Abstract
Tropical forests are subject to seasonal hurricanes resulting in cycles of canopy opening and deposition of litter, followed by periods of recovery and canopy closure. Herein, we review two studies of litter-based communities in Puerto Rico; (i) a survey of bromeliad invertebrates in three montane forest types along an elevational gradient in 1993–1997, during a period of canopy recovery after two severe hurricanes, and the results compared with those from a resurvey in 2010, and (ii) a large scale canopy trimming experiment in the lower montane (Tabonuco) forest designed to simulate an hurricane event, and to separate the effects of canopy opening from debris deposition. Measurements of changes in invertebrate community parameters and decay rates of litter were made in a litter bag experiment as part of this major experiment. As the canopy closed, during the periods of study, bromeliad density reduced, especially in the Tabonuco forest. This was associated with a decline in both alpha and gamma invertebrate diversity, which appears to have involved the loss of rarer species. In the Tabonuco forest, two endemic bromeliad specialists were not found during resampling in 2010, though the most common species were remarkably stable over the two decades. Canopy opening significantly altered the diversity, biomass, and composition of litter communities, irrespective of litter deposition. It particularly reduced organisms responsible for comminution of litter and increased the activity of fungivores and microbiovores. Both studies showed that canopy disturbance, either indirectly or directly, adversely affects invertebrate diversity and detrital processing.
1. Introduction
Caribbean forests are shaped by hurricanes that cause structural damage to the canopy through defoliation, branch, and tree falls, with consequential changes in light regime, microclimate, and increased heterogeneity in the understorey [1]. Changes in light regime are necessarily cyclical, as pioneers and other tree species fill gaps and the remaining canopy recovers between successive hurricanes, resulting in the suppression of the understorey. There is also evidence of generally increasing liana abundance and biomass in New World forests due to increasing forest disturbance, fragmentation, and elevated atmospheric CO2 [2]. This increase in lianas can add to shading and result in decreased tree diversity, recruitment, growth, and survival [2], and could also reduce understorey bromeliad density. In 1989, the Luquillo Experimental Forest (LEF) in Puerto Rico was severely damaged by Hurricane Hugo, and again by Hurricane Georges in 1998. Herein, we review the changes in two litter-based invertebrate communities, one in bromeliads in three forest types along an elevational gradient following canopy recovery after two severe hurricanes, and forest floor litter invertebrates in an experimental canopy trimming experiment (CTE), designed to simulate a hurricane in the Caribbean island of Puerto Rico.
The three forest types are:
- (i)
- Tabonuco forest, with Tabonuco (Dacryodes excelsa Vahl), motillo (Sloanea berteriana Choisy), and sierra palm (Prestoea montana (Graham) Nicholson) as the dominants in the canopy. The trees are tall, the canopy is dense, with thin, large, simple, or compound leaves, and little understorey in the mature forest, except in riparian areas. It has the highest diversity of tree species of the three forests. Tank bromeliads in the lower canopy and forest floor are infrequent, except in riparian areas and tree gaps.
- (ii)
- Palo colorado (Cyrilla racemiflora L. dominant). Trees are lower in height, with a more open canopy and simple leaves. Bromeliads are generally present in the lower canopy and forest floor.
- (iii)
- Elfin (dwarf) forest (Tabebuia rigida Urban). Trees are short (<5 m, smaller on exposed summits) and slow growing in extremely wet anaerobic soils, with microphyllous and coriaceous leaves. Frequently above the cloud condensation level. Bromeliads are extremely abundant, and adapted to high light intensity with red pigmentation.
Rainfall increases with elevation (approximately 3500, 4000, and 4500 mm/year−1 respectively at our three sites), and temperature decreases with elevation (ca 24 °C, 21.5 °C, and 19.5 °C mean daily temperature). From 1988–2008, which covers most of the study period, annual rainfall varied over 5–7 year cycles, but did not show any long term trend [3].
