Towards Harmonizing Leaf Litter Decomposition Studies Using Standard Tea Bags—A Field Study and Model Application
Abstract
:1. Introduction
2. Materials and Methods
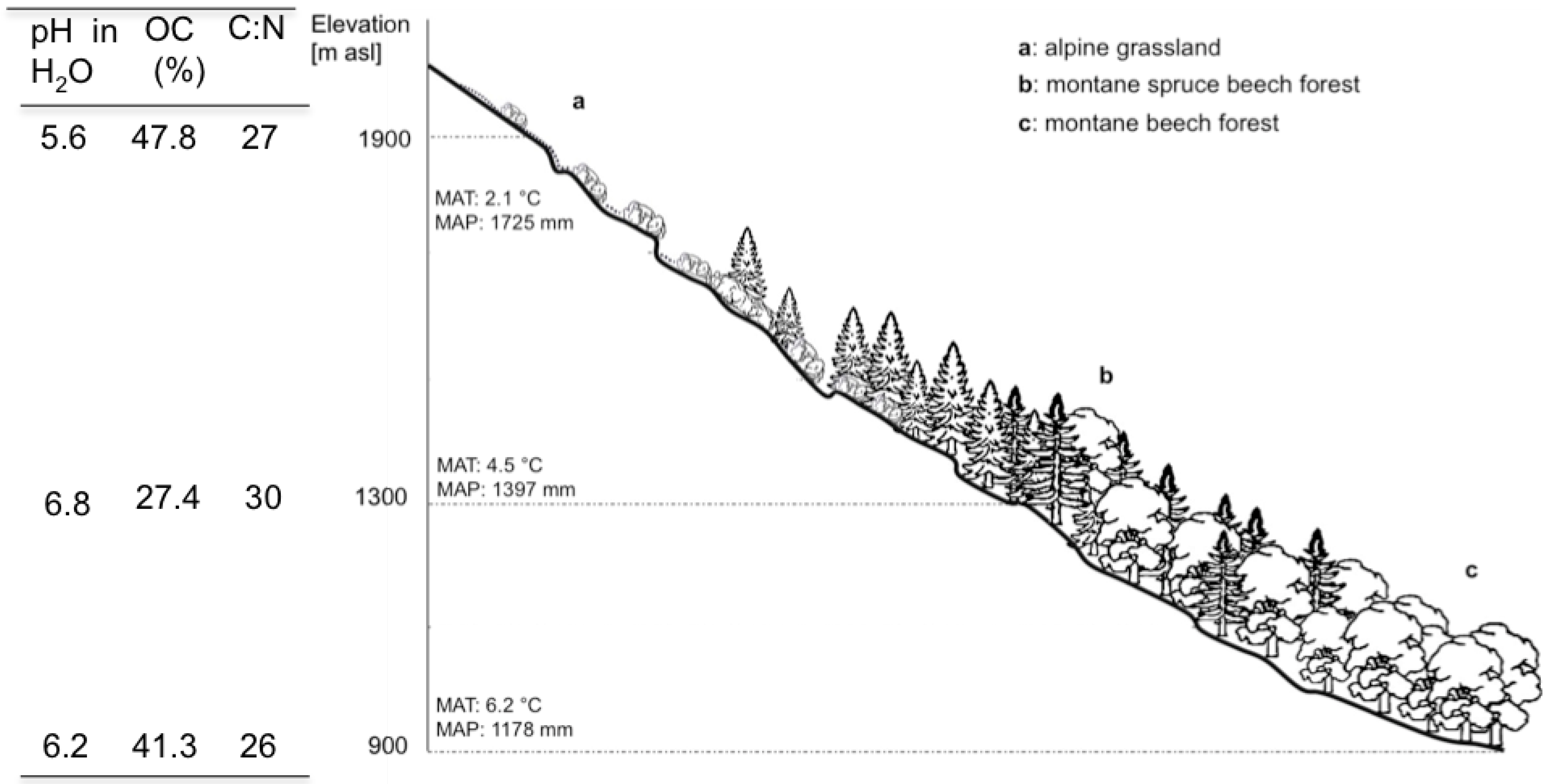
2.1. Study Area
2.2. Litter Decomposition Measurements
2.2.1. Local Litter
2.2.2. Tea Bag Litter
2.3. Litter Decomposition Simulations
2.4. Data Analysis
3. Results
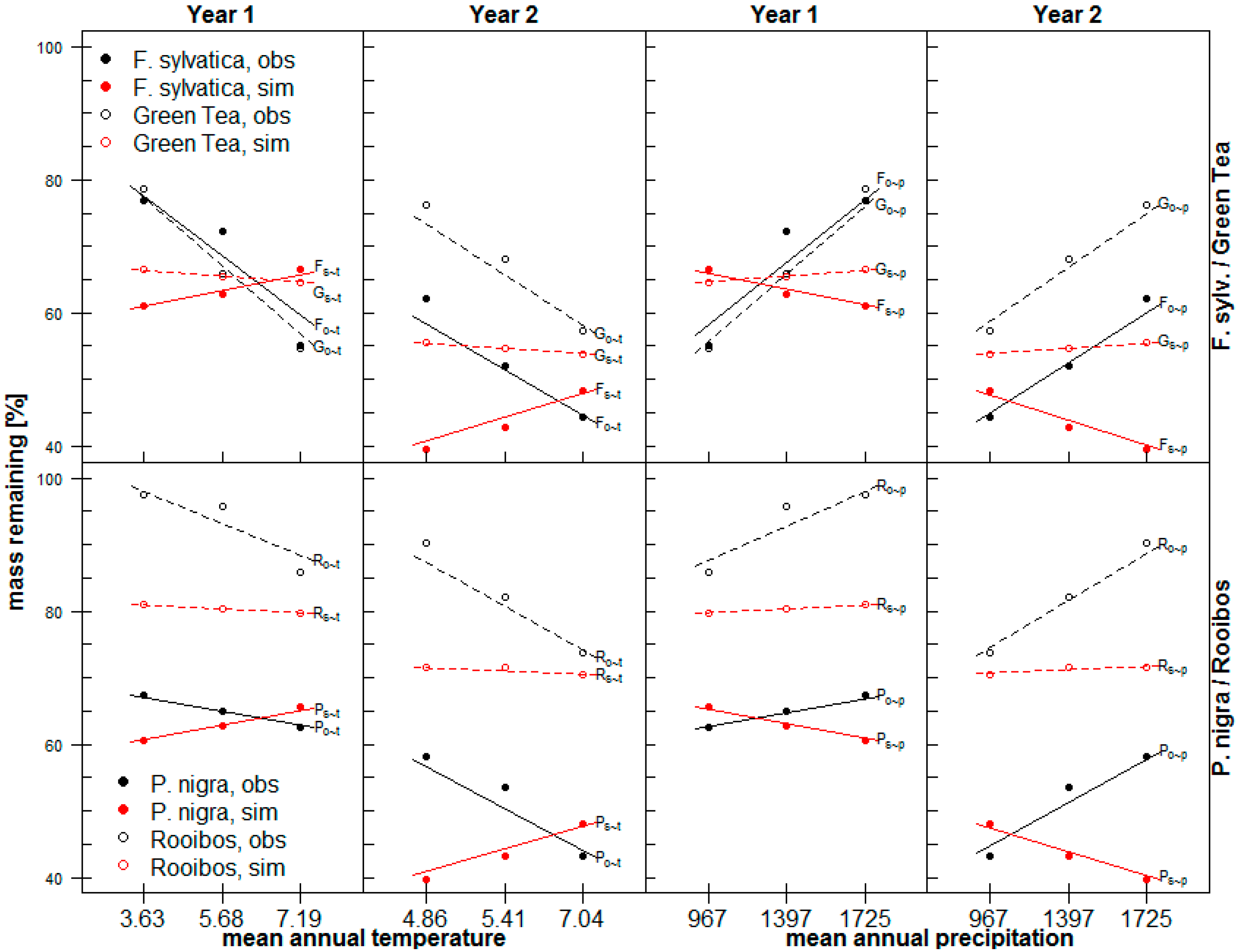
3.1. Observed Decomposition of Standard and Local Litter
3.2. Simulated Decomposition of Standard and Local Litter
4. Discussion
4.1. Observed Decomposition of Standard and Local Litter
4.2. Simulated Decomposition of Standard and Local Litter
4.3. Observed Versus Simulated Decomposition
4.4. Potentials of the Use of Standardized Litter
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Gavazov, K.S. Dynamics of alpine plant litter decomposition in a changing climate. Plant Soil 2010, 337, 19–32. [Google Scholar] [CrossRef]
- Houghton, R.A. Balancing the global carbon budget. Annu. Rev. Earth Planet. Sci. 2007, 35, 313–347. [Google Scholar] [CrossRef]
- Freschet, G.T.; Cornwell, W.K.; Wardle, D.A.; Elumeeva, T.G.; Liu, W.; Jackson, B.G.; Onipchenko, V.G.; Soudzilovskaia, N.A.; Tao, J.; Cornelissen, J.H.C. Linking litter decomposition of above- and below-ground organs to plant-soil feedbacks worldwide. J. Ecol. 2013, 101, 943–952. [Google Scholar] [CrossRef]
- Bradford, M.A.; Berg, B.; Maynard, D.S.; Wieder, W.R.; Wood, S.A. Understanding the dominant controls on litter decomposition. J. Ecol. 2016, 104, 229–238. [Google Scholar] [CrossRef]
