Disentangling Contributions of CO2 Concentration and Climate to Changes in Intrinsic Water-Use Efficiency in the Arid Boreal Forest in China’s Altay Mountains
Abstract
:1. Introduction
2. Data and Methods
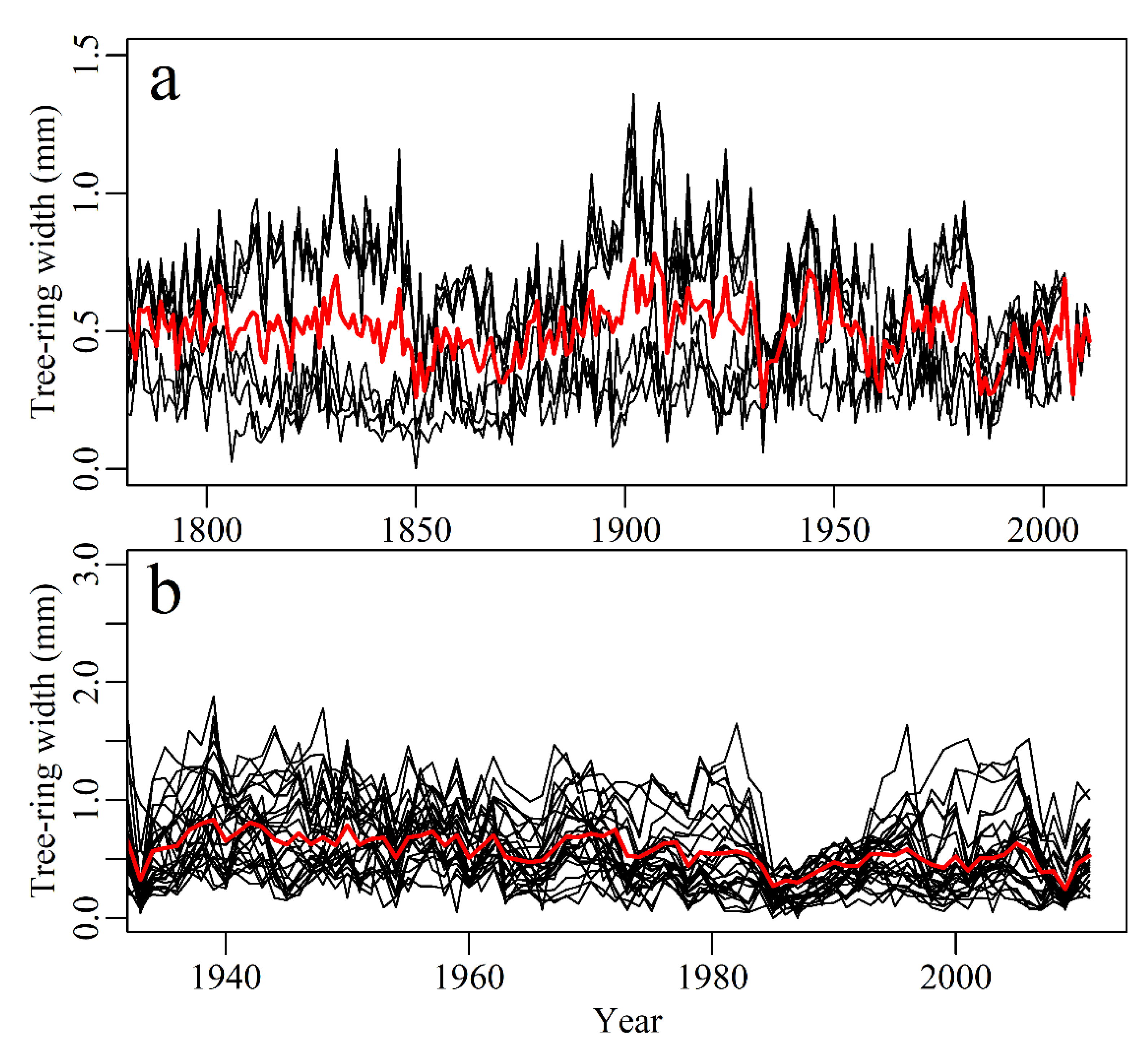
2.1. Sampling Site and Tree-Ring Width Measurements
2.2. Climate Data
2.3. Tree-Ring Stable Isotope Measurements
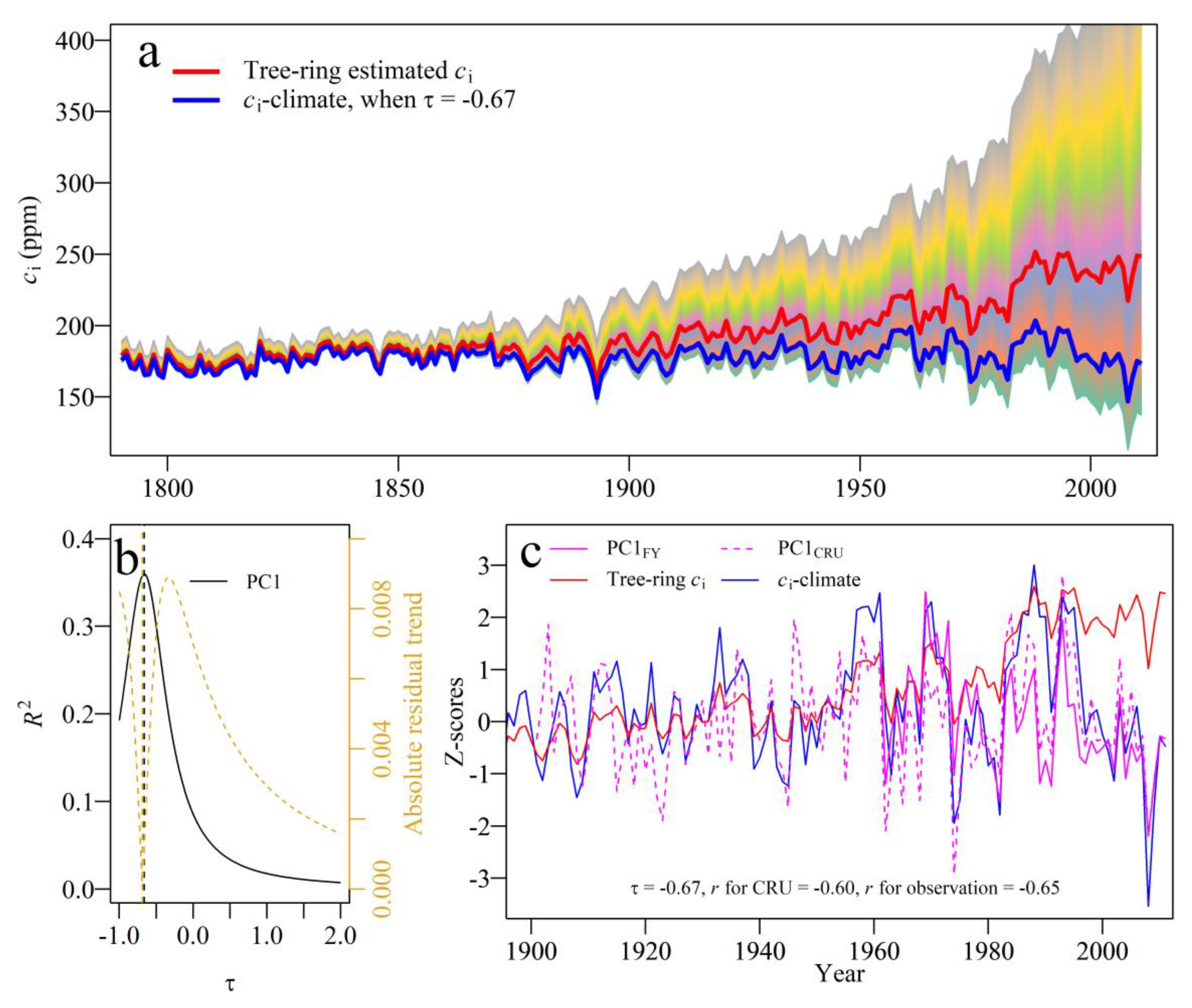
2.4. IWUE Estimation from Tree-Ring Δ13C and Climate Optimization in ci
2.5. Data Analysis
2.6. Climatic Signals from Tree-Ring δ18O
2.7. Tree-ring Growth and IWUE
3. Results
3.1. Climate Variability in the Study Region
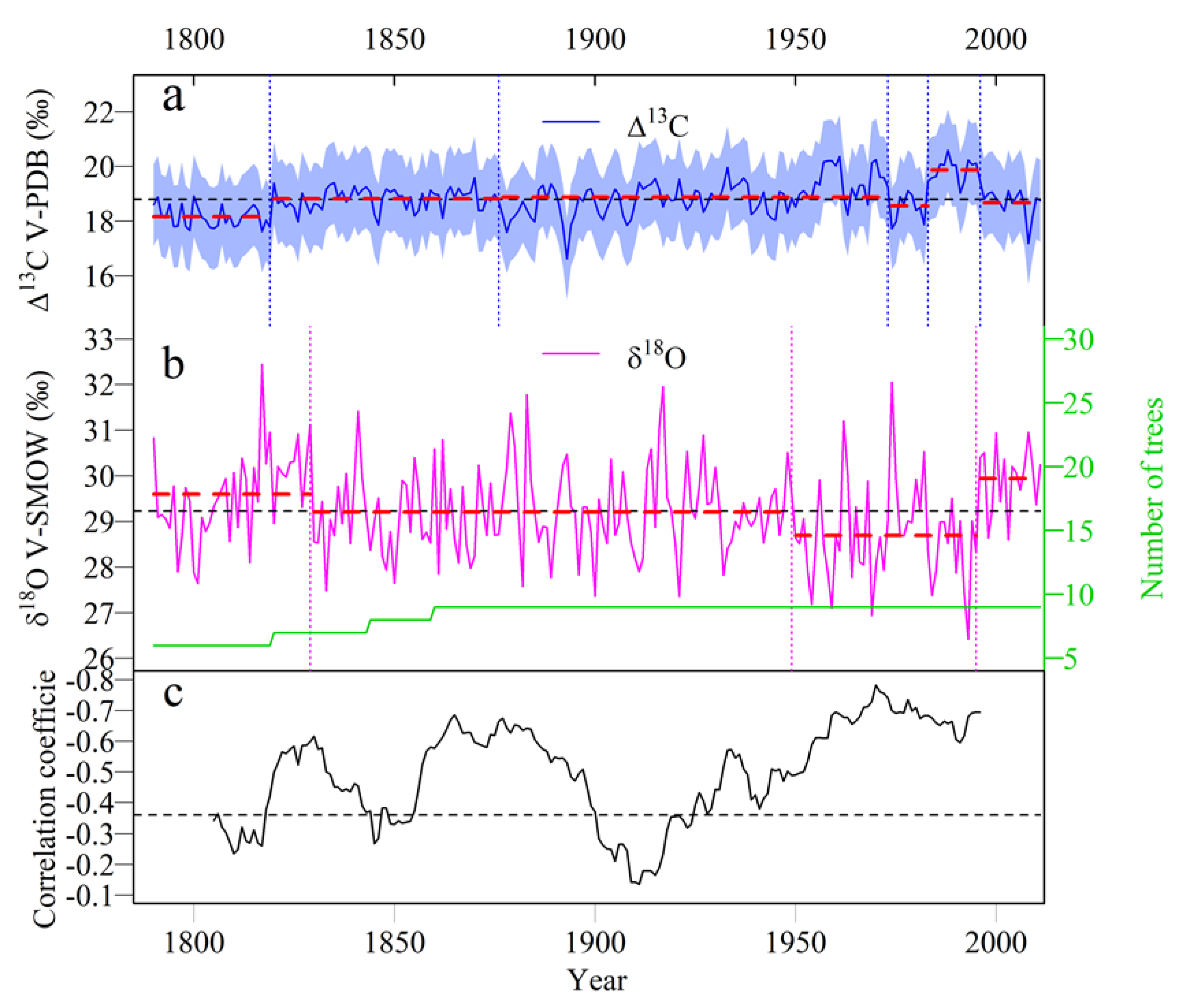
3.2. Temporal Variations of the Tree-Ring Δ13C and δ18O
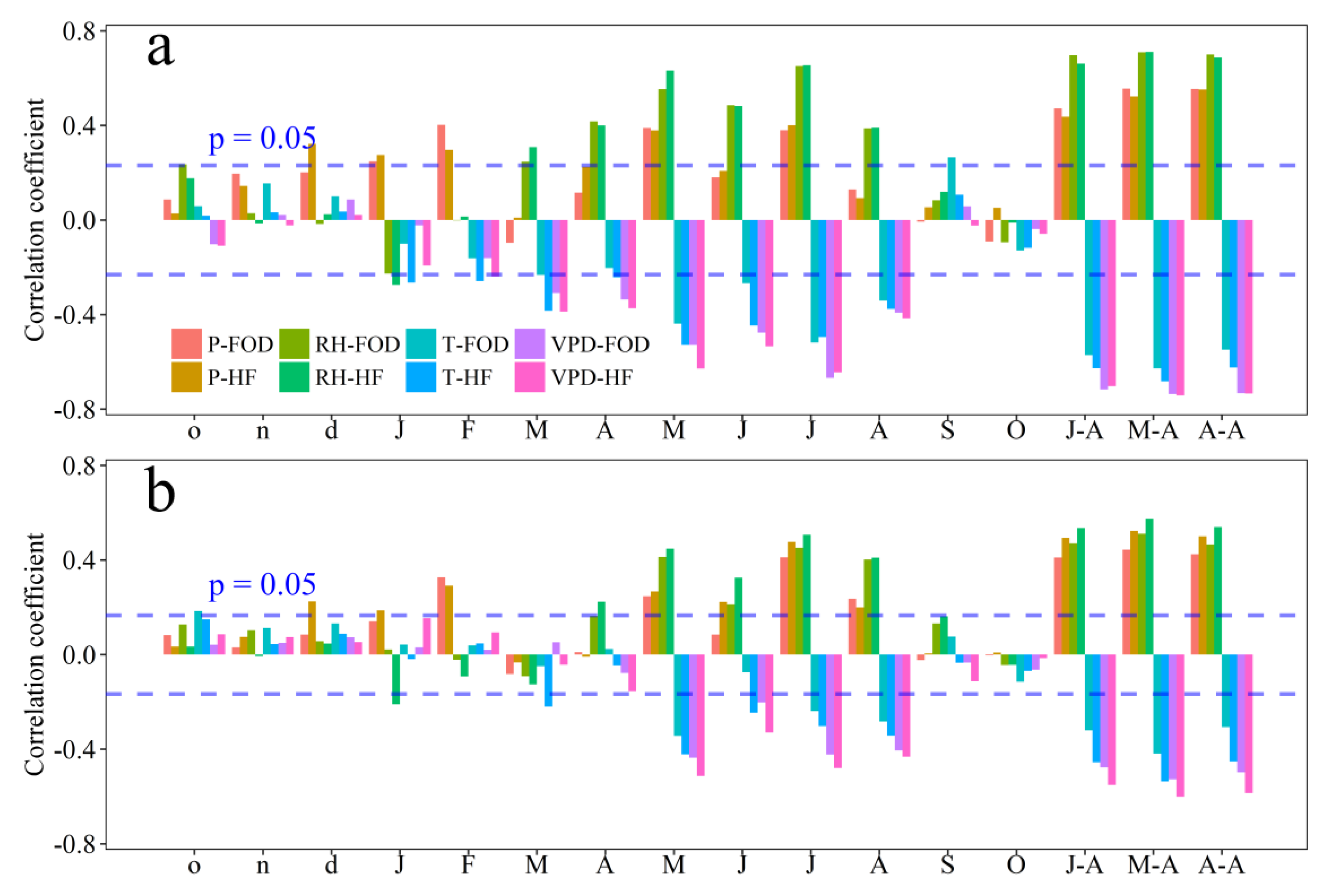
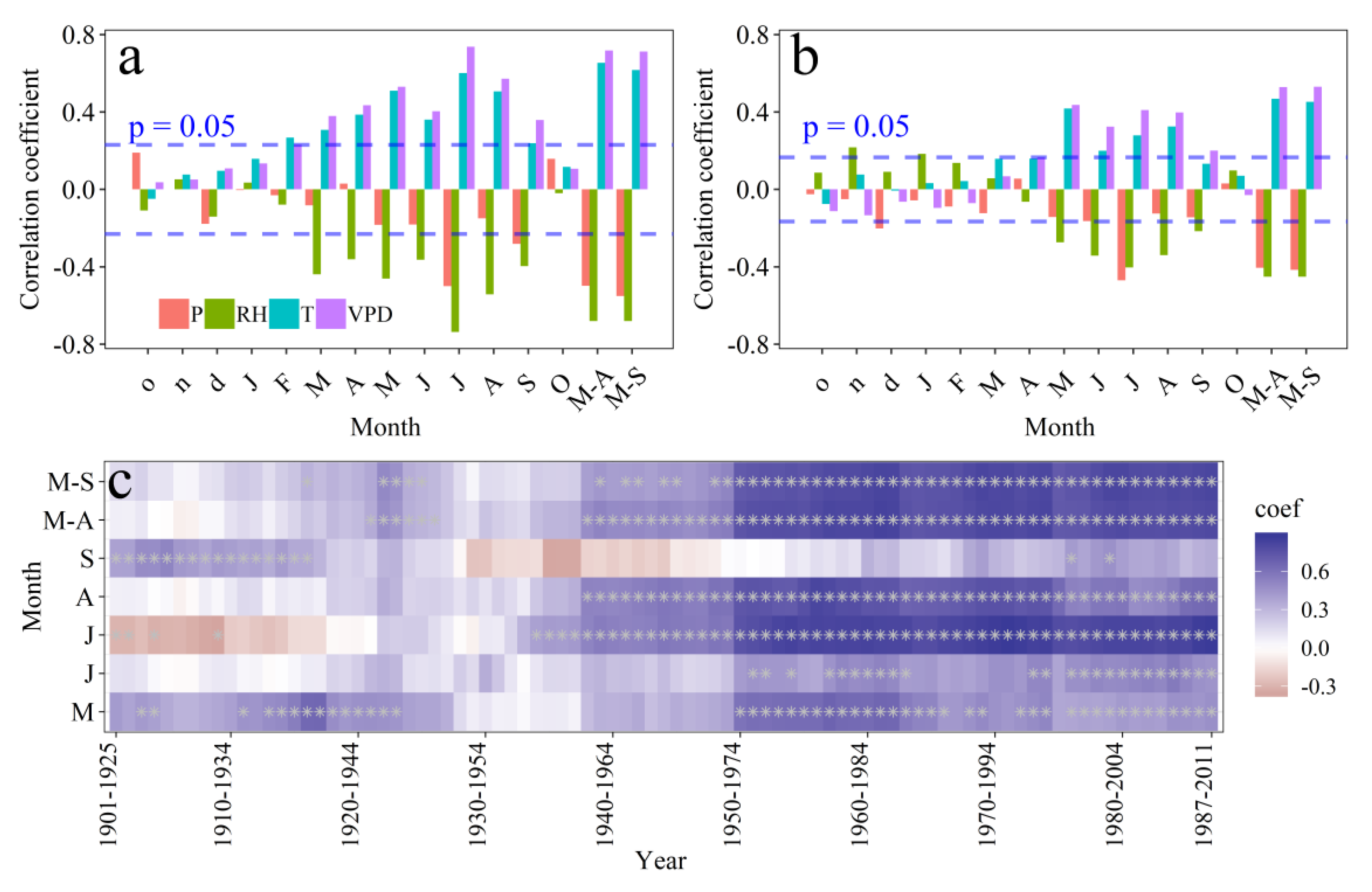
3.3. Climate Response of ci and Tree-Ring Width
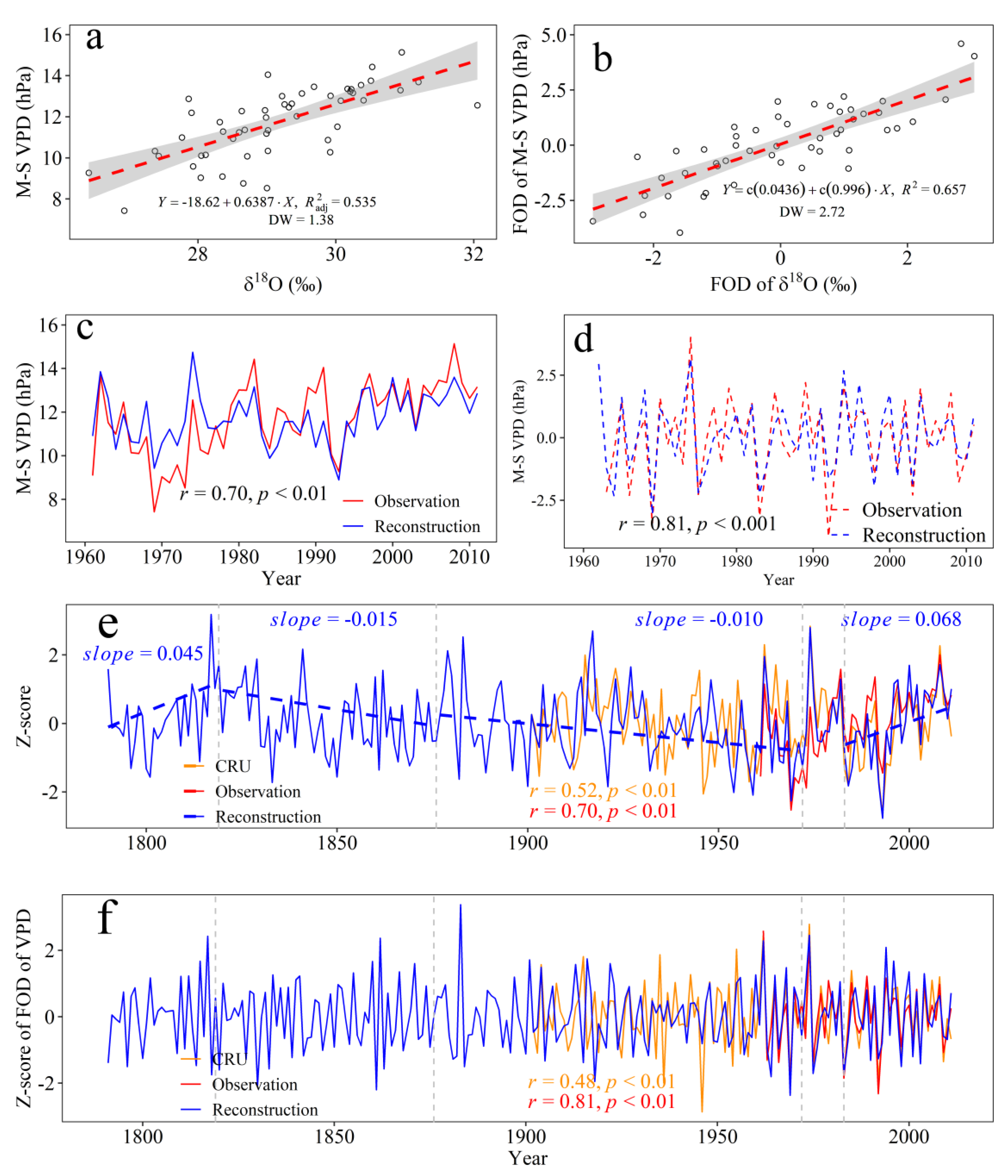
3.4. Climate Response of the Tree-Ring δ18O and VPD Reconstruction
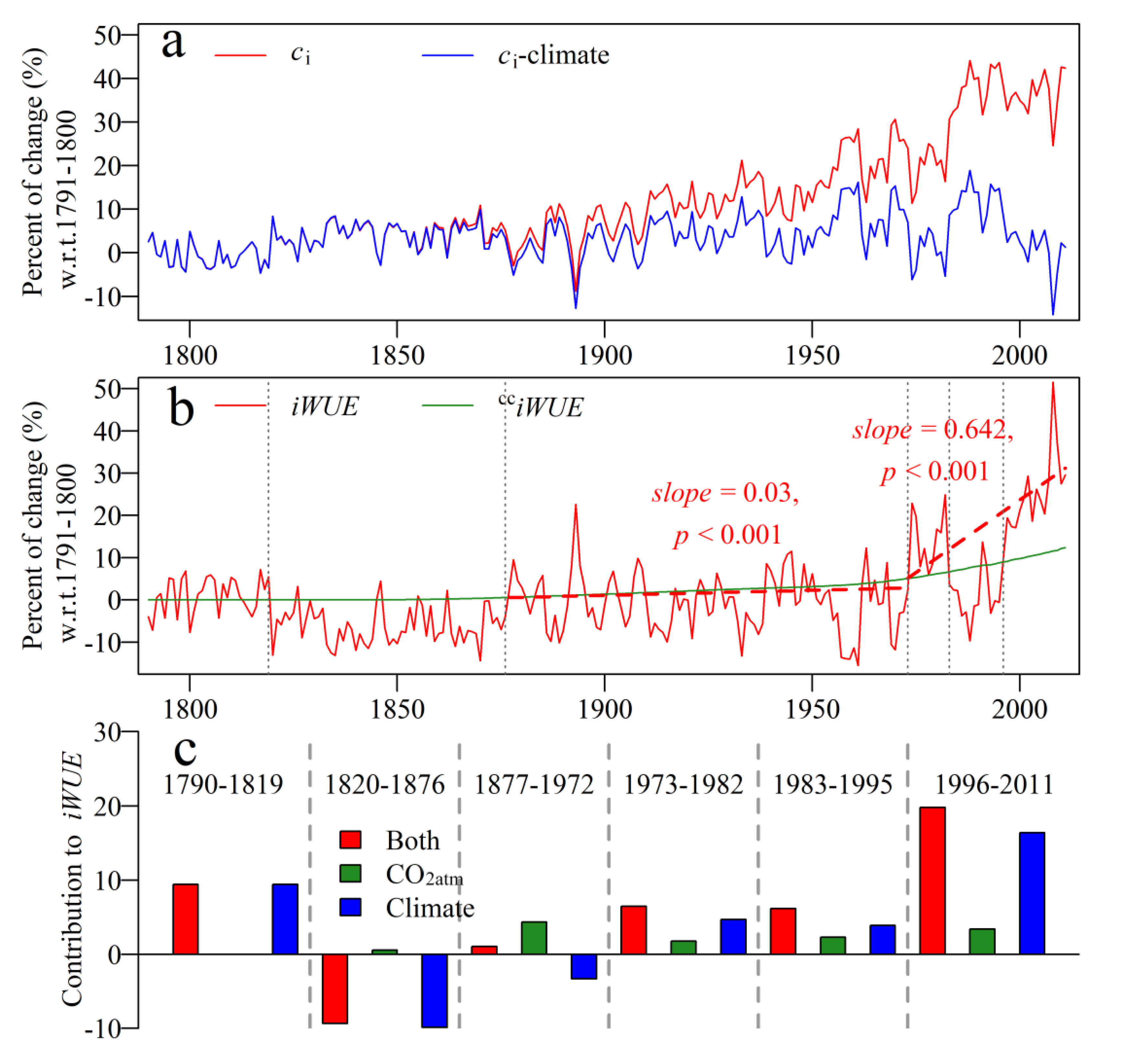
3.5. Percent Changes of ci and IWUE, and Contributions of Climate and CO2atm
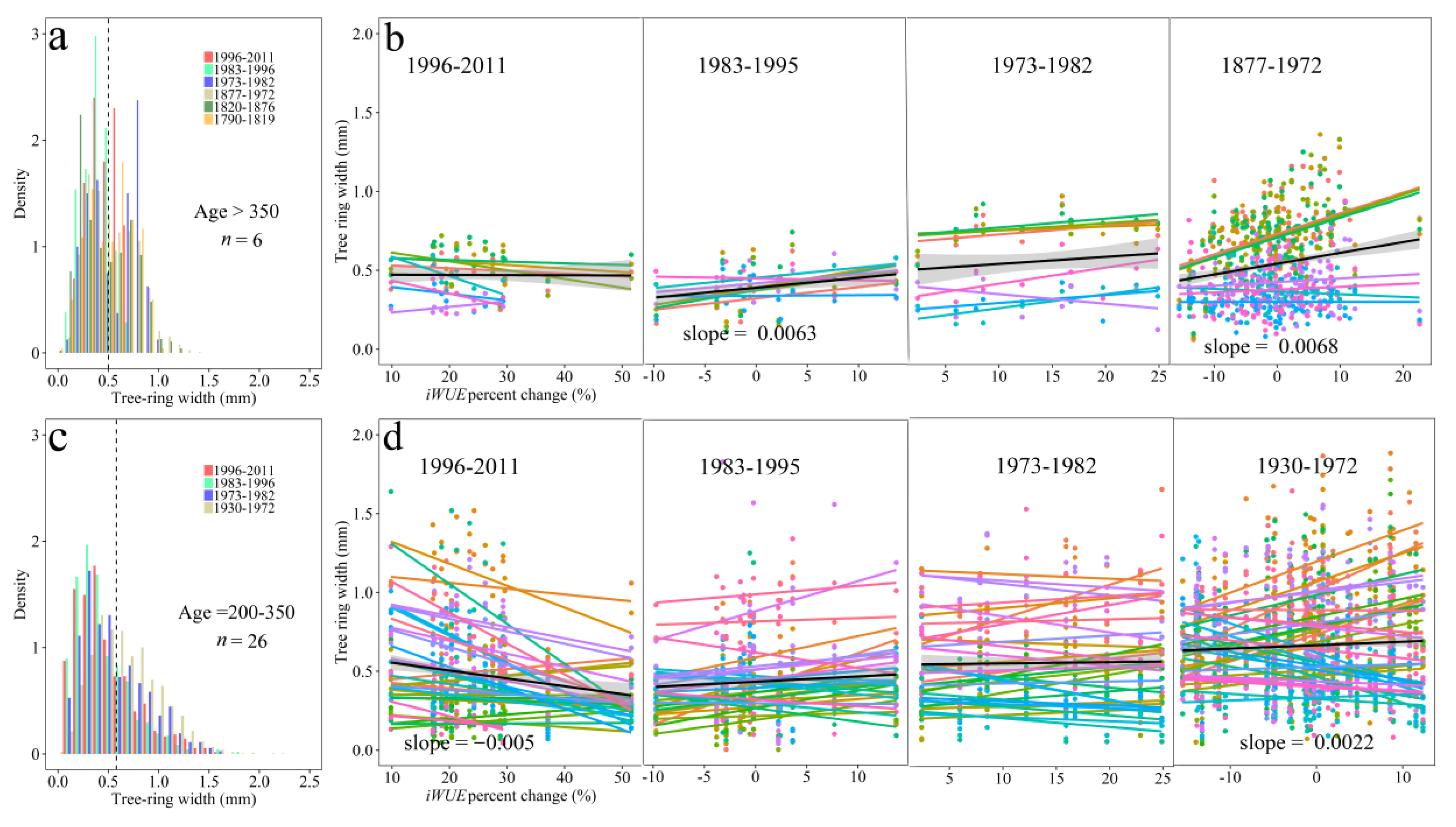
3.6. IWUE and Tree-ring Growth
4. Discussion
4.1. Climate Drivers of Tree’s Physiology
4.2. Contributions of CO2atm and Climate to Changes in IWUE
4.3. Implications of IWUE for Tree-ring Growth
5. Conclusions
Supplementary Materials
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Chen, B.; Chen, J.M.; Huang, L.I.N.; Tans, P.P. Modeling dynamics of stable carbon isotopic exchange between a boreal forest ecosystem and the atmosphere. Glob. Chang. Biol. 2006, 12, 1842–1867. [Google Scholar] [CrossRef]
