Delphinella Shoot Blight on Abies lasiocarpa Provenances in Norway
Abstract
:1. Introduction
2. Materials and Methods
2.1. Isolation and Identification of D. abietis
2.2. Inoculation Test
2.3. Seed Tests
2.4. Provenance Trial
2.4.1. Study Sites, Experimental Design and Provenaces
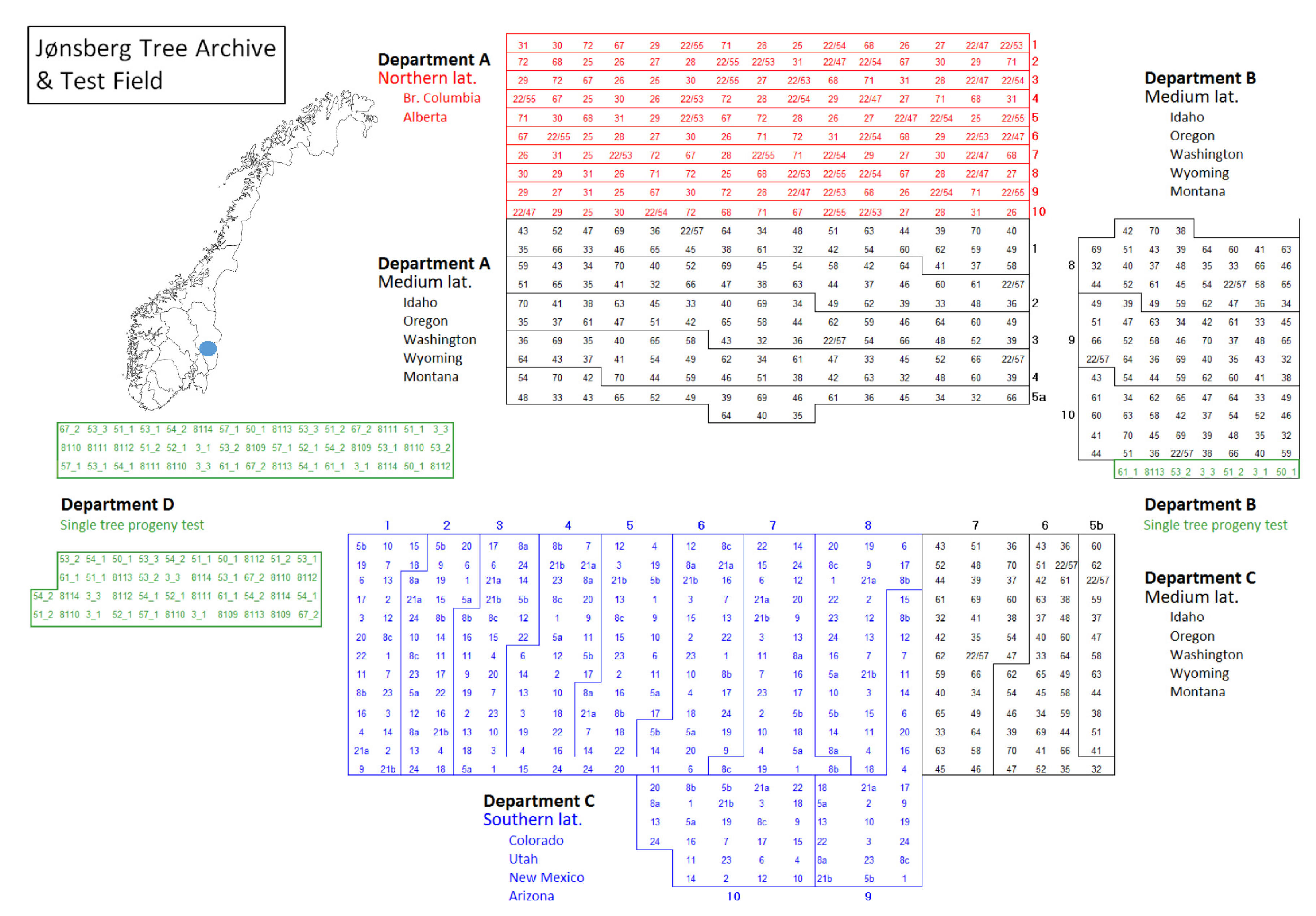
2.4.2. Rating of Needle Color and Disease Symptoms in the Provenance Trial at Jønsberg

2.4.3. Statistics Used to Analyse Field Data
2.5. Studies of Cuticular Thickness of Needles
3. Results and Discussion
3.1. Disease Symptoms Observed in 2008–2015