- Berg, B. Decomposition patterns for foliar litter—A theory for influencing factors. Soil Biol. Biochem. 2014, 78, 222–232. [Google Scholar] [CrossRef]
- Coûteaux, M.-M.; Bottner, P.; Berg, B. Litter decomposition, climate and litter quality. Trends Ecol. Evol. 1995, 10, 63–66. [Google Scholar] [CrossRef]
- Hararuk, O.; Luo, Y. Improvement of global litter turnover rate predictions using a Bayesian MCMC approach. Ecosphere 2014, 5. [Google Scholar] [CrossRef]
- Cornwell, W.K.; Cornelissen, J.H.C.; Amatangelo, K.; Dorrepaal, E.; Eviner, V.T.; Godoy, O.; Hobbie, S.E.; Hoorens, B.; Kurokawa, H.; Pérez-Harguindeguy, N.; et al. Plant species traits are the predominant control on litter decomposition rates within biomes worldwide. Ecol. Lett. 2008, 11, 1065–1071. [Google Scholar] [CrossRef] [PubMed]
- Berger, T.W.; Duboc, O.; Djukic, I.; Tatzber, M.; Gerzabek, M.H.; Zehetner, F. Decomposition of beech (Fagus sylvatica) and pine (Pinus nigra) litter along an Alpine elevation gradient: Decay and nutrient release. Geoderma 2015, 251–252. [Google Scholar] [CrossRef] [PubMed]
- Heim, A.; Frey, B. Early stage litter decomposition rates for Swiss forests. Biogeochemistry 2004, 70, 299–313. [Google Scholar] [CrossRef]
- Lorenz, K.; Preston, C.; Krumrei, S.; Feger, K.-H. Decomposition of needle/leaf litter from Scots pine, black cherry, common oak and European beech at a conurbation forest site. Eur. J. For. Res. 2004, 123, 177–188. [Google Scholar] [CrossRef]
- Duboc, O.; Zehetner, F.; Djukic, I.; Tatzber, M.; Berger, T.W.; Gerzabek, M.H. Decomposition of European beech and Black pine foliar litter along an Alpine elevation gradient: Mass loss and molecular characteristics. Geoderma 2012, 189, 522–531. [Google Scholar] [CrossRef]