- Jasechko, S.; Sharp, Z.D.; Gibson, J.J.; Birks, S.J.; Yi, Y.; Fawcett, P.J. Terrestrial water fluxes dominated by transpiration. Nature 2013, 496, 347–350. [Google Scholar] [CrossRef] [PubMed]
- Keenan, T.F.; Hollinger, D.Y.; Bohrer, G.; Dragoni, D.; Munger, J.W.; Schmid, H.P.; Richardson, A.D. Increase in forest water-use efficiency as atmospheric carbon dioxide concentrations rise. Nature 2013, 499, 324–327. [Google Scholar] [CrossRef] [PubMed]
- Wieser, G.; Oberhuber, W.; Waldboth, B.; Gruber, A.; Matyssek, R.; Siegwolf, R.T.W.; Grams, T.E.E. Long-term trends in leaf level gas exchange mirror tree-ring derived intrinsic water-use efficiency of Pinus cembra at treeline during the last century. Agric. For. Meteorol. 2018, 248, 251–258. [Google Scholar] [CrossRef]
- Charney, N.D.; Babst, F.; Poulter, B.; Record, S.; Trouet, V.M.; Frank, D.; Enquist, B.J.; Evans, M.E.K. Observed forest sensitivity to climate implies large changes in 21st century North American forest growth. Ecol. Lett. 2016, 19, 1119–1128. [Google Scholar] [CrossRef] [PubMed]
- Gagen, M.; Finsinger, W.; Wagner-Cremer, F.; McCarroll, D.; Loader, N.J.; Robertson, I.; Jalkanen, R.; Young, G.; Kirchhefer, A. Evidence of changing intrinsic water-use efficiency under rising atmospheric CO2 concentrations in Boreal Fennoscandia from subfossil leaves and tree ring δ13C ratios. Glob. Chang. Biol. 2011, 17, 1064–1072. [Google Scholar] [CrossRef]