Bromeliads, as epiphytic and saxicolous plants, are dependent on their invertebrate fauna to obtain litter minerals. Their roots do not absorb minerals, but act as holdfasts. Bromeliads absorb minerals through specialized trichomes at their leaf bases, from the breakdown of intercepted litter by invertebrates living in and above the retained water of the phytotelm. The effects of canopy disturbance on litter invertebrates may be direct, as a result of changes in litter and soil moisture, temperature, nutrient resources, and light regime, as a consequence of canopy opening and litter deposition, or indirect, through changes in the forest affecting the distribution of bromeliads, and thus, their invertebrate communities.
2. Canopy Cover and Bromeliad Ecology
We studied bromeliad invertebrate diversity, abundance, and community composition from 1993–1997, during the recovery period, and again in 2010 [4,5,6,7]. The hurricanes had noticeably affected bromeliad distribution and density in some areas, and the opportunity was taken to study the long-term effects on bromeliad invertebrate communities during canopy recovery, along an elevational gradient, over a period of 13–17 years.
Bromeliad plant populations (Guzmania berteroniana (Schult. & Schult.f.) Mez, Guzmania lingulata (L.) Mez, and Werauhia (Vriesea) sintenisii (Baker) J.R. Grant) in the lower canopy and forest floor were reduced as supporting branches fall to the ground. Their numbers increased after the canopy opened, and then decreased as the canopy recovered over the longer term because of changes in the light regime. Bromeliads, even shade-tolerant ones such as Guzmania lingulata, were not regarded as true shade plants [8], but rather as plants able to tolerate high light levels but requiring a higher humidity than the lower canopy. This assertion was substantiated in physiological experiments [9] that demonstrated the ubiquitous capacity of bromeliads to tolerate high light intensity. This dual requirement for light and high humidity also controls the distribution of bromeliads along the elevational gradient in the LEF. The highest density was in the Elfin forest, with little effective canopy, with small branches and sclerophyllous leaves providing little shade, and often immersed in cloud. In 2010 the density was 36,900 ha−1, 15% more than in 1993–1997 (Table 1). Bromeliads have become noticeably less frequent in the Tabonuco forest. In 1993–1997 they mainly occurred in the riparian zones, and were only found near ground level a short distance into the forest, no further than 12 m in from the edge of rivers. The mean density in this zone was 940 ha−1 (=45 ha−1 averaged over the Tabonuco as a whole [4]). In 2010 they were confined even more closely to the zone at the river edge, mostly <5 m from the bank, to give an overall density along the river edge of 135 ha−1, an 85% reduction (ca 2 ha−1 in the Tabonuco forest as a whole). In 2010 the Palo colorado forest populations were approximately half of what they were in 1993–1997 (1600 ha−1 cf. 3100 ha−1 in 1993–1997). Densiometer readings above bromeliads, in the Tabonuco forest only, gave a mean of 80 percent canopy cover (mean min. 70% towards the river, mean max. 91% towards the forest). In parts of the Tabonuco forest where no bromeliads occurred, canopy cover was 91–95% [7].

Table 1.
Canopy cover, bromeliad density, and animal abundance and diversity in Luquillo Experimental Forest (LEF) bromeliads at two different times. SE in parentheses.
The effect of post-hurricane forest recovery on the bromeliad fauna was unknown prior to our recent research. On the one hand, any change to the forest structure affecting understorey bromeliad density could affect their specialized invertebrate communities because of loss or isolation of suitable plants. On the other hand, the aquatic and moist litter habitat in bromeliads buffers any changes in the microclimate, invertebrate communities in bromeliads may be predicted to remain stable despite changes in the forest. We tested the stability of LEF bromeliad communities in the three forest types along the elevational gradient by comparing the 1993–1997 samples with those taken in 2010 [6].
The multiple years of observation within the 1990s allowed us to evaluate whether the observed communities in 2010 were within the known annual variation in this system. We considered three questions: (1) Did the diversity of species per bromeliad (alpha diversity) change between the 1990s and 2010? (2) Did the diversity of species within each forest (gamma diversity) change between the 1990s and 2010? (3) If species diversity changed from the time of our first sampling, which types of species increased or decreased the most? For all questions, we needed to account for the covariate of bromeliad size, known to have strong effects on abundance and diversity in this system [7,10,11].
