Species Diversity, Stand Structure, and Species Distribution across a Precipitation Gradient in Tropical Forests in Myanmar
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Sampling Procedures and Data Analysis
3. Results
3.1. Species Diversity
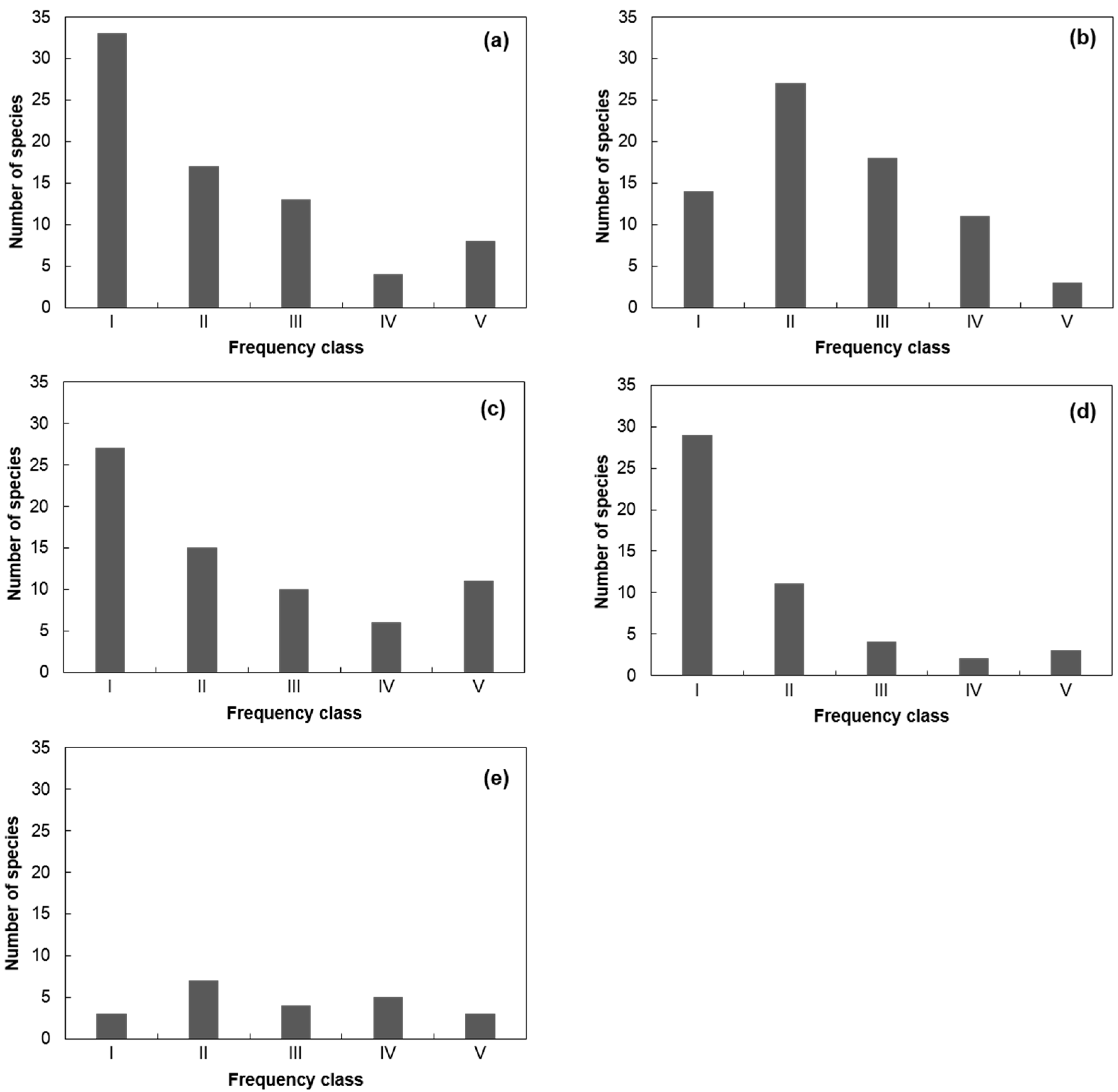
3.2. Species Composition and Distribution
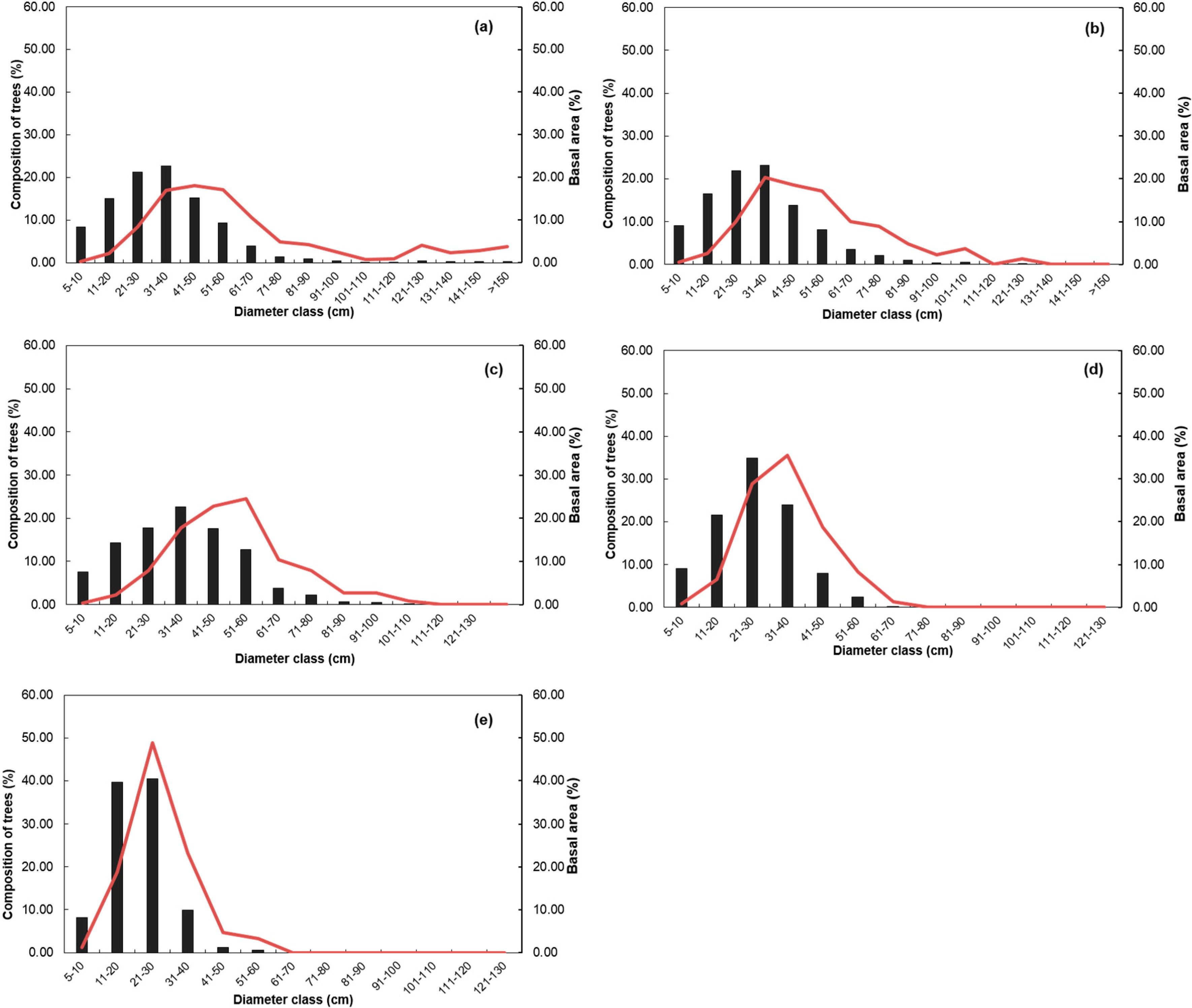
3.3. Stand Structure
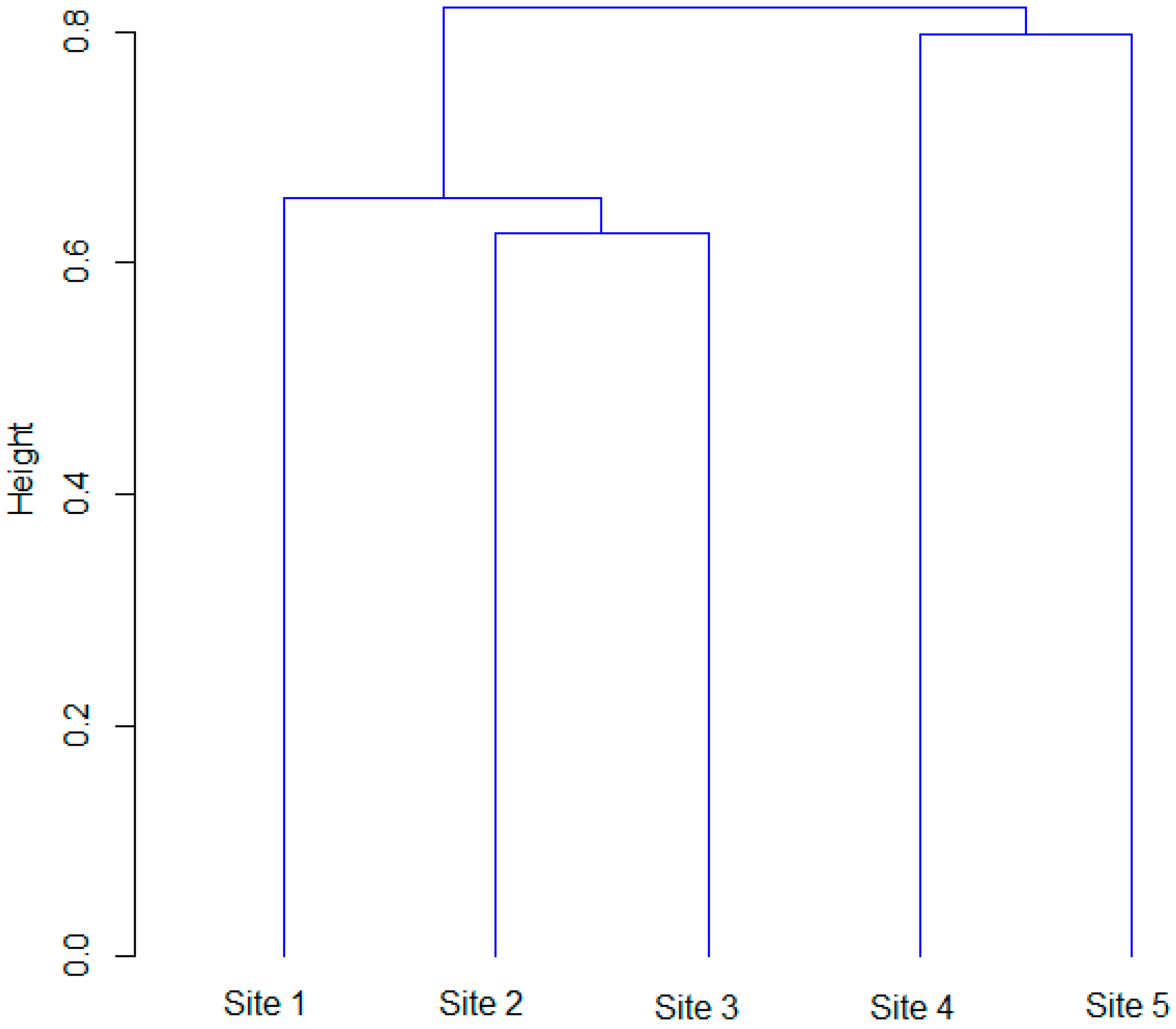
3.4. Similarity of Forests
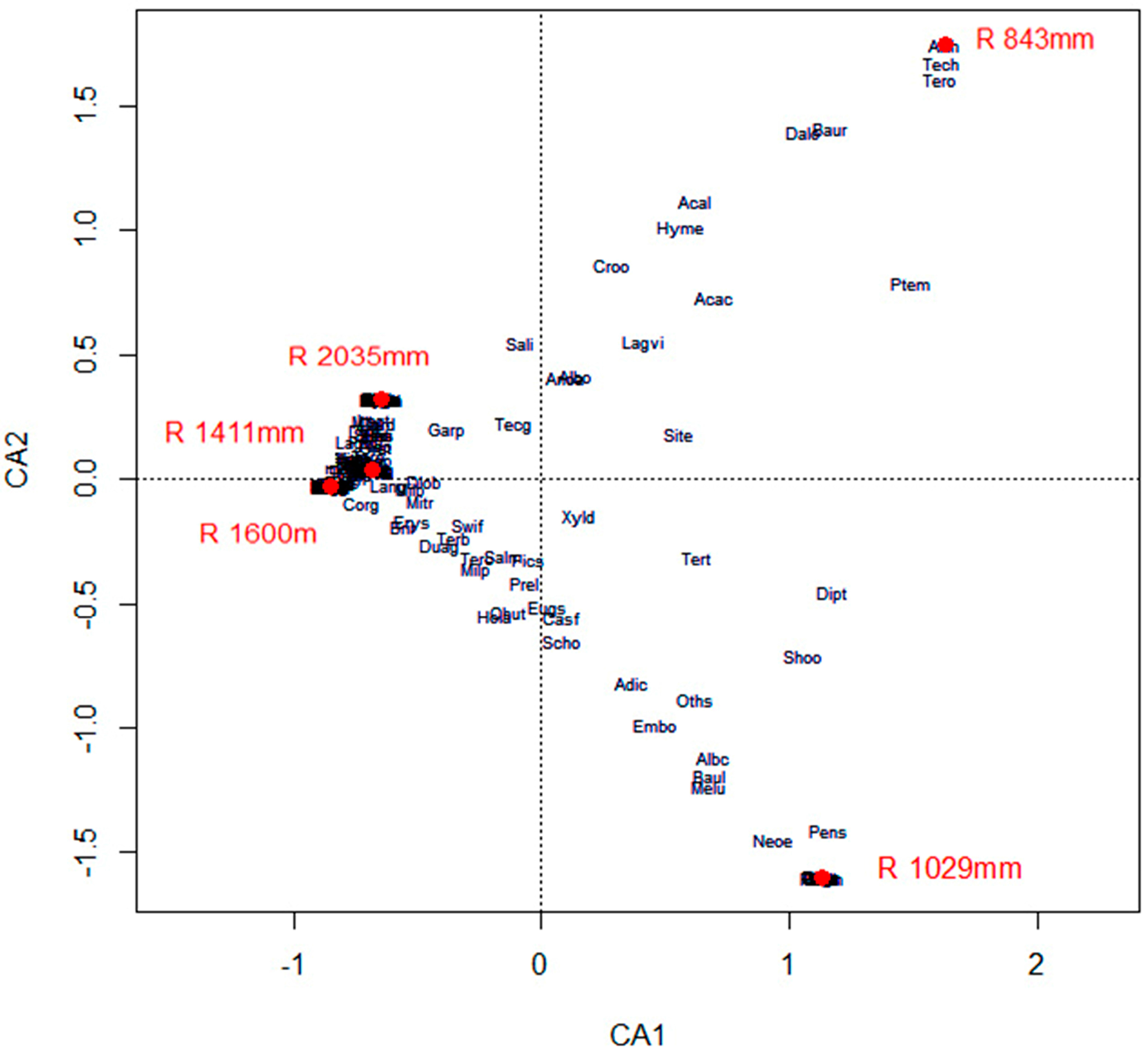
3.5. Effects of Climate on Diversity, Distribution, Diameter and Height of Forest
4. Discussion
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Cannon, C.H.; Peart, D.R.; Leighton, M. Tree species diversity in commercially logged Bornean rainforest. Science 1998, 281, 1366–1368. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.; Pohjonen, V.; Johansson, S.; Nashanda, M.; Katigula, M.I.L.; Luukkanen, O. Species diversity, forest structure and species composition in Tanzania tropical forests. For. Ecol. Manag. 2003, 173, 11–24. [Google Scholar] [CrossRef]
- Reilly, M.J.; Spies, T.A. Regional variation in stand structure and development in forests of Oregon, Washington, and inland Northern California. Ecosphere 2015, 6, 1–27. [Google Scholar] [CrossRef]
- Amissah, L.; Mohren, G.M.J.; Bongers, F.; Hawthorne, W.D.; Poorter, L. Rainfall and temperature affect tree species distribution in Ghana. J. Trop. Ecol. 2014, 30, 435–446. [Google Scholar] [CrossRef]
