. Plant Soil 2004, 265, 31–46. [Google Scholar] [CrossRef]
- Finér, L.; Ohashi, M.; Noguchi, K.; Hirano, Y. Fine root production and turnover in forest ecosystems in relation to stand and environmental characteristics. For. Ecol. Manag. 2011, 262, 2008–2023. [Google Scholar] [CrossRef]
- Meng, S.; Jia, Q.; Zhou, G.; Zhou, H.; Liu, Q.; Yu, J. Fine root biomass and its relationship with aboveground traits of Larix gmelinii trees in Northeastern China. Forests 2018, 9, 35. [Google Scholar] [CrossRef]
- Sun, Z.; Liu, X.; Schmid, B.; Bruelheide, H.; Bu, W.; Ma, K. Positive effects of tree species richness on fine-root production in a subtropical forest in SE-China. J. Plant Ecol. 2017, 10, 146–157. [Google Scholar] [CrossRef]
- Zhang, Y.; Cai, X.; Lv, J. Size and dynamics of soil organic carbon stock in cropland of the Eastern Qinghai-Tibetan Plateau. Agric. Ecosyst. Environ. 2016, 222, 125–132. [Google Scholar] [CrossRef]
- Zhang, Y.; Gu, F.; Liu, S.; Liu, Y.; Li, C. Variations of carbon stock with forest types in subalpine region of southwestern China. For. Ecol. Manag. 2013, 300, 88–95. [Google Scholar] [CrossRef]
- Chen, B.; Liu, S.; Ge, J.; Chu, J. Annual and seasonal variations of Q10 soil respiration in the sub-alpine forests of the Eastern Qinghai-Tibet Plateau, China. Soil Biol. Biochem. 2010, 42, 1735–1742. [Google Scholar] [CrossRef]
- Meng, Z. A study on community structure and species diversity of subalpine forests in western Sichuan. J. Sichuan Agric. Univ. 2007, 25, 441–446. (In Chinese) [Google Scholar]
- Fang, X.; Yu, D.; Zhou, W.; Zhou, L.; Dai, L. The effects of forest type on soil microbial activity in Changbai Mountain, Northeast China. Ann. For. Sci. 2016, 73, 473–482. [Google Scholar] [CrossRef] [Green Version]
- Iuss Working Group Wrb. World Reference Base for Soil Resources 2014, Update 2015 International Soil Classification System for Naming Soils and Creating Legends for Soil Maps; World Soil Resources Reports No. 106; FAO: Rome, Italy, 2015. [Google Scholar]
- Xu, Z.; Zhu, J.; Wu, F.; Liu, Y.; Tan, B.; Yang, W. Effects of litter quality and climate change along an elevational gradient on litter decomposition of subalpine forests, Eastern Tibetan Plateau, China. J. For. Res. 2016, 27, 505–511. [Google Scholar] [CrossRef]
- Pang, X.; Ning, W.; Qing, L.; Bao, W. The relation among soil microorganism, enzyme activity and soil nutrients under subalpine coniferous forest in Western Sichuan. Acta Ecol. Sin. 2009, 29, 286–292. [Google Scholar] [CrossRef]
- Huang, G.; Zhao, X.Y.; Su, Y.G.; Zhao, H.L.; Zhang, T.H. Vertical distribution, biomass, production and turnover of fine roots along a topographical gradient in a sandy shrubland. Plant Soil 2008, 308, 201–212. [Google Scholar] [CrossRef]
- Xiong, Y.; Liu, X.; Guan, W.; Liao, B.; Chen, Y.; Li, M.; Zhong, C. Fine root functional group based estimates of fine root production and turnover rate in natural mangrove forests. Plant Soil 2017, 413, 83–95. [Google Scholar] [CrossRef]
- Konôpka, B.; Noguchi, K.; Sakata, T.; Takahashi, M.; Konôpková, Z. Fine root dynamics in a Japanese cedar (Cryptomeria japonica) plantation throughout the growing season. For. Ecol. Manag. 2006, 225, 278–286. [Google Scholar] [CrossRef]
