Physical, Chemical, and Biological Properties of Soil under Decaying Wood in a Tropical Wet Forest in Puerto Rico
Abstract
:1. Introduction
2. Materials and Methods
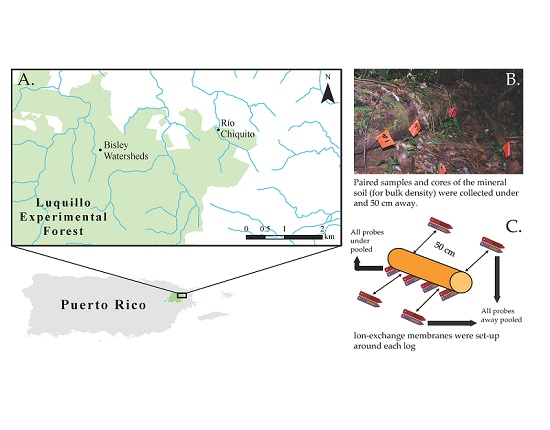
2.1. Study Area
2.2. Sampling Design
2.3. Soil Sampling and Analysis
2.4. Data Analysis
3. Results
3.1. Soil Physical Properties
3.1.1. Soil Temperature
3.1.2. Soil Moisture
3.2. Soil Chemical Properties
3.3. Soil Biota
3.3.1. Soil Microbial Biomass
3.3.2. Roots
4. Discussion
4.1. Effects on Soil Physical Properties
4.2. Effects On Soil Chemical Properties
4.3. Effects on Soil Biota
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Harmon, M.E.; Sexton, J. Guidelines for Measurements of Woody Detritus in Forest Ecosystems; US LTER Network Office: Seattle, WA, USA, 1996; Volume 20, Available online: http://www.lternet.edu/documents/Publications/woodydetritus/ (accessed on 31 May 2016).
- Delaney, M.; Brown, S.; Lugo, A.E.; Torres-Lezama, A.; Bello-Quintero, N. The quantity and turnover of dead wood in permanent forest plots in six life zones of Venezuela. Biotropica 1998, 30, 2–11. [Google Scholar] [CrossRef]
- Clark, D.B.; Clark, D.A.; Brown, S.; Oberbauer, S.F.; Veldkamp, E. Stocks and flows of coarse woody debris across a tropical rain forest nutrient and topography gradient. For. Ecol. Manag. 2002, 164, 237–248. [Google Scholar] [CrossRef]
- González, G.; Luce, M.M. Woody debris characterization along an elevation gradient in northeastern Puerto Rico. In Ecological Gradient Analyses in a Tropical Landscape. Ecological Bulletins 54; González, G., Willig, M.R., Waide, R.B., Eds.; 2013; pp. 181–193. [Google Scholar]
- Lambert, R.; Lang, G.E.; Reiners, W.A. Loss of mass and chemical change in decaying boles of a subalpine balsam fir forest. Ecology 1980, 61, 1460–1473. [Google Scholar] [CrossRef]
- Harmon, M.E.; Franklin, J.F.; Swanson, F.J.; Sollins, P.; Gregory, S.V.; Lattin, J.D.; Anderson, N.H.; Cline, S.P.; Aumen, N.G.; Sedell, J.R.; et al. Ecology of coarse woody debris in temperate ecosystems. Adv. Ecol. Res. 1986, 15, 133–302. [Google Scholar]
- Stevens, V. The Ecological Role of Coarse Woody Debris: An Overview of the Ecological Importance of CWD in BC Forests; Ministry of Forests, Research Program: Victoria, BC, Canada, 1997. Available online: http://www.for.gov.bc.ca/hfd/pubs/docs/Wp/Wp30.pdf (accessed on 12 November 2005).