- Condit, R.; Engelbrecht, B.M.J.; Pino, D.; Pérez, R.; Turner, B.L. Species distributions in response to individual soil nutrients and seasonal drought across a community of tropical trees. Proc. Natl. Acad. Sci. USA 2013, 110, 5064–5068. [Google Scholar] [CrossRef] [PubMed]
- Toledo, M.; Peña-Claros, M.; Bongers, F.; Alarcón, A.; Balcázar, J.; Chuviña, J.; Leaño, C.; Licona, J.C.; Poorter, L. Distribution patterns of tropical woody species in response to climatic and edaphic gradients. J. Ecol. 2012, 100, 253–263. [Google Scholar] [CrossRef]
- Khaine, I.; Woo, S.Y. An overview of interrelationship between climate change and forests. For. Sci. Technol. 2015, 11, 11–18. [Google Scholar] [CrossRef]
- Sevegnani, L.; Uhlmann, A.; Gasper, A.L.; de Meyeer, L.; Vibrans, A.C. Climate affects the structure of mixed rain forest in southern sector of Atlantic domain in Brazil. Acta Oecol. 2016, 77, 109–117. [Google Scholar] [CrossRef]
- Kalacska, M.; Sanchez-Azofeifa, G.A.; Calvo-Alvarado, J.C.; Quesada, M.; Rivard, B.; Janzen, D.H. Species compostion, similarity and diversity in three successional stages of a seasonally dry tropical forest. For. Ecol. Manag. 2004, 200, 227–247. [Google Scholar] [CrossRef]
- Feroz, S.M.; Kabir, M.E.; Hagihara, A. Species composition, diversity and stratification in subtropical evergreen broadleaf forests along a latitudinal thermal gradient in the Ryukyu Archipelago, Japan. Glob. Ecol. Conserv. 2015, 4, 63–72. [Google Scholar] [CrossRef]
- Johnson, C.; Chhin, S.; Zhang, J. Effects of climate on competitive dynamics in mixed conifer forests of the Sierra Nevada. For. Ecol. Manag. 2017, 394, 1–12. [Google Scholar] [CrossRef]