- Vogt, K.A.; Persson, H. Measuring growth and development of roots. In Techniques and Approaches in Forest Tree Ecophysiology; Lassoie, J.P., HIckler, T.M., Eds.; CRC Press: Boca Raton, FL, USA, 1991. [Google Scholar]
- Sparks, D.L.; Page, A.L.; Helmke, P.A.; Loeppert, R.H.; Soltanpour, P.N.; Tabatabai, M.A.; Johnston, C.T.; Sumner, M.E. Methods of Soil Analysis; ASA, CSSA and SSSA: Madison, WI, USA, 1996; pp. 961–1010. [Google Scholar]
- Brunner, I.; Bakker, M.R.; Björk, R.G.; Hirano, Y.; Lukac, M.; Aranda, X.; Børja, I.; Eldhuset, T.D.; Helmisaari, H.S.; Jourdan, C.; et al. Fine-root turnover rates of European forests revisited: An analysis of data from sequential coring and ingrowth cores. Plant Soil 2013, 362, 357–372. [Google Scholar] [CrossRef] [Green Version]
- Fairley, R.I.; Alexander, I.J. Methods of calculating fine root production in forests. In Ecological Interactions in Soil: Plants, Microbes and Animals; Fitter, A.H., Atkinson, D., Read, D., Eds.; Blackwell Scientific Publications: Oxford, UK, 1985; pp. 37–42. [Google Scholar]
- Wang, C.; Han, S.; Zhou, Y.; Zhang, J.; Zheng, X.; Dai, G.; Li, M.-H. Fine root growth and contribution to soil carbon in a mixed mature Pinus koraiensis forest. Plant Soil 2016, 400, 275–284. [Google Scholar] [CrossRef]
- Zhou, Y.; Su, J.; Janssens, I.A.; Zhou, G.; Xiao, C. Fine root and litterfall dynamics of three Korean pine (Pinus koraiensis) forests along an altitudinal gradient. Plant Soil 2014, 374, 19–32. [Google Scholar] [CrossRef]
- Wang, S.; Wang, Z.; Gu, J. Variation patterns of fine root biomass, production and turnover in Chinese forests. J. For. Res. 2017, 28, 1185–1194. [Google Scholar] [CrossRef]
- Peichl, M.; Arain, M.A. Above- and belowground ecosystem biomass and carbon pools in an age-sequence of temperate pine plantation forests. Agric. For. Meteorol. 2006, 140, 51–63. [Google Scholar] [CrossRef]
- McCormack, M.L.; Adams, T.S.; Smithwick, E.A.H.; Eissenstat, D.M. Variability in root production, phenology, and turnover rate among 12 temperate tree species. Ecology 2014, 95, 2224–2235. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Xiang, W.H.; Tian, D.L.; Fang, X.; Peng, C.H. Overyielding of fine root biomass as increasing plant species richness in subtropical forests in central southern China. Chin. J. Plant Ecol. 2011, 35, 539–550, (In Chinese with English Abstract). [Google Scholar] [CrossRef]
- Meinen, C.; Hertel, D.; Leuschner, C. Biomass and morphology of fine roots in temperate broad-leaved forests differing in tree species diversity: Is there evidence of below-ground overyielding? Oecologia 2009, 161, 99–111. [Google Scholar] [CrossRef] [PubMed]
- Domisch, T.; Finér, L.; Dawud, S.M.; Vesterdal, L.; Raulund-Rasmussen, K. Does species richness affect fine root biomass and production in young forest plantations? Oecologia 2015, 177, 581–594. [Google Scholar] [CrossRef] [PubMed]
- Brassard, B.W.; Chen, H.Y.H.; Bergeron, Y. Influence of environmental variability on root dynamics in northern forests. Crit. Rev. Plant Sci. 2009, 28, 179–197. [Google Scholar] [CrossRef]
- Quan, X.; Wang, C.; Zhang, Q.; Wang, X.; Luo, Y.; Bond-Lamberty, B. Dynamics of fine roots in five Chinese temperate forests. J. Plant Res. 2010, 123, 497–507. [Google Scholar] [CrossRef] [PubMed]
- Persson, H.Å.; Stadenberg, I. Spatial distribution of fine-roots in boreal forests in eastern Sweden. Plant Soil 2009, 318, 1–14. [Google Scholar] [CrossRef]