- Torres, J.A.; González, G. Wood decomposition of Cyrilla racemiflora (Cyrillaceae) in Puerto Rican dry and wet forests: A 13-year case study. Biotropica 2005, 37, 452–456. [Google Scholar] [CrossRef]
- Creed, I.F.; Morrison, D.L.; Nicholas, N.S. Is coarse woody debris a net sink or source of nitrogen in the red spruce-Fraser fir forest of the southern Appalachians, USA? Can. J. For. Res. 2004, 34, 716–727. [Google Scholar] [CrossRef]
- Harmon, M.E.; Sexton, J. Water balance of conifer logs in early stages of decomposition. Plant Soil 1995, 172, 141–152. [Google Scholar] [CrossRef]
- Durbak, I.; Green, D.W.; Highley, T.L.; Howard, J.L.; McKeever, D.B.; Miller, R.B.; Pettersen, R.C.; Rowell, R.M.; Simpson, W.T.; Skog, K.E.; et al. Kirk-Othmer, Encyclopedia of Chemical Technology, 4th ed.; Wiley-Interscience: New York, NY, USA, 1998; Volume 25, pp. 627–664. [Google Scholar]
- Boddy, L. Carbon dioxide release from decomposing wood: Effect of water content and temperature. Soil Biol. Biochem. 1983, 15, 501–510. [Google Scholar] [CrossRef]
- Temnuhin, V.B. Preliminary quantitative estimation of wood decomposition by fungi in a Russian temperate pine forest. For. Ecol. Manag. 1996, 81, 249–257. [Google Scholar] [CrossRef]
- Vogt, K.A.; Vogt, D.J.; Asbjornsen, H.; Dahlgren, R.A. Roots, nutrients and their relationship to spatial patterns. Plant Soil 1995, 168, 113–123. [Google Scholar] [CrossRef]
- Busse, M.D. Downed bole-wood decomposition in lodgepole pine forests of central Oregon. Soil Sci. Soc. Am. J. 1994, 581, 221–227. [Google Scholar] [CrossRef]
- Spears, J.D.H.; Holub, S.M.; Harmon, M.E.; Lajtha, K. The influence of decomposing logs on soil biology and nutrient cycling in an old-growth mixed coniferous forest in Oregon, USA. Can. J. For. Res. 2003, 33, 2193–2201. [Google Scholar] [CrossRef]
- Kayahara, G.J.; Klinka, K.; Lavkulich, L.M. Effects of decaying wood on eluviation, podzolization, acidification, and nutrition in soils with different moisture regimes. Environ. Monit. Assess. 1996, 39, 485–492. [Google Scholar] [CrossRef] [PubMed]
- Krzyszowska-Waitkus, A.J.; Vance, G.F. Influence of coarse woody debris on soil organic substances in a lodgepole pine forest. Agron. Abstr. 1999, 91, 306–308. [Google Scholar]
- Hafner, S.D.; Groffman, P.M.; Mitchell, M.J. Leaching of dissolved organic carbon, dissolved organic nitrogen, and other solutes from coarse woody debris and litter in a mixed forest in New York State. Biogeochemistry 2005, 74, 257–282. [Google Scholar] [CrossRef]
- Keenan, R.J.; Prescott, C.E.; Kimmins, J.P. Mass and nutrient content of woody debris and forest floor in western red cedar and western hemlock forests on northern Vancouver Island. Can. J. For. Res. 1993, 23, 1025–1059. [Google Scholar] [CrossRef]
- Preston, C.M.; Trofymow, J.A.; Niu, J.; Fyfe, C.A. CPMAS-NMR spectroscopy and chemical analysis of coarse woody debris in coastal forests of Vancouver Islands. For. Ecol. Manag. 1998, 111, 51–68. [Google Scholar] [CrossRef]
- Krankina, O.N.; Harmon, M.E.; Griazkin, A.V. Nutrient stores and dynamics of woody detritus in a boreal forest: Modeling potential implications at the stand level. Can. J. For. Res. 1999, 29, 20–32. [Google Scholar] [CrossRef]
- Cotrufo, M.F.; Ineson, P. Does elevated atmospheric CO2 concentrations affect wood decomposition? Plant Soil 2000, 224, 51–57. [Google Scholar] [CrossRef]
- Holub, S.M.; Spears, J.D.H.; Lajtha, K. A reanalysis of nutrient dynamics in coniferous coarse woody debris. Can. J. For. Res. 2001, 31, 1894–1902. [Google Scholar] [CrossRef]
- Zimmerman, J.K.; Pulliam, W.M.; Lodge, D.J.; Quiñones-Orfila, V.; Fetcher, N.; Guzmán-Grajáles, S.; Parrotta, J.A.; Asbury, C.E.; Walker, L.R.; Waide, R.B. Nitrogen immobilization by decomposing woody debris and the recovery of tropical wet forest from hurricane damage. Oikos 1995, 72, 314–322. [Google Scholar] [CrossRef]