- Nlung-Kweta, P.; Leduc, A.; Bergeron, Y. Climate and disturbance regime effects on aspen (Populus tremuloides Michx.) stand structure and composition along an east-west transect in Canada’s boreal forest. Forestry 2017, 90, 70–81. [Google Scholar] [CrossRef]
- Field, R.; O’Brien, E.M.; Whittaker, R.T. Global models for predicting woody plant richness from climate: Development and evaluation. Ecology 2005, 86, 2263–2277. [Google Scholar] [CrossRef]
- Francis, A.P.; Currie, D.J. A globally consistent richness-climate relationship for angiosperms. Am. Nat. 2003, 161, 523–536. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, E.M. Water-Energy dynamics, climate, and prediction of woody plant sciences richness: An interim general model. J. Biogeogr. 1998, 25, 379–398. [Google Scholar] [CrossRef]
- Gentry, A.H. Changes in plant community diversity and floristic composition on environmental and geographical gradients. Ann. Mo. Bot. Gard. 1988, 75, 1–34. [Google Scholar] [CrossRef]
- Gillman, L.N.; Wright, S.D. Species richness and evolutionary speed: The influence of temperature, water and area. J. Biogeogr. 2014, 41, 39–51. [Google Scholar] [CrossRef]
- Goldie, X.; Gillman, L.; Crisp, M.; Wright, S. Evolutionary speed limited by water in arid Australia. Proc. R. Soc. B 2010, 277, 2645–2653. [Google Scholar] [CrossRef] [PubMed]
- Toledo, M.; Poorter, L.; Pena-Claros, M.; Alarcon, A.; Balcazar, J.; Leano, C.; Licona, C.; Lianque, O.; Vroomans, V.; Zuidema, P.; et al. Climate is a stronger driver of tree and forest growth rates than soil and disturbance. J. Ecol. 2011, 99, 254–264. [Google Scholar] [CrossRef]