- Farquhar, G.D.; Ehleringer, J.R.; Hubick, K.T. Carbon isotope discrimination and photosynthesis. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1989, 40, 503–537. [Google Scholar] [CrossRef]
- Farquhar, G.D.; O’Leary, M.H.; Berry, J.A. On the relationship between carbon isotope discrimination and the intercellular carbon dioxide concentration in leaves. Aust. J. Plant Physiol. 1982, 9, 121–137. [Google Scholar] [CrossRef]
- Frank, D.C.; Poulter, B.; Saurer, M.; Esper, J.; Huntingford, C.; Helle, G.; Treydte, K.; Zimmermann, N.E.; Schleser, G.H.; Ahlstrӧm, A.; et al. Water-use efficiency and transpiration across European forests during the Anthropocene. Nat. Clim. Chang. 2015, 5, 579–583. [Google Scholar] [CrossRef]
- Voelker, S.L.; Brooks, J.R.; Meinzer, F.C.; Anderson, R.; Bader, M.K.; Battipaglia, G.; Becklin, K.M.; Beerling, D.; Bert, D.; Betancourt, J.L.; et al. A dynamic leaf gas-exchange strategy is conserved in woody plants under changing ambient CO2: Evidence from carbon isotope discrimination in paleo and CO2 enrichment studies. Glob. Chang. Biol. 2016, 22, 889–902. [Google Scholar] [CrossRef] [PubMed]
- McCarroll, D.; Loader, N.J. Stable isotopes in tree rings. Quat. Sci. Rev. 2004, 23, 771–801. [Google Scholar] [CrossRef] [Green Version]
- Roden, J.S.; Lin, G.; Ehleringer, J.R. A mechanistic model for interpretation of hydrogen and oxygen isotope ratios in tree-ring cellulose. Geochim. Cosmochim. Acta 2000, 64, 21–35. [Google Scholar] [CrossRef]