Generalized linear models (GLM) were used to examine year effects on alpha diversity (species richness per bromeliad) and total abundance per bromeliad, using bromeliad diameter as a covariate. If year effects were significant, we then examined whether the years in the 1990s could be pooled without affecting the model, indicating that the largest temporal difference was between decades, not within the 1990s. The GLMs for alpha diversity were based on a Poisson error distribution (or quasiPoisson in the case of overdispersion) with a natural log link. The GLMs were based on quartic-root transformed abundance. Different transformations were tested, and the quartic-root provided the best fit to the data. Finally, we used the ‘year x diameter’ models to predict species richness and abundance for the average diameter of bromeliad samples in each forest type, in each year. For more details of this analysis, and the results illustrated in Figure 1, see [7].
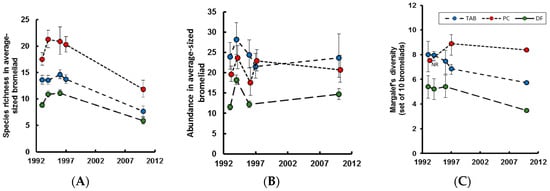
Figure 1.
Changes in bromeliad invertebrate communities over time. Predicted means with 95% confidence intervals (for details see [6]). (A) Species richness; (B) Animal abundance; (C) Gamma diversity. See [7]. NR = no replication for this data point; TAB = Tabonuco; PC = Palo colorado; DF = Elfin forest.
Alpha diversity (species per bromeliad) was significantly lower in the Tabonuco and Palo colorado in 2010, as compared to the 1990s (Figure 1A), despite broadly similar total numbers of individuals per plant (Figure 1B). Similar trends were seen in Elfin forest, but variance between years in the 1990s rendered the reduced diversity in 2010 nonsignificant (Table 1, Figure 1A) [7]. As shown previously, bromeliads in the Elfin forest had the fewest invertebrate species and individuals, in part because they had only half the plant diameter of bromeliads in the other forest types (Figure 1A,B) [4]. Palo colorado bromeliads consistently had the most species, even though the mean diameter of sampled bromeliads in this zone (79 cm) was similar to that in the Tabonuco (75 cm). Gamma diversity, the diversity of bromeliad-associated species per forest, as estimated from the combined contents of ten bromeliads, was significantly lower in both the Tabonuco and Elfin forest in 2010 than in any of the sampled years from the 1990s (2010 below 95% CI of all years in the 1990s: Figure 1C). In the Palo colorado forest, gamma diversity tended to be lower in 2010 than in 1997, but this difference was not significant (2010 within 95% CI of 1997: Figure 1C); comparisons were not possible with remaining years in the 1990s as the sampled size range of bromeliads did not overlap sufficiently with that in 2010 [7].
Within any one forest-type there were relatively few differences between the mean abundances or frequencies of common taxa in samples collected between 1993–1997 and those collected in 2010. In the Tabonuco forest, two taxa that were frequent in the early years were absent in 2010. Both species are bromeliad specialists, a hydrophilid beetle (Omicrus ingens M. Hansen & B.A. Richardson) whose larvae and adults were newly described from Puerto Rico in the earlier studies [12], and appear to be endemic both to Puerto Rico and bromeliads, and a pseudoscorpion (Macrochernes attenuatus Muchmore), also endemic and found only in bromeliads. Incidence of these two species, after accounting for bromeliad size, differed primarily between the 1990s and 2010 (O. ingens: 2010 vs. 1990s, χ2 = 5.68, p = 0.017, M. attenuatus: χ2 = 6.98, p = 0.008) rather than within the 1990s (no significant difference between models with all years versus those with 1990s vs. 2010, ANOVAs, p > 0.05). Similar changes in the occurrence of O. ingens and M. attenuatus were not seen in the Palo colorado forest, and neither occurred in the Elfin forest. Wyeomyia mosquito larvae, which normally have highest incidence in smaller bromeliads (a pattern also reported in Costa Rica) [13], were largely absent from small bromeliads in the Palo colorado in 2010 (decade x bromeliad diameter: χ2 = 6.68, p = 0.007), reducing their overall incidence in this habitat. The only common species to increase in frequency over time was the chironomid Tanytarsus bromelicola P.S. Cranston (decade: χ2 = 12.1, p = 0.0003), whose larvae were more common in Palo colorado in 2010 than the 1990s.