- Beard, K.H.; Vogt, K.A.; Vogt, D.J.; Scatena, F.N.; Covich, A.P.; Sigurdardottir, R.; Siccama, T.G.; Crowl, T.A. Structural and functional responses of a subtropical forest to 10 years of hurricanes and droughts. Ecol. Monogr. 2005, 75, 345–361. [Google Scholar] [CrossRef]
- Walker, L.R.; Zimmerman, J.K.; Lodge, D.J.; Guzmán-Grajales, S. An altitudinal comparison of growth and species composition in hurricane-damaged forests in Puerto Rico. J. Ecol. 1996, 84, 877–889. [Google Scholar] [CrossRef]
- Sanford, R.L., Jr.; Parton, W.J.; Ojima, D.S.; Lodge, D.J. Hurricane effects on soil organism altered dynamics and forest production in the Luquillo Experimental Forest, Puerto Rico: Results of simulation modelling. Biotropica 1991, 23, 364–372. [Google Scholar] [CrossRef]
- Hart, S.C. Nitrogen transformations in fallen tree boles and mineral soil of an old-growth forest. Ecology 1999, 80, 1385–1394. [Google Scholar] [CrossRef]
- Klinka, K.; Lavkulich, L.M.; Wang, Q.; Feller, M.C. Influence of decaying wood on chemical properties of forest floors and surface mineral soils: A pilot study. Ann. Sci. For. 1995, 52, 523–533. [Google Scholar] [CrossRef]
- Boddy, L. The micro-environment of basidiomycete mycelia in temperate deciduous woodlands. In The Ecology and Physiology of the Fungal Mycelium; Jennings, D.H., Rayner, A.D.M., Eds.; Cambridge University Press: Cambridge, UK, 1984; pp. 261–289. [Google Scholar]
- Boddy, L. Water and decomposition processes. In Water, Fungi and Plants; Ayres, P.G., Boddy, L., Eds.; Cambridge University Press: Cambridge, UK, 1986; pp. 375–398. [Google Scholar]
- Kavanagh, T.Y.; Kellman, M. Seasonal pattern of fine root proliferation in a tropical dry forest. Biotropica 1992, 24, 157–165. [Google Scholar] [CrossRef]
- Lodge, D.J.; Winter, D.; Clum, N.C. Competition by soil microbes with roots under decomposing logs. Inoculum 2001, 52, 49. [Google Scholar]
- Vitousek, P.M.; Matson, P.A. Mechanisms of nitro-gen retention in forest ecosystems: A field experiment. Science 1984, 225, 51–52. [Google Scholar] [CrossRef] [PubMed]
- Schimel, J.P.; Firestone, M.K. Nitrogen incorporation and flow through a coniferous forests oil profile. Soil Sci. Soc. Am. J. 1989, 53, 779–784. [Google Scholar] [CrossRef]
- Preston, C.M.; Marshall, V.G.; McCullogh, K.F.; Mead, D.J. Fate of 15N-labelled fertilizer applied on snow at two forested sites in British Columbia. Can. J. For. Res. 1990, 20, 1583–1592. [Google Scholar] [CrossRef]
- Zak, D.R.; Groffman, P.M.; Pregitzes, K.S.; Christensen, S.; Tiedje, J.M. The vernal dam: Plant-microbe competition for nitrogen in northern hardwood forests. Ecology 1990, 71, 651–656. [Google Scholar] [CrossRef]
- Groffman, P.M.; Zak, D.R.; Christensen, S.; Mosier, A.; Tiedje, J.M. Early spring nitrogen dynamics in a temperate forest landscape. Ecology 1993, 74, 1579–1585. [Google Scholar] [CrossRef]
- Lugo, A.E. Comparison of tropical tree plantations with secondary forests of similar age. Ecol. Monogr. 1992, 62, 1–41. [Google Scholar] [CrossRef]
- Scatena, F. An Introduction to the Physiography and History of the Bisley Experimental Watersheds in the Luquillo Mountains of Puerto Rico; USDA-FS-Technical Report No. SO-72; United States Department of Agriculture, Forest Service, Southern Research Station: New Orleans, LA, USA, 1989. [Google Scholar]
- Zalamea, M.; González, G.; Ping, C.L.; Michaelson, G. Soil organic matter dynamics under decaying Wood in a subtropical wet Forest: Effect of tree species and decay stage. Plant Soil 2007, 296, 173–185. [Google Scholar] [CrossRef]
- Cuevas, E.; Brown, S.; Lugo, A.E. Above and belowground organic matter storage and production in a tropical pine plantation and a paired broadleaf secondary forest. Plant Soil 1991, 135, 257–268. [Google Scholar] [CrossRef]
- Gonzalez, G. Bisley Tower (TOWER I) Meteorological Station. Long Term Ecological Research Network. Available online: http://dx.doi.org/10.6073/pasta/ac2730ec2c56ffe08302a8c3cc50cd90 (accessed on 27 July 2016).