- Scheidegger, Y.; Saurer, M.; Bahn, M.; Siegwolf, R. Linking stable oxygen and carbon isotopes with stomatal conductance and photosynthetic capacity: A conceptual model. Oecologia 2000, 125, 350–357. [Google Scholar] [CrossRef] [PubMed]
- Barbour, M.M.; Roden, J.S.; Farquhar, G.D.; Ehleringer, J.R. Expressing leaf water and cellulose oxygen isotope ratios as enrichment above source water reveals evidence of a Péclet effect. Oecologia 2004, 138, 426–435. [Google Scholar] [CrossRef] [PubMed]
- Giammarchi, F.; Cherubini, P.; Pretzsch, H.; Tonon, G. The increase of atmospheric CO2 affects growth potential and intrinsic water-use efficiency of Norway spruce forests: Insights from a multi-stable isotope analysis in tree rings of two Alpine chronosequences. Trees 2017, 31, 503–515. [Google Scholar] [CrossRef]
- Levesque, M.; Siegwolf, R.; Saurer, M.; Eilmann, B.; Rigling, A. Increased water-use efficiency does not lead to enhanced tree growth under xeric and mesic conditions. New Phytol. 2014, 203, 94–109. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peñuelas, J.; Canadell, J.G.; Ogaya, R. Increased water-use efficiency during the 20th century did not translate into enhanced tree growth. Glob. Ecol. Biogeogr. 2011, 20, 597–608. [Google Scholar] [CrossRef]
- Trahan, M.W.; Schubert, B.A. Temperature-induced water stress in high-latitude forests in response to natural and anthropogenic warming. Glob. Chang. Biol. 2016, 22, 782–791. [Google Scholar] [CrossRef] [PubMed]