Despite decreases in three species, and increases in another, the 12 most common species were generally remarkably constant in occurrences over the 17 years period of this study. The observed declines in alpha and gamma diversity must, therefore, have involved the loss of some of the rarer species. Certainly, preferential loss of rare species is what is expected from standard metapopulation theory—Any increase in extinction rates will drive those species extinct that initially occur in the fewest patches.
Habitat fragmentation and loss bring about a reduction in resources and their dependant populations, increased risks of extinction of small populations, and isolation which can prevent recruitment from other populations [14]. Approximately 80% of bromeliad invertebrates are larval or nymphal forms that, as adults, pass into the general forest ecosystem before returning to breed [5]. All life-stages of the endemic and bromeliad specialist beetle, Omicrus ingens, and the pseudoscorpion, Macrochernes attenuatus, however, live permanently in bromeliad plants and this lack of vagility makes them especially vulnerable, with increasing isolation of bromeliads in the Tabonuco forest.
3. Canopy Trimming Experiment
Canopy leaves, twigs, fruits, and seeds inevitably become litter, which either lodges in epiphytes, branches, tree holes, or mycelial webs in the understorey [15], or falls to the forest floor, to provide a habitat for mainly detritivore and fungivore invertebrate communities which aid its breakdown. The decomposition and mineralization of litter provides the major pathway for transferring nutrients from vegetation to soil and epiphytes such as bromeliads, with their invertebrate communities. Knowledge of nutrient pools, the fluxes between them, and losses from the system, is essential for an understanding of nutrient recycling and forest productivity.
The effect of canopy opening on forest floor litter invertebrates was studied as part of a large Canopy Trimming Experiment (CTE), designed to simulate a hurricane, and to separate the effects of canopy opening (changes in temperature, humidity, throughfall, and light) from debris deposition (changes in nutrient levels and the physical structure of the forest floor), in a study on the effects of an increasing frequency of storms, as a possible consequence of climate change [16,17,18].
In each of three blocks there were four randomized 30 × 30 m treatment plots, which were treated as follows:
- Canopy trimmed and trimmed biomass distributed on forest floor.
- Canopy trimmed and trimmed biomass removed from the plot.
- Canopy not trimmed but canopy biomass from a trimmed plot distributed on forest floor.
- Canopy not trimmed and no canopy biomass added to forest floor.
Each plot was divided into subplots and replicate litter bags were placed in three subplots within each plot.
Removing the canopy increased soil moisture and reduced litter moisture at the start of the experiment and, over the duration of the experiment the differences were reduced as the canopy closed (Figure 2A,B). The canopy trimming, and redistribution as required, took from October 2004 to June 2005. Litter bags were placed in the plots in June 2005 and retrieved at intervals. We studied the colonisation and decay of leaf litter in litter bags by invertebrates from June 2005 to January 2007 [19,20]. Invertebrates were recovered from the bags using Tullgren funnels and were identified, counted, and measured for calculation of diversity and biomass. Seven recoveries of litter bags (1008 total) and extractions of invertebrates (52,035 total) were made over a period of 19 months. During that time canopy openness in trimmed plots decreased linearly, from approximately 20% after trimming (much less might have been expected, but the canopy from the surrounding forest was still providing some oblique cover), to the same level as in the untrimmed plots, approx. 7% (Figure 3A).