- Berg, B.; Staaf, H. Decomposition rate and chemical changes of scots pine needle litter. II. Influence of chemical composition. Ecol. Bull. 1980, 32, 373–390. [Google Scholar]
- Jansson, P.-E.; Reurslag, A. Climatic influence on litter decomposition: Methods and some Results of a NW- European transect. In Responses of Forest Ecosystems to Environmental Changes; Teller, A., Mathy, P., Jeffers, J.N.R., Eds.; Springer: Dordrecht, The Netherlands, 1992; pp. 351–358. [Google Scholar]
- Keuskamp, J.A.; Dingemans, B.J.J.; Lehtinen, T.; Sarneel, J.M.; Hefting, M.M. Tea bag index: A novel approach to collect uniform decomposition data across ecosystems. Methods Ecol. Evol. 2013, 4, 1070–1075. [Google Scholar] [CrossRef]
- Teabag Index. Available online: http://www.decolab.org/tbi/ (accessed on 26 July 2016).
- Cotrufo, M.F.; Wallenstein, M.D.; Boot, C.M.; Denef, K.; Paul, E. The Microbial Efficiency-Matrix Stabilization (MEMS) framework integrates plant litter decomposition with soil organic matter stabilization: Do labile plant inputs form stable soil organic matter? Glob. Chang. Biol. 2013, 19, 988–995. [Google Scholar] [CrossRef] [PubMed]
- Järvenpää, M.; (Tampere University of Technology, Department of Mathematics, Tampere, Finland); Repo, A.; (Finnish Environment Institute, Ecosystem Processes, Helsinki, Finland); Liski, J.; (Finnish Environment Institute, Ecosystem Processes, Helsinki, Finland); Kaasalainen, M.; (Tampere University of Technology, Department of Mathematics, Tampere, Finland). Unpublished work. 2016.