- Saurer, M.; Spahni, R.; Frank, D.C.; Joos, F.; Leuenberger, M.; Loader, N.J.; McCarroll, D.; Gagen, M.; Poulter, B.; Siegwolf, R.T.; et al. Spatial variability and temporal trends in water-use efficiency of European forests. Glob. Chang. Biol. 2014, 20, 3700–3712. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Andreu-Hayles, L.; Planells, O.; Gutierrez, E.; Muntan, E.; Helle, G.; Anchukaitis, K.J.; Schleser, G.H. Long tree-ring chronologies reveal 20th century increases in water-use efficiency but no enhancement of tree growth at five Iberian pine forests. Glob. Chang. Biol. 2011, 17, 2095–2112. [Google Scholar] [CrossRef]
- Knorre, A.A.; Siegwolf, R.T.W.; Saurer, M.; Sidorova, O.V.; Vaganov, E.A.; Kirdyanov, A.V. Twentieth century trends in tree ring stable isotopes (δ13C and δ18O) of Larix sibirica under dry conditions in the forest steppe in Siberia. J. Geophys. Res. 2010, 115. [Google Scholar] [CrossRef]
- Timofeeva, G.; Treydte, K.; Bugmann, H.; Rigling, A.; Schaub, M.; Siegwolf, R.; Saurer, M. Long-term effects of drought on tree-ring growth and carbon isotope variability in Scots pine in a dry environment. Tree Physiol. 2017, 37, 1028–1041. [Google Scholar] [CrossRef] [PubMed]
- Xu, G.; Liu, X.; Qin, D.; Chen, T.; An, W.; Wang, W.; Wu, G.; Zeng, X.; Ren, J. Climate warming and increasing atmospheric CO2 have contributed to increased intrinsic water-use efficiency on the northeastern Tibetan Plateau since 1850. Trees 2013, 27, 465–475. [Google Scholar] [CrossRef]