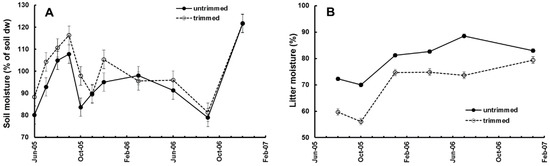
Figure 2.
Habitat parameters over time. (A) Soil moisture; (B) Litter moisture. See [19].
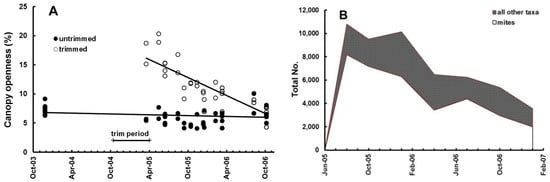
Figure 3.
Changes over time. (A) Canopy openness; (B) No. of invertebrates in litter bags at each recovery. See [19].
Invertebrate community parameters changed significantly during the course of the experiment. At the first collection, 2 months after placing, the litter bags were well-colonized by invertebrates from their surrounding environment, mainly fungivores, mites, ants, and collembola which together comprised 95% of all organisms by number. Animal abundance declined in the following 17 months, as litter in the bags was consumed and decayed. Other taxa increased proportionately during that period, as mites, ants, and collembola counts decreased to 80% (Figure 3B). Animal abundance, biomass, and diversity in the litter bags were significantly lower in trimmed plots than those with intact canopy, irrespective of litter addition (Figure 4A,B). There was also a reduction in the abundance of coqui frogs (Eleutherodactylus coqui Thomas) in these same plots [21]. Individual taxa responded differently to the trimming of the canopy, causing a major shift in community composition. Mites, Collembola, Psocoptera, and all fungivores, responded positively in trimmed plots, whereas Homoptera and Hemiptera, isopods, and millipedes responded negatively (Figure 5A,B), as did all other taxonomic groups.
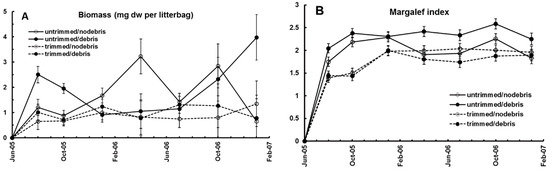
Figure 4.
The effect of canopy trimming on biomass and diversity in litterbags. (A) Invertebrate biomass; (B) Margalef diversity index. See [19].
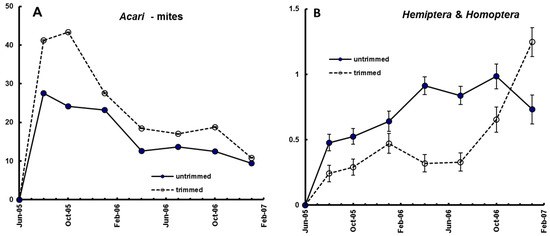
Figure 5.
Examples of the effect of canopy trimming on mean number of animals per litterbag. (A) Acari; (B) Hemiptera & Homoptera. For other taxonomic groups see [19].