- Fischer, R.; Armstrong, A.; Shugart, H.H.; Huth, A. Simulating the impacts of reduced rainfall on carbon stocks and net ecosystem exchange in a tropical forest. Environ. Model. Softw. 2014, 52, 200–206. [Google Scholar] [CrossRef]
- Wang, Z.; Brown, J.H.; Tang, Z.; Fang, J. Temperature dependence, spatial scale, and tree species diversity in eastern Asia and North America. Proc. Natl. Acad. Sci. USA 2009, 106, 13388–13392. [Google Scholar] [CrossRef] [PubMed]
- Gillman, L.N.; Wright, S.D.; Cusens, J.; McBride, P.D.; Malhi, Y.; Whittaker, R.J. Latitude, productivity and species richness. Glob. Ecol. Biogeogr. 2015, 24, 107–117. [Google Scholar] [CrossRef]
- Sullivan, M.J.P.; Talbot, J.; Lewis, S.L.; Phillips, O.L.; Qie, L.; Begne, S.K.; Chave, J.; Cuni-Sanchez, A.; Hubau, W.; Lopez-Gonzalez, G.; et al. Diversity and carbon storage across the tropical forest biome. Sci. Rep. 2017, 7, 39102. [Google Scholar] [CrossRef] [PubMed]
- Thomas, CD.; Cameron, A.; Green, R.E.; Bakkenes, M.; Beaumont, L.J.; Collingham, Y.C.; Erasmus, B.F.N.; Siqueira, M.F.; Grainger, A.; Hannah, L.; et al. Extinction risk from climate change. Nature 2004, 427, 145–148. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Diekmann, M. Species indicator values as an important tool in applied plant ecology—A review. Basic Appl. Ecol. 2003, 4, 493–506. [Google Scholar] [CrossRef]