- Girardin, M.P.; Bouriaud, O.; Hogg, E.H.; Kurz, W.; Zimmermann, N.E.; Metsaranta, J.M.; de Jong, R.; Frank, D.C.; Esper, J.; Büntgen, U.; et al. No growth stimulation of Canada’s boreal forest under half-century of combined warming and CO2 fertilization. Proc. Natl. Acad. Sci. USA 2016, 113, E8406–E8414. [Google Scholar] [CrossRef] [PubMed]
- Ward, E.J.; Domec, J.-C.; Laviner, M.A.; Fox, T.R.; Sun, G.; McNulty, S.; King, J.; Noormets, A. Fertilization intensifies drought stress: Water use and stomatal conductance of Pinus taeda in a midrotation fertilization and throughfall reduction experiment. For. Ecol. Manag. 2015, 355, 72–82. [Google Scholar] [CrossRef]
- Gessler, A.; Cailleret, M.; Joseph, J.; Schönbeck, L.; Schaub, M.; Lehmann, M.; Treydte, K.; Rigling, A.; Timofeeva, G.; Saurer, M. Drought induced tree mortality—A tree-ring isotope based conceptual model to assess mechanisms and predispositions. New Phytol. 2018, 219, 485–490. [Google Scholar] [CrossRef] [PubMed]
- Xu, G.; Liu, X.; Qin, D.; Chen, T.; Wang, W.; Wu, G.; Sun, W.; An, W.; Zeng, X. Relative humidity reconstruction for northwestern China’s Altay Mountains using tree-ring δ18O. Chin. Sci. Bull. 2014, 59, 190–200. [Google Scholar] [CrossRef]
- Rinn, F. TSAPWin: Time Series Analysis and Presentation for Dendrochronology and Related Applications; Rinn Tech: Heidelberg, Germany, 2003; Available online: http://www.rinntech.de/content/view/17/48/lang,english/index.html (accessed on 9 October 2018).