It is known that fungal biomass in the LEF changes rapidly in response to moisture [15]. In the CTE fungal biomass in the trimmed and untrimmed plots was similar, but fungal community composition was not [22]. Trimmed plots, with lower litter moisture, had lower basidiomycete white-rot activity, suggesting a shift to microfungi. Greater growth of microfungi in the drier cut plot litter could explain the increase of microbiovores—mites and collembola (Figure 4A,B). Changes in invertebrate communities in the trimmed plots may be explained by three mechanisms: migration from adverse conditions of light, heat, and drought; differential mortality of taxa sensitive to disturbance; and interaction with the changing fungal community. There was a negative correlation between Margalef’s diversity index of litter arthropods and Percentage Mass Remaining (PMR) in the litter bags suggesting that functional complexity is an important determinant of decay in this forest [20]. In trimmed plots with lower invertebrate diversity mean PMR was 19.5% and 14.3% (SEs 1.5%) in untrimmed plots. During this decay process the percentages of N, Al, Ca, Mg, and Fe in the litter increased over time as the litter in the bags decayed in trimmed plots. After 1.6 year nitrogen mineralization was still reduced in trimmed plots compared to untrimmed plots, and P and Ca were significantly immobilized in the first 0.6 year [20]. Canopy opening led to increased seedling recruitment, particularly of trees and shrubs [23,24], and was associated with increased herbivory on these highly nutritious pioneer plants [25]. Herbivorous phasmids were found to reduce the decomposition rate of litter by 50% because of reduction in high quality litter abundance through selective feeding and, consequently, lowered bacterial richness and abundance [25]. Litter snails, once assumed to be comminuters of litter, did not affect decomposition rates [26]. These results, from a simulated hurricane, demonstrate that canopy opening can adversely impact litter decomposition, and associated nutrient release from litter.
4. Conclusions
In forests the canopy is of prime importance in circulation of nutrients. Its production of leaves, flowers, fruits, and small wood falls to the ground, or is trapped by lower canopy and saxicolous bromeliads. This litter is processed by invertebrate detritivores, bacteria, and fungi, so returning minerals to the ecosystem. Litterfall varies annually and is higher prior to the dry season, and declines with elevation, but increases during hurricanes. Changes in canopy cover determine the structure of the understorey and distribution of plants, such as bromeliads, indirectly affecting their invertebrate communities, or directly affecting decomposition process on the forest floor, as shown in the CTE. Canopy opening was the major determinant of changes in forest floor invertebrate communities, bringing about reduction in diversity and biomass, and causing changes in community composition. These changes adversely affected decay rates of litter and associated nutrient release and cycling. As habitat monitors, surveys of bromeliad faunas would appear to be suitable tools. This study has shown that the data from bromeliads, from three forest types with different faunal communities, can be used for capturing their non-random temporal changes in abundance, frequency of occurrence, and diversity. Specifically, it demonstrated the decline in invertebrate species richness and loss of species between decades during a period of canopy recovery as bromeliad density declined.
Both studies showed the importance of changes in canopy cover resulting from disturbance, such as hurricanes, in determining forest processes and ecology. Habitat fragmentation and loss bring about a reduction in resources for dependant populations, increased risks of extinction of small populations, and isolation which can prevent recruitment from other populations. Hurricane Maria severely damaged the LEF in October 2017. There will be a good opportunity to monitor the recovery and resilience of the forest following that event, which has had several thousand years of evolution in a hurricane regime.
Author Contributions
B.R. and M.R. conceived and carried out the bromeliad studies; G.G. conceived the litter bag experiment in the CTE; B.R. and M.R. processed the invertebrates and, with G.G., analyzed the data; B.R. and M.R wrote the paper. G.G. reviewed a previous version of the manuscript.
Funding
The original bromeliad studies were carried out with funding to B.R. from the Royal Society of Edinburgh, the Carnegie Trust for the Universities of Scotland, and Edinburgh Napier University for travel. The studies in 2010 were performed by M.R. and B.R. under grant DEB-0620910 from the National Science Foundation to the Institute for Tropical Ecosystems Studies, University of Puerto Rico, and the International Institute of Tropical Forestry, as part of the Luquillo Long-Term Ecological Research programme in the Luquillo Experimental Forest, and USDA IITF Grant 01-1G11120101-001. The CTE study was supported by Grants DEB-0218039 and DEB-0620910 from the National Science Foundation to the Institute for Tropical Ecosystem Studies (ITES), University of Puerto Rico (UPR), and the International Institute for Tropical Forestry USDA Forest Service. These grants support the Luquillo Long-Term Ecological Research Program in Puerto Rico.