- Toledo, M.; Poorter, L.; Peña-Claros, M.; Alarcón, A.; Balcázar, J.; Chuviña, J.; Leaño, C.; Licona, J.C.; ter Steege, H.; Bongers, F. Patterns and determinants of floristic variation across lowland forests of Bolivia. Biotropica 2011, 43, 405–413. [Google Scholar] [CrossRef]
- Htun, N.Z.; Mizoue, N.; Yoshida, S. Tree species composition and diversity at different levels of disturbance in Popa Mountain Park, Myanmar. Biotropica 2011, 43, 597–603. [Google Scholar] [CrossRef]
- Khai, T.C.; Mizoue, N.; Kajisa, T.; Ota, T.; Yoshida, S. Stand structure, composition and illegal logging in selectively logged production forests of Myanmar: Comparison of two compartments subject to different cutting frequency. Glob. Ecol. Conserv. 2016, 7, 132–140. [Google Scholar] [CrossRef]
- Tun, K.S.W.; Stefano, J.D.; Volkova, L. Forest management influences above ground carbon and tree species diversity in Myanmar’s mixed deciduous forests. Forests 2016, 7, 217. [Google Scholar]
- Khaing, N.; Mitloehner, R. Structure and Composition of Dry Deciduous Forests in Central Myanmar; Annual Conference of Forest Research Institute: Nay Pyi Taw, Myanmar; Forest Research Institute: Nay Pyi Taw, Myanmar, 2014; Leaflet No. 4. [Google Scholar]
- FAO. Global Forest Resources Assessment 2015: How Are The World’s Forests Changing; Food and Agriculture Organization of the United Nations: Rome, Italy, 2015.
- The IUCN Red List of Threatened Species. Version 2017-1. Available online: http://www.webcitation.org/query?url=http%3A%2F%2Fwww.iucnredlist.org%2Fsearch&date=2017-07-09 (accessed on 12 May 2017).