- Cook, E.R.; Kairiukstis, L.A. Methods of Dendrochronology: Applications in the Environmental Sciences; Kluwer Academic Publishers: Boston, MA, USA, 1990. [Google Scholar]
- Bolton, D. The computation of equivalent potential temperature. Mon. Weather Rev. 1980, 108, 1046–1053. [Google Scholar] [CrossRef]
- Harris, I.; Jones, P.D.; Osborn, T.J.; Lister, D.H. Updated high-resolution grids of monthly climatic observations—The CRU TS3.10 Dataset. Int. J. Climatol. 2014, 34, 623–642. [Google Scholar] [CrossRef] [Green Version]
- Leavitt, S.W. Tree-ring isotopic pooling without regard to mass: No difference from averaging δ13C values of each tree. Chem. Geol. 2008, 252, 52–55. [Google Scholar] [CrossRef]
- Laumer, W.; Andreu, L.; Helle, G.; Schleser, G.H.; Wieloch, T.; Wissel, H. A novel approach for the homogenization of cellulose to use micro-amounts for stable isotope analyses. Rapid Commun. Mass Spec. 2009, 23, 1934–1940. [Google Scholar] [CrossRef] [PubMed]
- O’Leary, M.H. Carbon isotope fractionation in plants. Phytochemistry 1981, 20, 553–567. [Google Scholar] [CrossRef]
- Saurer, M.; Siegwolf, R.T.W.; Schweingruber, F.H. Carbon isotope discrimination indicates improving water-use efficiency of trees in northern Eurasia over the last 100 years. Glob. Chang. Biol. 2004, 10, 2109–2120. [Google Scholar] [CrossRef]
- Treydte, K.S.; Frank, D.C.; Saurer, M.; Helle, G.; Schleser, G.H.; Esper, J. Impact of climate and CO2 on a millennium-long tree-ring carbon isotope record. Geochim. Cosmochim. Acta 2009, 73, 4635–4647. [Google Scholar] [CrossRef]
- Zang, C.; Biondi, F. Treeclim: An R package for the numerical calibration of proxy-climate relationships. Ecography 2015, 38, 431–436. [Google Scholar] [CrossRef]
- Killick, R.; Eckley, I.A. Changepoint: An R package for changepoint analysis. J. Stat. Softw. 2014, 58, 1–19. [Google Scholar] [CrossRef]
- Salzer, M.W.; Hughes, M.K.; Bunn, A.G.; Kipfmueller, K.F. Recent unprecedented tree-ring growth in bristlecone pine at the highest elevations and possible causes. Proc. Natl. Acad. Sci. USA 2009, 106, 20348–20353. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- R-Core-Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2016. [Google Scholar]
- Roden, J.S.; Farquhar, G.D. A controlled test of the dual-isotope approach for the interpretation of stable carbon and oxygen isotope ratio variation in tree rings. Tree Physiol. 2012, 32, 490–503. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barbour, M.M.; Walcroft, A.S.; Farquhar, G.D. Seasonal variation in δ13C and δ18O of cellulose from growth rings of Pinus radiata. Plant Cell Environ. 2002, 25, 1483–1499. [Google Scholar] [CrossRef]
- Vygodskaya, N.N.; Milyukova, I.; Varlagin, A.; Tatarinov, F.; Sogachev, A.; Kobak, K.I.; Desyatkin, R.; Bauer, G.; Hollinger, D.Y.; Kelliher, F.M.; et al. Leaf conductance and CO2 assimilation of Larix gmelinii growing in an eastern Siberian boreal forest. Tree Physiol. 1997, 17, 607–615. [Google Scholar] [CrossRef] [PubMed]
- Novick, K.A.; Ficklin, D.L.; Stoy, P.C.; Williams, C.A.; Bohrer, G.; Oishi, A.C.; Papuga, S.A.; Blanken, P.D.; Noormets, A.; Sulman, B.N.; et al. The increasing importance of atmospheric demand for ecosystem water and carbon fluxes. Nat. Clim. Chang. 2016, 6, 1023–1027. [Google Scholar] [CrossRef] [Green Version]
- Belmecheri, S.; Wright, W.E.; Szejner, P.; Morino, K.A.; Monson, R.K. Carbon and oxygen isotope fractionations in tree rings reveal interactions between cambial phenology and seasonal climate. Plant Cell Environ. 2018. [Google Scholar] [CrossRef] [PubMed]
- Sidorova, O.; Siegwolf, R.; Saurer, M.; Shashkin, A.; Knorre, A.; Prokushkin, A.; Vaganov, E.; Kirdyanov, A. Do centennial tree-ring and stable isotope trends of Larix gmelinii (Rupr.) Rupr. indicate increasing water shortage in the Siberian north? Oecologia 2009, 161, 825–835. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Zhang, X.; Zhao, L.; Xu, G.; Wang, L.; Sun, W.; Zhang, Q.; Wang, W.; Zeng, X.; Wu, G. Tree ring δ18O reveals no long-term change of atmospheric water demand since 1800 in the northern Great Hinggan Mountains, China. J. Geophys. Res. Atmos. 2017, 122, 6697–6712. [Google Scholar] [CrossRef]
- Eilmann, B.; Rigling, A. Tree-growth analyses to estimate tree species’ drought tolerance. Tree Physiol. 2012, 32, 178–187. [Google Scholar] [CrossRef] [PubMed]
- Oberhuber, W.; Kofler, W.; Schuster, R.; Wieser, G. Environmental effects on stem water deficit in co-occurring conifers exposed to soil dryness. Int. J. Biometeorol. 2015, 59, 417–426. [Google Scholar] [CrossRef] [PubMed]
- Gessler, A.; Ferrio, J.P.; Hommel, R.; Treydte, K.; Werner, R.A.; Monson, R.K. Stable isotopes in tree rings: Towards a mechanistic understanding of isotope fractionation and mixing processes from the leaves to the wood. Tree Physiol. 2014, 34, 796–818. [Google Scholar] [CrossRef] [PubMed]
- Aizen, V.B.; Aizen, E.; Fujita, K.; Nikitin, S.A.; Kreutz, K.J.; Takeuchi, L.N. Stable-isotope time series and precipitation origin from firn-core and snow samples, Altai glaciers, Siberia. J. Glaciol. 2005, 51, 637–654. [Google Scholar] [CrossRef] [Green Version]