- Torres, J.A. Wood decomposition of Cyrilla racemiflora in a tropical montane forest. Biotropica 1994, 26, 124–140. [Google Scholar] [CrossRef]
- Goodell, B.; Quian, Y.; Jellison, J. Fungal decay of wood: Soft rot—Brown rot—White rot. In Development of Commercial Wood Preservatives; Oxford University Press: Oxford, UK, 2008; Volume 982, pp. 9–31. [Google Scholar]
- Day, P.R. Particle fractionation and particle-size analysis, Hydrometer method. In American Society of Agronomy, Methods of Soil Analysis, Part 1, Agronomy 9; American Society of Agronomy/Soil Science Society of America: Madison, WI, USA, 1965; pp. 562–566. [Google Scholar]
- Tabatabai, M.A.; Bremmer, J.M. Automated instruments for determination of total carbon, nitrogen, and sulfur in soils by combustion techniques. In Soil Analysis, Modern Instruments Techniques; Marcel Dekker, Inc.: New York, NY, USA, 1991; pp. 261–286. [Google Scholar]
- Luh Huang, C.Y.; Schulte, E.E. Digestion of plant tissue for analysis by ICO emission spectroscopy. Commun. Soil Sci. Plant Anal. 1985, 16, 943–958. [Google Scholar] [CrossRef]
- Hunter, A.H. International Soil Fertility and Improvement: Laboratory Procedures; Department of Soil Science, North Carolina State University: Raleigh, NC, USA, 1982. [Google Scholar]
- Qian, P.; Schoenau, J.J. Practical applications of ion exchange resins in agriculture and environmental soil research. Can. J. Soil Sci. 2002, 82, 9–21. [Google Scholar] [CrossRef]
- Lin, Q.; Brookes, P.C. An evaluation of the substrate-induced respiration method. Soil Biol. Biochem. 1999, 31, 1969–1983. [Google Scholar] [CrossRef]
- Zalamea, M.; González, G. Substrate-induced respiration in Puerto Rican soils: Minimum glucose amendment. Acta Cient. 2007, 21, 11–17. [Google Scholar]
- Ruan, H.H.; Zou, X.M.; Scatena, F.N.; Zimmerman, J.K. Asynchronous rhythms of soil microbial biomass and plant litterfall in a tropical wet forest. Plant Soil 2004, 260, 147–154. [Google Scholar] [CrossRef]
- Anderson, J.P.E.; Domsch, K.H. A physiological method for the quantitative measurement of microbial biomass in soil. Soil Biol. Biochem. 1978, 10, 215–221. [Google Scholar] [CrossRef]
- Bouma, T.J.; Nielsen, K.L.; Koutstaal, B. Sample preparation and scanning protocol for computerised analysis of root length and diameter. Plant Soil 2000, 218, 185–196. [Google Scholar] [CrossRef]
- SPSS. SPSS 11.5 for Windows; SPSS: Chicago, IL, USA, 2002. [Google Scholar]
- Cox, S.B.; Willig, M.R.; Scatena, F.N. Variation in nutrient characteristics of surface soils from the Luquillo Experimental Forest of Puerto Rico—A multivariate perspective. Plant Soil 2002, 247, 189–198. [Google Scholar] [CrossRef]
- Basnet, K. Effect of topography on the pattern of trees in tabonuco (Dacryodes excelsa) dominated rain forest of Puerto Rico. Biotropica 1992, 24, 31–42. [Google Scholar] [CrossRef]
- Sánchez, M.J. Estudio Comparativo de Algunas Propiedades Químicas y Físicas de Suelos de Bosque Bajo Uso Natural y Plantaciones Silvestres. Master’s Thesis, University of Puerto Rico, Mayagüez, Puerto Rico, 1989. [Google Scholar]
- Sánchez, M.J.; López, E.; Lugo, A.E. Chemical and Physical Analyses of Selected Plants and Soils from Puerto Rico (1981–1990); Research Note 1; USDA Forest Service—IITF: Río Piedras, Puerto Rico, 1997; p. 112. [Google Scholar]
- Cox, S.B. Soil Properties and Microbial Functional Diversity of Surface Soils in the Luquillo Experimental Forest of Puerto Rico. Ph.D. Thesis, Texas Tech University, Lubbock, TX, USA, 1999. [Google Scholar]
- Lodge, D.J.; Zou, X.; Clum, N.C. Effect of Decomposing Coarse Woody Debris on Soil Carbon, Nutrients, and Microbial Biomass. In Oral Presentation at 2001 LTER All Scientist Meeting, Snowbird, UT, USA, 2–4 August 2000.