Acknowledgments
Thanks to D.S. Srivastava for the interdecadal statistical analysis of the bromeliad data. Thanks also to the staff of El Verde Field Station, Institute for Tropical Ecosystems Studies, and the International Institute of Tropical Forestry, Río Piedras, for continued help and support, and to two anonymous reviewers for helpful comments.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Brokaw, N.V.L.; Zimmerman, J.K.; Willig, M.R.; Camilo, G.R.; Covich, A.P.; Crowl, T.A.; Fetcher, N.; Haines, B.L.; Lodge, D.J.; Lugo, A.E.; et al. Response to disturbance. In A Caribbean Forest Tapestry; Brokaw, N., Crowl, T.A., Lugo, A.E., McDowell, W.H., Scatena, F.N., Waide, R.B., Willig, M.R., Eds.; Oxford University Press: New York, NY, USA, 2012; pp. 201–271. [Google Scholar]
- Schnitzer, F.A.; Bongers, F. Increasing liana abundance and biomass in tropical forests: Emerging patterns and putative mechanism. Ecol. Lett. 2011, 14, 397–406. [Google Scholar] [CrossRef] [PubMed]
- Harris, N.L.; Lugo, A.E.; Brown, S.; Heartsill Scalley, T. Luquillo Experimental Forest: Research History and Opportunities; EFR-1 Department of Agriculture: Washington, DC, USA, 2012; p. 152.
- Richardson, B.A. The bromeliad microcosm and the assessment of faunal diversity in a Neotropical forest. Biotropica 1999, 31, 321–336. [Google Scholar] [CrossRef]
- Richardson, B.A.; Richardson, M.J.; Scatena, F.N.; McDowell, W.H. Effects of nutrient availability and other elevational changes on bromeliad populations and their invertebrate communities in a humid tropical forest in Puerto Rico. J. Trop. Ecol. 2000, 16, 167–188. [Google Scholar] [CrossRef]
- Richardson, B.A.; Richardson, M.J. Litter-based invertebrate communities in forest floor and bromeliad microcosms along an elevational gradient in Puerto Rico. In Ecological Gradient Analyses in a Tropical Landscape; González, G., Willig, M.R., Waide, R.B., Eds.; Wiley-Blackwell: Hoboken, NJ, USA, 2013; Volume 54, pp. 101–115. ISBN 978-1-118-65932-8. [Google Scholar]
- Richardson, M.J.; Richardson, B.A.; Srivastava, D.S. The Stability of Invertebrate Communities in Bromeliad Phytotelmata in a Rain Forest Subject to Hurricanes. Biotropica 2015, 47, 201–207. [Google Scholar] [CrossRef]
- Pittendrigh, C.S. The bromeliad-Anopheles malaria complex in Trinidad. I-The bromeliad flora. Evolution 1948, 13, 998–1003. [Google Scholar] [CrossRef]
- Griffiths, H.; Maxwell, K. In memory of C.S. Pittendrigh: Does exposure in forest canopies relate to photoprotective strategies in epiphytic bromeliads? Funct. Ecol. 1999, 13, 15–23. [Google Scholar] [CrossRef]
- Araújo, V.A.; Melo, S.K.; Araújo, A.P.A.; Gomes, M.L.M.; Carneiro, M.A.A. Relationship between invertebrate fauna and bromeliad size. Braz. J. Biol. 2007, 67, 611–617. [Google Scholar] [CrossRef] [PubMed]
- Dézerald, O.; Leroy, C.; Corbara, B.; Carrias, J.-F.; Pélozuelo, L.; Dejean, A.; Céréghino, R. Food-web structure in relation to environmental gradients and predator-prey ratios in tank-bromeliad ecosystems. PLoS ONE 2013, 8, e71735. [Google Scholar] [CrossRef] [PubMed]
- Hansen, M.; Richardson, B.A. A new species of Omicrus Sharp (Coleoptera: Hydrophilidae) from Puerto Rico and its larva, the first known larva of Omicrini. Syst. Entomol. 1998, 23, 1–8. [Google Scholar] [CrossRef]