- Forest Department of MNREC. Plant Species of IUCN Red List in Myanmar; Ministry of Natural Resources and Environmental Conservation (MNREC): Nay Pyi Taw, Myanmar, 2016.
- Forest Department of MNREC. National Biodiversity Strategy and Action Plan; Ministry of Natural Resources and Environmental Conservation (MNREC): Nay Pyi Taw, Myanmar, 2011.
- Cain, S.A. The species-area curve. Am. Midl. Nat. 1938, 19, 573–581. [Google Scholar] [CrossRef]
- Cencini, M.; Pigolotti, S.; Muñoz, M.A. What ecological factors shape species-area curves in neutral models? PLoS ONE 2012, 7, e38232. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oo, T.N. Carbon Sequestration of Tropical Deciduous Forests and Forest Plantations in Myanmar. Ph.D. Thesis, Seoul National University, Seoul, Korea, 2009. [Google Scholar]
- Lamprecht, H. Silviculture in the Tropics; Springer: Eschborn, Germany; Rossdorf, Germany, 1989; p. 296. [Google Scholar]
- Kurz, S. Forest Flora of British Burma; Office of the superintendent of government printing: Calcutta, India, 1877. [Google Scholar]
- Heltshe, J.F.; Forrester, N.E. Estimating species richness using Jackknife procedure. Biometrics 1983, 39, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Shannon, C.E.; Weaver, W.J. The Mathematical Theory of Communication; University of Illinois Press: Urbana, IL, USA; Chicago, IL, USA, 1949. [Google Scholar]
- Simpson, E. Measurement of diversity. Nature 1949, 163, 688. [Google Scholar] [CrossRef]
- Hakkenberg, C.R.; Song, C.; Peet, R.K.; White, P.S. Forest structure as a predictor of tree species diversity in the North Carolina Piedmont. J. Veg. Sci. 2016, 27, 1151–1163. [Google Scholar] [CrossRef]
- Heip, C.H.R.; Herman, P.M.J.; Soetaert, K. Indices of diversity and evenness. Oceanis 1998, 24, 61–87. [Google Scholar]
- Magurran, A.E. Ecological Diversity and It's Measurement; Princeton University Press: Princeton, NJ, USA, 1988; p. 179. [Google Scholar]
- Kershaw, J.A.; Ducey, M.J.; Beers, T.W.; Husch, B. Forest Mensuration, 5th ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA; Chichester, UK, 2017. [Google Scholar]
- Newton, A.C. Techniques in ecology and conservation series. In Forest Ecology and Conservation: A Handbook of Techniques; Oxford University Press: New York, NY, USA, 2007. [Google Scholar]
- Maçaneiro, J.P.D.; Oliveira, L.Z.; Seubert, R.C.; Eisenlohr, P.V.; Schorn, L.A. More than environmental control at local scales: Do spatial processes play an important role in floristic variation in subtropical forests? Acta Bot. Bras. 2016, 30, 183–192. [Google Scholar] [CrossRef]
- Saiter, F.Z.; Eisenlohr, P.V.; França, G.S.; Stehmann, J.R.; Thomas, W.W.; Oliveira-Filho, A.T. Floristic units and their predictors unveiled in part of the Atlantic forest hotspot: Implications for conservation planning. Ann. Braz. Acad. Sci. 2015, 87, 2031–2046. [Google Scholar] [CrossRef] [PubMed]
- Kyaw, N.N. Site Influence on Growth and Phenotype of Teak (Tectona grandis Linn. F.) in Natural Forests of Myanmar. Ph.D. Thesis, Der Georg-August-Universität Göttingen, Göttingen, Germany, 2003. [Google Scholar]
- Sagar, R.; Raghubanshi, A.S.; Singh, J.S. Tree species composition, dispersion and diversity along a disturbance gradient in a dry tropical forest region of India. For. Ecol. Manag. 2003, 186, 61–71. [Google Scholar] [CrossRef]
- Trejo, I.; Dirzo, R. Floristic diversity of Mexican seasonally dry tropical forests. Biodivers. Conserv. 2002, 11, 2063–2084. [Google Scholar] [CrossRef]