- Lodge, D.J.; United States Department of Agriculture, Forest Service, Forest Products Laboratory (now Northern Recearch Station), Luquillo, Puerto Rico. Unpublished work. 2000.
- Paul, E.A.; Clark, F.E. Soil microbiology and Biochemistry; Academic Press Inc.: San Diego, CA, USA, 1989. [Google Scholar]
- Robertson, G.P. Factors regulating nitrification in primary and secondary succession. Ecology 1982, 63, 1561–1573. [Google Scholar] [CrossRef]
- Sulewski, C.A.; Greer, K.J.; Schoenau, J.J.; Baron, V.S. Factors affecting nutrient supply rate measurements with PRS™-probes. In Proceedings of the Soils and Crops Workshop, Saskatoon, SK, Canada, 2002.
- Yano, Y.; Lajtha, K.; Sollins, P.; Caldwell, B.A. Chemistry and dynamics of dissolved organic matter in a temperate coniferous forest on andic soils: Effect of litter quality. Ecosystems 2005, 8, 286–300. [Google Scholar] [CrossRef]
- Silver, W.L.; Lugo, A.E.; Keller, M. Soil oxygen availability and biogeochemistry along rainfall and topographic gradients in upland wet tropical forest soils. Biogeochemistry 1999, 44, 301–328. [Google Scholar] [CrossRef]
- Yavitt, J.B.; Fahey, T.J. Chemical composition of interstitial water in decaying lodgepole pine bole wood. Can. J. For. Res. 1985, 15, 1149–1153. [Google Scholar] [CrossRef]
- Linnik, P.M. Zinc, lead and cadmium speciation in Dnieper water-bodies. Lakes Reserv. Res. Manag. 2000, 5, 261–265. [Google Scholar] [CrossRef]
- Limbert, E.S.B.; Betts, W.B. Influences of substrate chemistry and microbial metabolic diversity on the bioremediation of xenobiotic contamination. Genet. Eng. Biotechnol. 1996, 16, 159–180. [Google Scholar]
- Sparling, G.P. The substrate-induced respiration method. In Methods in Applied Soil Microbiology and Biochemistry; Alef, K., Nannipieri, P., Eds.; Academic Press: New York, NY, USA, 1995; pp. 397–404. [Google Scholar]
- Griffith, G.W.; Easton, G.L.; Jones, A.W. Ecology and diversity of waxcap (Hygrocybe spp.) fungi. Bot. J. Scotl. 2002, 54, 7–22. [Google Scholar] [CrossRef]
- Lodge, D.J. Nutrient cycling by fungi in wet tropical forests. In Aspects of Tropical Mycology; Isaac, S., Frankland, J.C., Watling, R., Whalley, A.J.S., Eds.; BMS Symposium Series; Cambridge University Press: Cambridge, UK, 1993; Chapter 19; pp. 37–57. [Google Scholar]
- Lodge, D.J.; Ingham, E.R. A comparison of agar film techniques for estimating fungal biovolumes in litter and soil. Agric. Ecosyst. Environ. 1991, 34, 131–144. [Google Scholar] [CrossRef]
- Qian, P.; Schoenau, J.J. Assessing nitrogen mineralization from soil organic matter using anion exchange membranes. Fertil. Res. 1995, 40, 143–148. [Google Scholar] [CrossRef]