- Gilbert, B.; Srivastava, D.S.; Kirby, K.R. Niche partitioning at multiple scales facilitates coexistence among mosquito larvae. Oikos 2008, 117, 944–950. [Google Scholar] [CrossRef]
- Brown, J.H.; Lomolino, M.V. Biogeography, 2nd ed.; Sinauer Associates: Sunderland, MA, USA, 1998; ISBN 0-87893-073-6. [Google Scholar]
- Lodge, D.J. Microorganisms. In The Food Web of a Tropical Rain Forest; Reagan, D.P., Waide, R.B., Eds.; University of Chicago Press: Chicago, IL, USA, 1996; pp. 53–108. ISBN 0-226-70600-1. [Google Scholar]
- Shiels, A.B.; González, G. Understanding the key mechanisms of tropical forest responses to canopy loss and biomass deposition from experimental hurricane effects. For. Ecol. Manag. 2014, 332, 1–10. [Google Scholar] [CrossRef]
- Shiels, A.B.; González, G.; Willig, M.R. Responses to canopy loss and biomass deposition in a tropical forest ecosystem: Synthesis from an experimental manipulation simulating effects of hurricane disturbance. For. Ecol. Manag. 2014, 332, 124–133. [Google Scholar] [CrossRef]
- Shiels, A.B.; González, G.; Lodge, D.J.; Willig, M.R.; Zimmerman, J.K. Cascading Effects of Canopy Opening and Debris Deposition from a Large-Scale Hurricane Experiment in a Tropical Rain Forest. BioScience 2015, 65, 871–881. [Google Scholar] [CrossRef]
- Richardson, B.A.; Richardson, M.J.; González, G.; Shiels, A.B.; Srivastava, D.S. A canopy trimming experiment in Puerto Rico: The response of litter invertebrate communities to canopy loss and debris deposition in a tropical forest subject to hurricanes. Ecosystems 2010, 13, 286–301. [Google Scholar] [CrossRef]
- González, G.; Lodge, D.J.; Richardson, B.A.; Richardson, M.J. A canopy trimming experiment in Puerto Rico: The response of litter decomposition and nutrient release to canopy opening and debris deposition in a subtropical wet forest. For. Ecol. Manag. 2014, 332, 32–46. [Google Scholar] [CrossRef]
- Klawinski, P.D.; Dalton, D.J.; Shiels, A.B. Coqui frog populations are negatively affected by canopy opening but not detritus deposition following an experimental hurricane in a tropical rain forest. For. Ecol. Manag. 2014, 332, 118–123. [Google Scholar] [CrossRef]
- Rivera-Figueroa, F.J. Efecto de un Disturbio Natural en el Perfil de Ácidos Grasos de Comunidades Microbianas en el Bosque Experimental de Luquillo en Puerto Rico. Master’s Thesis, Universidad del Turabo, Gurabo, Puerto Rico, 2008. [Google Scholar]
- Shiels, A.B.; Zimmerman, J.K.; Garcia-Montiel, D.C.; Jonckheere, I.; Holm, J.; Horton, D.; Brokaw, N. Plant responses to simulated hurricane impacts in a subtropical wet forest, Puerto Rico. J. Ecol. 2010, 98, 659–673. [Google Scholar] [CrossRef]
- Zimmerman, J.K.; Hogan, J.A.; Shiels, A.B.; Bithorn, J.E.; Matta Carmona, S.; Brokaw, N. Seven-year responses of trees to experimental hurricane effects in a tropical rainforest, Puerto Rico. For. Ecol. Manag. 2014, 332, 64–74. [Google Scholar] [CrossRef]
- Prather, C. Divergent responses of leaf herbivory to simulated hurricane effects in a rainforest understory. For. Ecol. Manag. 2014, 332, 87–92. [Google Scholar] [CrossRef]
- Prather, C.M.; Belovsky, G.E.; Cantrell, S.A.; González, G. Tropical herbivorous phasmids, but not litter snails, alter decomposition rates by modifying litter bacteria. Ecology 2018, 99, 782–791. [Google Scholar] [CrossRef] [PubMed]
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).