- Woo, S.Y.; Hung, T.T.; Park, P.S. Stand structure and natural regeneration of degraded forestland in the northern mountainous region of Vietnam. Landsc. Ecol. Eng. 2011, 7, 251–261. [Google Scholar] [CrossRef]
- Osland, M.; Feher, L.C.; Griffith, K.T.; Cavanaugh, K.C.; Enwright, N.M.; Day, R.H.; Stagg, C.L.; Krauss, K.W.; Howard, R.J.; Grace, J.B.; et al. Climatic controls on the global distribution, abundance, and species richness of mangrove forests. Ecol. Monogr. 2017, 87, 341–359. [Google Scholar] [CrossRef]
- Staver, A.C.; Archibald, S.; Levin, S. Tree cover in sub-Saharan Africa: Rainfall and fire constrain forest and savanna as alternative stable states. Ecology 2011, 92, 1063–1072. [Google Scholar] [CrossRef] [PubMed]
- Feroz, S.M.; Min, W.; Li, Y.; Sharma, S.; Suwa, R.; Nakamura, K.; Hagihara, A.; Denda, T.; Yokota, M. Floristic composition, woody species diversity and spatial distribution of trees based on architectural stratification in a subtropical evergreen broadleaf forest on Ishigaki Island in the Ryukyu Archipelago, Japan. Tropics 2009, 18, 103–114. [Google Scholar] [CrossRef]
- Réjou-Méchain, M.; Pélissier, R.; Gourlet-Fleury, S.; Couteron, P.; Nasi, R.; Thompson, J.D. Regional variation in tropical forest tree species composition in the central African Republic: An assessment based on inventories by forest companies. J. Trop. Ecol. 2008, 24, 663–674. [Google Scholar] [CrossRef]


| Site | Shannon Diversity Index | Simpson Diversity Index | Shannon Evenness | No. of Species Per Plot | Jackknife Species Index |
|---|---|---|---|---|---|
| Site 1 | 3.05 (0.25) a | 0.95 (0.02) a | 93.65 (1.84) a | 26.36 (5.24) a | 75.83 a,* |
| Site 2 | 3.16 (0.24) a | 0.96 (0.02) a | 94.74 (2.26) a | 28.44 (5.08) a | 73.79 a,* |
| Site 3 | 3.20 (0.06) a | 0.96 (0.01) a | 95.33 (1.52) a | 29.00 (2.26) a | 69.73 a,* |
| Site 4 | 2.26 (0.54) b | 0.87 (0.08) b | 87.93 (7.87) b | 14.10 (6.42) b | 49.81 b,* |
| Site 5 | 2.03 (0.41) b | 0.83 (0.10) b | 87.56 (7.65) b | 10.83 (3.76) b | 23.00 c,* |
| Site 1 | Site 2 | Site 3 | Site 4 | Site 5 | |||||
|---|---|---|---|---|---|---|---|---|---|
| Family | F (%) | Family | F (%) | Family | F (%) | Family | F (%) | Family | F (%) |
| Fabaceae | 12.2 | Fabaceae | 9.7 | Anacardiaceae | 8.7 | Combretaceae | 10.4 | Mimosaceae | 19 |
| Lythraceae | 8.1 | Bignoniaceae | 8.3 | Euphorbiaceae | 8.7 | Anacardiaceae | 8.3 | Combretaceae | 14.3 |
| Mimosaceae | 8.1 | Lythraceae | 8.3 | Fabaceae | 8.7 | Fabaceae | 8.3 | Dipterocarpaceae | 14.3 |
| Combretaceae | 6.8 | Mimosaceae | 8.3 | Rubiaceae | 8.7 | Mimosaceae | 8.3 | Verbenaceae | 9.5 |
| Anacardiaceae | 5.4 | Anacardiaceae | 6.9 | Bignoniaceae | 7.2 | Dipterocarpaceae | 6.3 | Euphorbiaceae | 4.8 |
| Site | Parameter | Upper Layer | Middle Layer | Lower Layer | Top Height |
|---|---|---|---|---|---|
| Site 1 | Avg. H (m) | 49.45 (10.09) a,A | 23.35 (4.17) b,A | 12.06 (3.98) b,A | 55.37 (7.80) A |
| Avg. D (cm) | 119.64 (26.74) a,A | 52.18 (10.55) b,A | 23.93 (9.87) b,A | ||
| Density per ha | 6.91 (5.25) c,C | 105.82 (38.09) b,C | 222.91 (61.58) a,A | ||
| BA per ha | 8.14 (8.63) b,B,C | 23.55 (7.05) a,A | 11.73 (4.02) b,A | ||
| Site 2 | Avg. H (m) | 29.98 (3.82) a,B | 17.82 (3.22) b,B | 8.57 (2.72) c,B | 38.52 (2.21) B |
| Avg. D (cm) | 76.77 (13.57) a,B | 40.15 (8.81) b,B | 16.67 (6.49) c,B | ||
| Density per ha | 24.44 (9.89) c,B | 193.33 (20.98) a,A | 150.67 (37.09) b,B | ||
| BA per ha | 11.66 (3.98) b,B | 25.66 (2.95) a,A | 3.79 (1.12) c,B | ||