| Soil Property | Bisley Experimental Watershed | Río Chiquito Plantation |
|---|---|---|
| Loam: | Loam/Silt Loam: | |
| Texture [40] | 45% Sand | 32% Sand |
| 34% Silt | 48% Silt | |
| 21% Clay | 20% Clay | |
| Bulk density (kg/L) * | 0.60 | 0.80 |
| %Organic matter [40] | 5.79 ± 2.5 | 5.11 ± 1.38 |
| %Nitrogen [42] * | 0.401 ± 0.01 | 0.408 ± 0.02 |
| Phosphorus (mgg−1) * | 0.3 ± 0.009 | 0.25 ± 0.02 |
| pH [40] | 4.6 | 4.9 |
| Calcium (mgg−1) * | 0.7 ± 0.2 | 4.2 ± 1.0 |
| Magnesium (mgg−1) * | 1.7 ± 0.4 | 3.0 ± 0.6 |
| Nutrient | D. excelsa | S. macrophylla | |||||||
|---|---|---|---|---|---|---|---|---|---|
| 7 Year-Old | 15 Year-Old | 7 Year-Old | 15 Year-Old | ||||||
| Al | Total | 42.3 | (3.2) | 25.5 | (2.5) | 21.4 | (2.1) | 49.5 | (2.5) |
| Extractable | 0.4 | (0.1) | 0.7 | (0.1) | 0.02 | (0.003) | 0.2 | (0.02) | |
| % | 1.0 | (0.2) | 3.3 | (0.6) | 0.1 | (0.03) | 0.3 | (0.1) | |
| Fe | Total | 53.3 | (1.4) | 46.5 | (2.2) | 37.8 | (3.8) | 70.2 | (3.2) |
| Extractable | 1.4 | (0.3) | 2.6 | (0.3) | 0.2 | (0.05) | 1.2 | (0.1) | |
| % | 2.8 | (0.6) | 5.8 | (0.7) | 0.7 | (0.1) | 1.6 | (0.2) | |
| Mn | Total | 0.6 | (0.2) | 0.1 | (0.02) | 1.5 | (0.2) | 0.5 | (0.1) |
| Extractable | 0.1 | (0.03) | 0.0 | (0.01) | 0.3 | (0.05) | 0.2 | (0.04) | |
| % | 28.3 | (3.5) | 36.8 | (5.3) | 24.9 | (4.6) | 25.6 | (3.2) | |
| Ca | Total | 0.7 | (0.2) | 0.44 | (0.1) | 4.2 | (1.0) | 0.9 | (0.2) |
| Extractable | 0.7 | (0.2) | 0.39 | (0.1) | 2.4 | (0.5) | 0.8 | (0.1) | |
| % | 100.0 | (3.9) | 88.4 | (9.0) | 58.8 | (33.4) | 91.5 | (3.0) | |
| Mg | Total | 1.7 | (0.4) | 0.6 | (0.1) | 3.0 | (0.6) | 0.8 | (0.03) |
| Extractable | 0.3 | (0.1) | 0.2 | (0.02) | 0.7 | (0.1) | 0.3 | (0.02) | |
| % | 21.4 | (3.3) | 27.4 | (3.1) | 37.0 | (10.8) | 32.9 | (1.9) | |
| Na | Total | 0.1 | (0.01) | 0.1 | (0.01) | 0.2 | (0.03) | 0.1 | (0.01) |
| Extractable | 0.1 | (0.01) | 0.1 | (0.01) | 0.2 | (0.1) | 0.1 | (0.01) | |
| % | 55.4 | (6.4) | 60.4 | (6.6) | 93.6 | (20.3) | 82.8 | (11.1) | |
| K | Total | 0.5 | (0.1) | 0.3 | (0.03) | 0.4 | (0.1) | 0.2 | (0.03) |
| Extractable | 0.2 | (0.02) | 0.1 | (0.02) | 0.1 | (0.02) | 0.1 | (0.02) | |
| % | 51.8 | (10.4) | 56.1 | (15.4) | 80.4 | (19.7) | 76.0 | (11.4) | |
| P | Total | 0.3 | (0.02) | 0.3 | (0.02) | 0.3 | (0.02) | 0.4 | (0.01) |
| Extractable | 0.04 | (0.02) | 0.1 | (0.02) | 0.02 | (0.02) | 0.03 | (0.01) | |
| % | 11.8 | (1.9) | 19.0 | (5.2) | 7.9 | (1.1) | 6.8 | (1.1) | |