- Chen, H.Y.H.; Brassard, B.W. Intrinsic and extrinsic controls of fine root life span. Crit. Rev. Plant Sci. 2013, 32, 151–161. [Google Scholar] [CrossRef]
- Persson, H.Å.; Stadenberg, I. Fine root dynamics in a Norway spruce forest (Picea abies (L.) Karst) in eastern Sweden. Plant Soil 2010, 330, 329–344. [Google Scholar] [CrossRef]
- Xiao, C.W.; Sang, W.G.; Wang, R.Z. Fine root dynamics and turnover rate in an Asia white birch forest of Donglingshan Mountain, China. For. Ecol. Manag. 2008, 255, 765–773. [Google Scholar] [CrossRef]
- Ma, Z.; Chen, H.Y.H. Effects of species diversity on fine root productivity in diverse ecosystems: A global meta-analysis. Glob. Ecol. Biogeogr. 2016, 25, 1387–1396. [Google Scholar] [CrossRef]
- Wardle, D.A. Communities and Ecosystems: Linking the Aboveground and Belowground Components; Princeton University Press: Princeton, NJ, USA, 2002; pp. 387–392. [Google Scholar]
- McCormack, L.M.; Adams, T.S.; Smithwick, E.A.H.; Eissenstat, D.M. Predicting fine root lifespan from plant functional traits in temperate trees. New Phytol. 2012, 195, 823–831. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, Y.S.; Chen, G.S.; Lin, P.; Xie, J.S.; Guo, J.F. Fine root distribution, seasonal pattern and production in four plantations compared with a natural forest in Subtropical China. Ann. For. Sci. 2004, 61, 617–627. [Google Scholar] [CrossRef] [Green Version]
- Liu, S.; Yang, H.; Luo, D.; Shi, Z.; Liu, Q.; Zhang, L. Seasonal dynamics of soil respiration and gross nitrification rate of different forests in the subalpine of western Sichuan. Acta Ecol. Sin. 2019, in press (In Chinese with English Abstract). [Google Scholar] [CrossRef]
- Zou, T.; Zhang, Z.; Li, N.; Yuan, Y.; Zheng, D.; Liu, Q.; Yin, H. Differential uptakes of different forms of soil nitrogen among major tree species in subalpine coniferous forests of western Sichuan, China. Chin. J. Plant Ecol. 2017, 41, 1051–1059, (In Chinese with English Abstract). [Google Scholar]
- Burton, A.J.; Pregitzer, K.S.; Hendrick, R.L. Relationships between fine root dynamics and nitrogen availability in Michigan northern hardwood forests. Oecologia 2000, 125, 389–399. [Google Scholar] [CrossRef] [PubMed]
- Tierney, G.L.; Fahey, T.J.; Groffman, P.M.; Hardy, J.P.; Fitzhugh, R.D.; Driscoll, C.T. Soil freezing alters fine root dynamics in a northern hardwood forest. Biogeochemistry 2001, 56, 175–190. [Google Scholar] [CrossRef]
- Silver, W.L.; Miya, R.K. Global patterns in root decomposition: Comparisons of climate and litter quality effects. Oecologia 2001, 129, 407–419. [Google Scholar] [CrossRef] [PubMed]
- Tierney, G.L.; Fahey, T.J.; Groffman, P.M.; Hardy, J.P.; Fitzhugh, R.D.; Driscoll, C.T.; Yavitt, J.B. Environmental control of fine root dynamics in a northern hardwood forest. Glob. Chang. Biol. 2003, 9, 670–679. [Google Scholar] [CrossRef]
- Majdi, H.; Pregitzer, K.; Moren, A.S.; Nylund, J.E.; Ågren, G.I. Measuring fine root turnover in forest ecosystems. Plant Soil 2005, 276, 1–8. [Google Scholar] [CrossRef]
- Kurz, W.A.; Kimmins, J.P. Analysis of some sources of error in methods used to determine fine roor production in forest ecosystems: A simulation approach. Can. J. For. Res. 1987, 17, 909–912. [Google Scholar] [CrossRef]





| Parameters | MF | NF | PF |
|---|---|---|---|