| Site 3 | Avg. H (m) | 25.86 (3.21) a,C | 16.61 (3.07) b,C | 7.23 (2.20) c,C | 33.50 (1.92) B |
| Avg. D (cm) | 63.58 (10.42) a,C | 36.99 (8.18) b,C | 13.46 (5.18) c,C | ||
| Density per ha | 50.40 (8.88) c,A | 194.40 (21.92) a,A | 80.40 (21.94) b,C | ||
| BA per ha | 16.43 (2.33) b,A | 21.92 (2.25) a,A | 1.31 (0.27) c,C | ||
| Site 4 | Avg. H (m) | 19.53 (2.11) a,D | 12.46 (2.29) b,D | 5.96 (1.28) c,D | 25.85 (0.54) C |
| Avg. D (cm) | 47.33 (5.55) a,D | 28.85 (5.95) b,D | 11.99 (3.32) c,C,D | ||
| Density per ha | 26.80 (13.47) c,B | 212.00 (29.15) a,A | 82.80 (20.75) b,C | ||
| BA per ha | 4.78 (2.66) b,C | 14.45 (1.64) a,B | 1.01 (0.35) c,C | ||
| Site 5 | Avg. H (m) | 14.17 (1.99) a,E | 8.96 (1.57) b,E | 5.09 (0.63) c,E | 18.15 (1.79) D |
| Avg. D (cm) | 36.00 (5.66) a,E | 21.29 (4.42) b,E | 10.46 (1.75) c,D | ||
| Density per ha | 26.67 (11.77) b,B | 154.00 (39.09) a,B | 48.00 (14.75) b,C | ||
| BA per ha | 2.78 (1.54) b,D | 5.72 (1.29) a,C | 0.42 (0.14) c,C |
| Site 5 | Site 4 | Site 3 | Site 2 | |
|---|---|---|---|---|
| Sorenson’s index | ||||
| Site 4 | 42.25 | |||
| Site 3 | 24.18 | 37.29 | ||
| Site 2 | 27.37 | 50.82 | 59.15 | |
| Site 1 | 26.80 | 43.55 | 56.94 | 59.46 |
| Lamprecht modification index | ||||
| Site 4 | 20.86 | |||
| Site 3 | 11.04 | 32.20 | ||
| Site 2 | 11.69 | 29.41 | 59.67 | |
| Site 1 | 9.74 | 25.68 | 50.50 | 55.68 |
| Sorensosn’s quantitative index | ||||
| Site 4 | 26.50 | |||
| Site 3 | 24.91 | 32.78 | ||
| Site 2 | 23.04 | 29.88 | 56.08 | |
| Site 1 | 23.38 | 29.07 | 51.32 | 48.97 |
| Parameter | Avg. R | Max. R | Avg. T | T dif |
|---|---|---|---|---|
| Diameter (upper) | 0.981 ** | 0.994 ** | 0.604 | 0.503 |
| Diameter (middle) | 0.995 ** | 0.971 ** | 0.643 | 0.631 |
| Diameter (lower) | 0.959 ** | 0.983 ** | 0.552 | 0.430 |
| Height (upper) | 0.974 ** | 0.991 ** | 0.580 | 0.472 |
| Height (middle) | 0.994 ** | 0.968 ** | 0.656 | 0.646 |
| Height (lower) | 0.980 ** | 0.993 ** | 0.603 | 0.500 |
| Species richness | 0.659 ** | 0.575 ** | 0.733 ** | 0.806 |
| Shannon diversity | 0.650 ** | 0.569 ** | 0.714 ** | 0.783 |
| Simpson diversity | 0.571 ** | 0.504 ** | 0.595 ** | 0.651 |
| Shannon evenness | 0.434 ** | 0.380 ** | 0.530 ** | 0.565 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khaine, I.; Woo, S.Y.; Kang, H.; Kwak, M.; Je, S.M.; You, H.; Lee, T.; Jang, J.; Lee, H.K.; Lee, E.; et al. Species Diversity, Stand Structure, and Species Distribution across a Precipitation Gradient in Tropical Forests in Myanmar. Forests 2017, 8, 282. https://doi.org/10.3390/f8080282
Khaine I, Woo SY, Kang H, Kwak M, Je SM, You H, Lee T, Jang J, Lee HK, Lee E, et al. Species Diversity, Stand Structure, and Species Distribution across a Precipitation Gradient in Tropical Forests in Myanmar. Forests. 2017; 8(8):282. https://doi.org/10.3390/f8080282
Chicago/Turabian StyleKhaine, Inkyin, Su Young Woo, Hoduck Kang, MyeongJa Kwak, Sun Mi Je, Hana You, Taeyoon Lee, Jihwi Jang, Hyun Kyung Lee, Euddeum Lee, and et al. 2017. "Species Diversity, Stand Structure, and Species Distribution across a Precipitation Gradient in Tropical Forests in Myanmar" Forests 8, no. 8: 282. https://doi.org/10.3390/f8080282
APA StyleKhaine, I., Woo, S. Y., Kang, H., Kwak, M., Je, S. M., You, H., Lee, T., Jang, J., Lee, H. K., Lee, E., Yang, L., Kim, H., Lee, J. K., & Kim, J. (2017). Species Diversity, Stand Structure, and Species Distribution across a Precipitation Gradient in Tropical Forests in Myanmar. Forests, 8(8), 282. https://doi.org/10.3390/f8080282