- Chen, F.; Yuan, Y.; Wei, W.; Zhang, T.; Shang, H.; Zhang, R. Precipitation reconstruction for the southern Altay Mountains (China) from tree rings of Siberian spruce, reveals recent wetting trend. Dendrochronologia 2014, 32, 266–272. [Google Scholar] [CrossRef]
- Zhang, T.; Yuan, Y.; Chen, F.; Yu, S.; Zhang, R.; Qin, L.; Jiang, S. Reconstruction of hydrological changes based on tree-ring data of the Haba River, northwestern China. J. Arid Land 2018, 10, 53–67. [Google Scholar] [CrossRef]
- Battipaglia, G.; Saurer, M.; Cherubini, P.; Calfapietra, C.; McCarthy, H.R.; Norby, R.J.; Cotrufo, M.F. Elevated CO2 increases tree-level intrinsic water use efficiency: Insights from carbon and oxygen isotope analyses in tree rings across three forest FACE sites. New Phytol. 2013, 197, 544–554. [Google Scholar] [CrossRef] [PubMed]
- Streit, K.; Siegwolf, R.T.W.; Hagedorn, F.; Schaub, M.; Buchmann, N. Lack of photosynthetic or stomatal regulation after 9 years of elevated [CO2] and 4 years of soil warming in two conifer species at the alpine treeline. Plant Cell Environ. 2014, 37, 315–326. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Liu, X.; Zhang, Q.; Zeng, X.; Xu, G.; Wu, G.; Wang, W. Species-specific tree growth and intrinsic water-use efficiency of Dahurian larch (Larix gmelinii) and Mongolian pine (Pinus sylvestris var. mongolica) growing in a boreal permafrost region of the Greater Hinggan Mountains, Northeastern China. Agric. For. Meteorol. 2018, 248, 145–155. [Google Scholar]
- Weigt, R.B.; Streit, K.; Saurer, M.; Siegwolf, R.T.W. The influence of increasing temperature and CO2 concentration on recent growth of old-growth larch: Contrasting responses at leaf and stem processes derived from tree-ring width and stable isotopes. Tree Physiol. 2018, 38, 706–720. [Google Scholar] [CrossRef] [PubMed]
- Körner, C. Paradigm shift in plant growth control. Curr. Opin. Plant Biol. 2015, 25, 107–114. [Google Scholar] [CrossRef] [PubMed]
- Fatichi, S.; Leuzinger, S.; Körner, C. Moving beyond photosynthesis: From carbon source to sink-driven vegetation modeling. New Phytol. 2014, 201, 1086–1095. [Google Scholar] [CrossRef] [PubMed]


| Interannual | Period | r | RE | CE | Period | r | RE | CE |
| Calibration | 1987–2011 | 0.82 | 1961–1986 | 0.64 | ||||
| Verification | 1961–1986 | 0.64 | 0.42 | −0.08 | 1987–2011 | 0.82 | 0.52 | −0.17 |
| Full period | 1961–2011 | 0.70 | ||||||
| Leave-one-out method | 1961–2011 | 0.67 | 0.45 | 0.45 | ||||
| FOD | Period | r | RE | CE | Period | r | RE | CE |
| Calibration | 1987–2011 | 0.79 | 1961–1986 | 0.81 | ||||
| Verification | 1961–1986 | 0.81 | 0.66 | 0.66 | 1987–2011 | 0.79 | 0.65 | 0.65 |
| Full period | 1961–2011 | 0.81 | ||||||
| Leave-one-out method | 1961–2011 | 0.79 | 0.63 | 0.63 |
| Old Trees (Age > 350) | Medium-Aged Trees (Age 200–350) | ||||||
|---|---|---|---|---|---|---|---|
| period 1 | period 2 | difference (period 1–period 2) | p-value | period 1 | period 2 | difference (period 1–period 2) | p-value |
| 1790–1819 | 1996–2011 | 0.039 | 0.74 | 1930–1972 | 1996–2011 b | 0.183 | 0.001 |
| 1983–1995 a | 0.120 | 0.001 | 1983–1995 b | 0.230 | 0.001 | ||
| 1973–1982 | −0.047 | 0.646 | 1973–1982 b | 0.109 | 0.001 | ||
| 1877–1972 | −0.027 | 0.63 | 1973–1982 | 1996–2011 b | 0.074 | 0.003 | |
| 1820–1876 | 0.044 | 0.166 | 1983–1995 b | 0.122 | 0.001 | ||
| 1820–1876 | 1996–2011 | −0.006 | 0.999 | 1983–1995 | 1996–2011 | −0.047 | 0.080 |
| 1983–1995 a | 0.075 | 0.042 | |||||
| 1973–1982 a | −0.092 | 0.018 | |||||
| 1877–1972 a | −0.072 | 0.001 | |||||
| 1877–1972 | 1996–2011 | 0.066 | 0.091 | ||||
| 1983–1995 a | 0.147 | 0.001 | |||||
| 1973–1982 | −0.019 | 0.980 | |||||
| 1973–1982 | 1996–2011 | 0.086 | 0.153 | ||||
| 1983–1995 a | 0.167 | 0.001 | |||||
| 1983–1995 | 1996–2011 | −0.081 | 0.143 | ||||
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, G.; Liu, X.; Belmecheri, S.; Chen, T.; Wu, G.; Wang, B.; Zeng, X.; Wang, W. Disentangling Contributions of CO2 Concentration and Climate to Changes in Intrinsic Water-Use Efficiency in the Arid Boreal Forest in China’s Altay Mountains. Forests 2018, 9, 642. https://doi.org/10.3390/f9100642
Xu G, Liu X, Belmecheri S, Chen T, Wu G, Wang B, Zeng X, Wang W. Disentangling Contributions of CO2 Concentration and Climate to Changes in Intrinsic Water-Use Efficiency in the Arid Boreal Forest in China’s Altay Mountains. Forests. 2018; 9(10):642. https://doi.org/10.3390/f9100642
Chicago/Turabian StyleXu, Guobao, Xiaohong Liu, Soumaya Belmecheri, Tuo Chen, Guoju Wu, Bo Wang, Xiaomin Zeng, and Wenzhi Wang. 2018. "Disentangling Contributions of CO2 Concentration and Climate to Changes in Intrinsic Water-Use Efficiency in the Arid Boreal Forest in China’s Altay Mountains" Forests 9, no. 10: 642. https://doi.org/10.3390/f9100642
APA StyleXu, G., Liu, X., Belmecheri, S., Chen, T., Wu, G., Wang, B., Zeng, X., & Wang, W. (2018). Disentangling Contributions of CO2 Concentration and Climate to Changes in Intrinsic Water-Use Efficiency in the Arid Boreal Forest in China’s Altay Mountains. Forests, 9(10), 642. https://doi.org/10.3390/f9100642