- Didion, M.; Frey, B.; Rogiers, N.; Thürig, E. Validating tree litter decomposition in the Yasso07 carbon model. Ecol. Model. 2014, 291, 58–68. [Google Scholar] [CrossRef]
- Gholz, H.L.; Wedin, D.A.; Smitherman, S.M.; Harmon, M.E.; Parton, W.J. Long-term dynamics of pine and hardwood litter in contrasting environments: Toward a global model of decomposition. Glob. Chang. Biol. 2000, 6, 751–765. [Google Scholar] [CrossRef]
- Austrian Federal Ministry of Finance. Available online: http://www.bundesfinanzministerium.de/Web/EN/Home/home.html (accessed on 26 July 2016).
- International Union of Soil Sciences (IUSS) Working Group World Reference Base for Soil Resources (WRB). World Reference Base for Soil Resources 2006; Food and Agriculture Organization of the United Nations: Rome, Italy, 2006; p. 128. [Google Scholar]
- Djukic, I.; Zehetner, F.; Watzinger, A.; Horacek, M.; Gerzabek, M.H. In situ carbon turnover dynamics and the role of soil microorganisms therein: A climate warming study in an Alpine ecosystem. FEMS Microbiol. Ecol. 2013, 83, 112–124. [Google Scholar] [CrossRef] [PubMed]
- Tuomi, M.; Rasinmäki, J.; Repo, A.; Vanhala, P.; Liski, J. Soil carbon model Yasso07 graphical user interface. Environ. Model. Softw. 2011, 26, 1358–1362. [Google Scholar] [CrossRef]
- Tuomi, M.; Laiho, R.; Repo, A.; Liski, J. Wood decomposition model for boreal forests. Ecol. Model. 2011, 222, 709–718. [Google Scholar] [CrossRef]
- Tuomi, M.; Thum, T.; Järvinen, H.; Fronzek, S.; Berg, B.; Harmon, M.; Trofymow, J.A.; Sevanto, S.; Liski, J. Leaf litter decomposition—Estimates of global variability based on Yasso07 model. Ecol. Model. 2009, 220, 3362–3371. [Google Scholar] [CrossRef]
- Tuomi, M.; Vanhala, P.; Karhu, K.; Fritze, H.; Liski, J. Heterotrophic soil respiration—Comparison of different models describing its temperature dependence. Ecol. Model. 2008, 211, 182–190. [Google Scholar] [CrossRef]
- Soil carbon model—Yasso. Available online: http://www.syke.fi/projects/yasso (accessed on 26 July 2016).
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2015. [Google Scholar]
- Duboc, O.; Dignac, M.-F.; Djukic, I.; Zehetner, F.; Gerzabek, M.H.; Rumpel, C. Lignin decomposition along an Alpine elevation gradient in relation to physicochemical and soil microbial parameters. Glob. Chang. Biol. 2014, 20, 2272–2285. [Google Scholar] [CrossRef] [PubMed]
- McGuire, K.L.; Treseder, K.K. Microbial communities and their relevance for ecosystem models: Decomposition as a case study. Soil Biol. Biochem. 2010, 42, 529–535. [Google Scholar] [CrossRef]
- Frey, B.; Swiss Federal Institute for Forest, Snow and Landscape Research WSL, Forest Soils and Biogeochemistry, CH-8903, Birmensdorf, Switzerland. Personal communication. 16 March 2016. [Google Scholar]
- Berg, B.; McClaugherty, C. Plant Litter. Decomposition, Humus Formation, Carbon Sequestration; Springer-Verlag: Berlin, Heidelberg, 2008. [Google Scholar]
- Didion, M.; Blujdea, V.; Grassi, G.; Hernández, L.; Jandl, R.; Kriiska, K.; Lehtonen, A.; Saint-André, L. Models for reporting forest litter and soil C pools in national greenhouse gas inventories: Methodological considerations and requirements. Carbon Manag. 2016, 1–14. [Google Scholar] [CrossRef]
- Evans, M.R. Modelling ecological systems in a changing world. Phil. Trans. R. Soc. B 2012, 367, 181–190. [Google Scholar] [CrossRef] [PubMed]
- International Long Term Ecological Research. Available online: http://www.ilternet.edu (accessed on 26 July 2016).