| Origin | Artificial + natural | Natural | Artificial |
| Average age (year) | ~28 | ~30 | ~30 |
| Altitude (m) | 2983~3046 | 2971~3029 | 2913~2955 |
| Slope | Southeast | Southeast | Southeast |
| Stand height (m) | 6.07 ± 0.39 b | 10.19 ± 0.96 a | 8.28 ± 0.33 a |
| Stand DBH (cm) | 9.02 ± 0.37 b | 9.50 ± 0.26 b | 11.40 ± 0.38 a |
| Stand density (stem ha−1) | 2266.67 ± 134.11 a | 2083.33 ± 151.61 a | 1433.33 ± 88.19 b |
| Stand basal area (m2 ha−1) | 14.41 ± 0.52 a | 14.67 ± 0.41 a | 14.65 ± 0.84 a |
| Aboveground biomass (kg m−2) | 6.75 ± 0.12 a | 7.02 ± 0.13 a | 7.28 ± 0.18 a |
| Dominant tree species 1 | Picea asperata Mast. (51.42) | Abies faxoniana Rehd. et Wils (36.54) | Picea asperata Mast. (100) |
| Betula albo-sinensis Burk. (23.47) | Betula albo-sinensis Burk. (28.09) | ||
| Cerasus duclouxii (Koehne) Yu et Li (11.64) | Sorbus koehneana Schneid. (10.50) | ||
| Sorbus koehneana Schneid. (5.35) | Cerasus duclouxii (Koehne) Yu et Li (8.53) | ||
| Acer L. (5.02) | Betula utilis D. Don (6.11) | ||
| Acer L. (5.56) | |||
| Tree species diversity | 0.75 ± 0.06 a | 0.91 ± 0.04 a | 0 |
| Soil C content (g kg−1) | |||
| 0–10 cm | 47.54 ± 6.89 a | 48.28 ± 7.84 a | 33.80 ± 7.59 a |
| 10–20 cm | 25.84 ± 3.56 a | 43.92 ± 5.07 a | 26.93 ± 5.70 a |
| 20–30 cm | 16.60 ± 2.67 a | 42.06 ± 8.74 a | 25.10 ± 5.29 a |
| Soil N content (g kg−1) | |||
| 0–10 cm | 3.02 ± 0.53 a | 2.73 ± 0.25 a | 1.95 ± 0.66 a |
| 10–20 cm | 1.51 ± 0.30 a | 2.38 ± 0.18 a | 1.30 ± 0.45 a |
| 20–30 cm | 0.78 ± 0.16 a | 2.25 ± 0.36 a | 1.30 ± 0.53 a |
| C:N ratio | |||
| 0–10 cm | 15.99 ± 0.66 a | 17.60 ± 1.80 a | 18.85 ± 2.34 a |
| 10–20 cm | 17.51 ± 1.00 a | 18.48 ± 1.72 a | 22.61 ± 2.86 a |
| 20–30 cm | 21.86 ± 1.22 a | 18.53 ± 1.71 a | 22.28 ± 3.75 a |
| Trend | Live | ||
|---|---|---|---|
| Increase | Decrease | ||
| Dead | ∆Blive < ∆Bdead | ∆Blive > ∆Bdead | |
| Increase | P = ∆Blive + ∆Bdead | P = ∆Blive + ∆Bdead | P = 0 |
| Decrease | P = ∆Blive | P = 0 | |
| Source | d.f. | FRB | FRN | FRB:FRN | FRP | FRT |
|---|---|---|---|---|---|---|
| Type | 2 | 96.42 *** | 61.69 *** | 21.17 *** | 37.25 *** | 59.56 *** |
| Depth | 2 | 417.04 *** | 217.87 *** | 11.14 ** | 699.38 *** | 68.43 *** |
| Type × Depth | 4 | 66.55 *** | 14.78 *** | 3.43 * | 54.20 *** | 1.15 NS |
| Fine Root Dynamics | Ammonium | Nitrate | Inorganic N |
|---|---|---|---|
| Fine root biomass | −0.96 ** | 0.07 | −0.91 ** |
| Fine root production | −0.88 ** | 0.04 | −0.86 ** |
| Fine root turnover rate | 0.88 ** | −0.16 | 0.80 ** |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, S.; Luo, D.; Yang, H.; Shi, Z.; Liu, Q.; Zhang, L.; Kang, Y. Fine Root Dynamics in Three Forest Types with Different Origins in a Subalpine Region of the Eastern Qinghai-Tibetan Plateau. Forests 2018, 9, 517. https://doi.org/10.3390/f9090517
Liu S, Luo D, Yang H, Shi Z, Liu Q, Zhang L, Kang Y. Fine Root Dynamics in Three Forest Types with Different Origins in a Subalpine Region of the Eastern Qinghai-Tibetan Plateau. Forests. 2018; 9(9):517. https://doi.org/10.3390/f9090517
Chicago/Turabian StyleLiu, Shun, Da Luo, Hongguo Yang, Zuomin Shi, Qianli Liu, Li Zhang, and Ying Kang. 2018. "Fine Root Dynamics in Three Forest Types with Different Origins in a Subalpine Region of the Eastern Qinghai-Tibetan Plateau" Forests 9, no. 9: 517. https://doi.org/10.3390/f9090517