| Soil Nutrient | p-Values | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| S | SP | DS | P | S * SP | S * DS | SP * DS | SP * P | DS * P | |
| Al | <0.001 | <0.001 | 0.03 | 0.78 | <0.001 | 0.12 | 0.01 | 0.25 | 0.26 |
| Fe | <0.001 | <0.001 | 0.13 | 0.08 | 0.001 | 0.13 | 0.18 | 0.09 | 0.16 |
| Mn | <0.001 | 0.001 | 0.43 | 0.55 | 0.01 | 0.39 | 0.02 | 0.98 | 0.27 |
| Ca | <0.001 | <0.001 | <0.001 | 0.85 | 0.03 | 0.44 | 0.06 | 0.69 | 0.02 |
| Mg | <0.001 | <0.001 | <0.001 | <0.001 | 0.76 | 0.85 | 0.19 | 0.19 | 0.28 |
| K | 0.02 | 0.98 | 0.84 | 0.78 | 0.93 | 0.97 | 0.57 | 0.99 | 0.13 |
| Total N | 0.18 | 0.05 | <0.001 | 0.002 | 0.08 | 0.04 | 0.85 | 0.20 | 0.10 |
| NO3− | 0.43 | 0.002 | <0.001 | 0.002 | 0.29 | 0.01 | 0.76 | 0.09 | 0.07 |
| NH4+ | 0.04 | 0.006 | 0.37 | 0.24 | 0.007 | 0.54 | 0.78 | 0.38 | 0.80 |
| S | <0.001 | 0.09 | 0.99 | 0.34 | 0.13 | 0.51 | 0.02 | 0.02 | 0.04 |
| Cu | <0.001 | 0.68 | 0.002 | 0.50 | 0.43 | 0.07 | 0.46 | 0.39 | 0.46 |
| Zn | <0.001 | 0.05 | <0.001 | 0.30 | 0.01 | 0.75 | <0.001 | 0.58 | 0.34 |
| B | 0.39 | <0.001 | 0.001 | 0.49 | 0.02 | 0.44 | 0.30 | 0.42 | 0.78 |
| Pb | 0.001 | 0.67 | 0.96 | 0.23 | 0.51 | 0.77 | 0.05 | 0.42 | 0.21 |
| Root Length Category | p-Values | ||||||
|---|---|---|---|---|---|---|---|
| S | SP | DS | P | S * SP | S * DS | SP * DS | |
| Live | |||||||
| Coarse | <0.001 | 0.73 | <0.001 | 0.02 | 0.49 | 0.04 | 0.09 |
| Fine | <0.001 | 0.32 | <0.001 | <0.001 | 0.03 | 0.37 | 0.92 |
| Total | <0.001 | 0.56 | <0.001 | <0.001 | 0.05 | 0.25 | 0.58 |
| Dead | |||||||
| Coarse | 0.20 | 0.05 | <0.001 | 0.01 | 0.82 | 0.21 | <0.001 |
| Fine | 0.02 | 0.04 | <0.001 | 0.01 | 0.009 | 0.78 | 0.02 |
| Total | 0.03 | 0.06 | <0.001 | 0.01 | 0.01 | 0.68 | 0.007 |
| Total coarse | 0.06 | 0.47 | <0.001 | <0.001 | 0.44 | 0.42 | <0.001 |
| Total fine | 0.009 | 0.05 | <0.001 | <0.001 | 0.006 | 0.83 | 0.07 |
| Total | <0.001 | 0.11 | <0.001 | <0.001 | 0.01 | 0.71 | 0.02 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zalamea, M.; González, G.; Lodge, D.J. Physical, Chemical, and Biological Properties of Soil under Decaying Wood in a Tropical Wet Forest in Puerto Rico. Forests 2016, 7, 168. https://doi.org/10.3390/f7080168
Zalamea M, González G, Lodge DJ. Physical, Chemical, and Biological Properties of Soil under Decaying Wood in a Tropical Wet Forest in Puerto Rico. Forests. 2016; 7(8):168. https://doi.org/10.3390/f7080168
Chicago/Turabian StyleZalamea, Marcela, Grizelle González, and Deborah Jean Lodge. 2016. "Physical, Chemical, and Biological Properties of Soil under Decaying Wood in a Tropical Wet Forest in Puerto Rico" Forests 7, no. 8: 168. https://doi.org/10.3390/f7080168