- Adair, E.C.; Parton, W.J.; del Grosso, S.J.; Silver, W.L.; Harmon, M.E.; Hall, S.A.; Burke, I.C.; Hart, S.C. Simple three-pool model accurately describes patterns of long-term litter decomposition in diverse climates. Glob. Chang. Biol. 2008, 14, 2636–2660. [Google Scholar] [CrossRef]
- Manzoni, S.; Piñeiro, G.; Jackson, R.B.; Jobbágy, E.G.; Kim, J.H.; Porporato, A. Analytical models of soil and litter decomposition: Solutions for mass loss and time-dependent decay rates. Soil Biol. Biochem. 2012, 50, 66–76. [Google Scholar] [CrossRef]
- Harmon, M.E.; Silver, W.L.; Fasth, B.; Chen, H.U.A.; Burke, I.C.; Parton, W.J.; Hart, S.C.; Currie, W.S. Long-term patterns of mass loss during the decomposition of leaf and fine root litter: An intersite comparison. Glob. Chang. Biol. 2009, 15, 1320–1338. [Google Scholar] [CrossRef]
- Berg, B.; Kjønaas, O.J.; Johansson, M.B.; Erhagen, B.; Åkerblom, S. Late stage pine litter decomposition: Relationship to litter N, Mn, and acid unhydrolyzable residue (AUR) concentrations and climatic factors. For. Ecol. Manag. 2015, 358, 41–47. [Google Scholar] [CrossRef]
- Bradford, M.A.; Tordoff, G.M.; Eggers, T.; Jones, T.H.; Newington, J.E. Microbiota, fauna, and mesh size interactions in litter decomposition. Oikos 2002, 99, 317–323. [Google Scholar] [CrossRef]
- Petersen, B.M.; Olesen, J.E.; Heidmann, T. A flexible tool for simulation of soil carbon turnover. Ecol. Model. 2002, 151, 1–14. [Google Scholar] [CrossRef]
- Chertov, O.G.; Komarov, A.S.; Nadporozhskaya, M.; Bykhovets, S.S.; Zudin, S.L. ROMUL—A model of forest soil organic matter dynamics as a substantial tool for forest ecosystem modeling. Ecol. Model. 2001, 138, 289–308. [Google Scholar] [CrossRef]
- Coleman, K.; Jenkinson, D.S. RothC-26.3—A model for the turnover of carbon in soil. In Evaluation of Soil Organic Matter Models Using Existing Long-Term Datasets; Powlson, D.S., Smith, P., Smith, J.U., Eds.; Springer: Heidelberg, Germany, 1996; pp. 237–246. [Google Scholar]
- Liski, J.; Palosuo, T.; Peltoniemi, M.; Sievänen, R. Carbon and decomposition model Yasso for forest soils. Ecol. Model. 2005, 189, 168–182. [Google Scholar] [CrossRef]

| A | W | E | N | |
|---|---|---|---|---|
| Green Tea | 0.283 | 0.493 | 0.066 | 0.156 |
| Rooibos Tea | 0.289 | 0.215 | 0.049 | 0.444 |
| Fagus sp. | 0.498 | 0.165 | 0.074 | 0.264 |
| Pinus sp. | 0.421 | 0.202 | 0.172 | 0.205 |
| Elevation | 900 m | 1300 m | 1900 m | |||
|---|---|---|---|---|---|---|
| Year | 2007 | 2008 | 2007 | 2008 | 2007 | 2008 |
| Mean annual temperature (°C) | 7.2 | 7.0 | 5.7 | 5.4 | 3.6 | 4.9 |
| Temperature amplitude (°C) | 7.6 | 6.9 | 5.9 | 5.7 | 6.9 | 5.8 |
| Annual precipitation (mm) | 967 | 967 | 1397 | 1397 | 1725 | 1725 |
| Elevation (m asl) | Green Tea against Fagus sylvatica Litter | Rooibos Tea against Pinus nigra Litter | ||
|---|---|---|---|---|
| Year 1 | Year 2 | Year 1 | Year 2 | |
| 1900 | 0.724 | 0.000 | 0.000 | 0.000 |
| 1300 | 0.165 | 0.001 | 0.000 | 0.000 |
| 900 | 0.999 | 0.086 | 0.000 | 0.000 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Didion, M.; Repo, A.; Liski, J.; Forsius, M.; Bierbaumer, M.; Djukic, I. Towards Harmonizing Leaf Litter Decomposition Studies Using Standard Tea Bags—A Field Study and Model Application. Forests 2016, 7, 167. https://doi.org/10.3390/f7080167
Didion M, Repo A, Liski J, Forsius M, Bierbaumer M, Djukic I. Towards Harmonizing Leaf Litter Decomposition Studies Using Standard Tea Bags—A Field Study and Model Application. Forests. 2016; 7(8):167. https://doi.org/10.3390/f7080167
Chicago/Turabian StyleDidion, Markus, Anna Repo, Jari Liski, Martin Forsius, Michael Bierbaumer, and Ika Djukic. 2016. "Towards Harmonizing Leaf Litter Decomposition Studies Using Standard Tea Bags—A Field Study and Model Application" Forests 7, no. 8: 167. https://doi.org/10.3390/f7080167