3.2. Damage by D. abietis at Jønsberg May Be Related to Increased Precipitation

3.3. D. abietis Was Readily Isolated from Ascospore Dispersal

3.4. Fulfilment of Koch’s Postulates for D. abietis

3.5. D. abietis May Be Seedborne
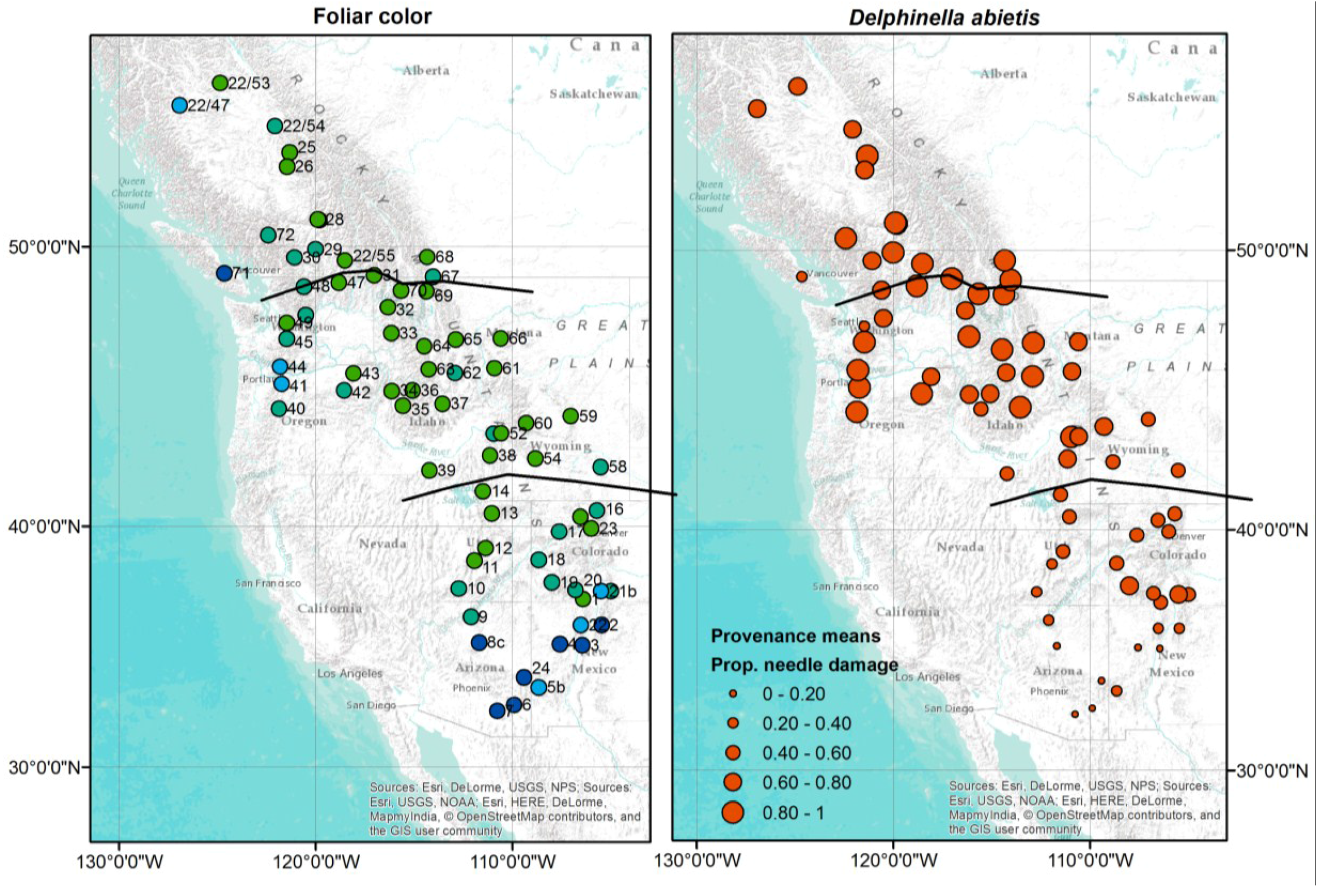
3.6. Some Provenances of Subalpine Fir Show Stronger Resistance against D. abietis
| Color | ||||||
|---|---|---|---|---|---|---|
| Region | Effect | Estimate | Num df | Den df | F or Z Value | p Value |
| South | Prov. | 27 | 235 | 20.61 | <0.0001 | |
| RP | 0.147(0.019) | 7.74 | <0.0001 | |||
| Error | 0.289(0.012) | 25.11 | <0.0001 | |||
| Middle | Prov. | 32 | 287 | 7.21 | <0.0001 | |
| RP | 0.150(0.024) | 6.34 | <0.0001 | |||
| Error | 0.463(0.022) | 21.38 | <0.0001 | |||
| North | Prov. | 14 | 126 | 11.78 | <0.0001 | |
| RP | 0.099(0.031) | 3.14 | 0.0008 | |||
| Error | 0.413(0.034) | 12.12 | <0.0001 | |||
| Needle damage | ||||||
| Region | Effect | Estimate | Num df | Den df | F or Z value | p value |
| South | X1 (Color) | 0.06(0.01) | 1 | 1261 | 24.16 | <0.0001 |
| Prov. | 27 | 235 | 6.48 | <0.0001 | ||
| Repl. | 0.003(0.002) | 1.59 | 0.056 | |||
| RP | 0.011(0.002) | 4.29 | <0.0001 | |||
| Error | 0.082(0.003) | 25.08 | <0.0001 | |||
| Middle | X1 (Color) | −0.038(0.01) | 1 | 917 | 11.46 | 0.0007 |
| Prov. | 32 | 287 | 4.49 | <0.0001 | ||
| Repl. | 0.006(0.003) | 1.85 | 0.032 | |||
| RP | 0.02(0.004) | 5.97 | <0.0001 | |||
| Error | 0.072(0.003) | 21.4 | <0.0001 | |||
| North | X1 (Color) | 0.057(0.02) | 1 | 293 | 8.30 | 0.0043 |
| Prov. | 14 | 126 | 3.07 | 0.0004 | ||
| Repl. | 0.001(0.002) | 0.61 | 0.27 | |||
| RP | 0.022(0.006) | 3.76 | <0.0001 | |||
| Error | 0.066(0.005) | 12.16 | <0.0001 | |||
3.7. Blue Needles on Subalpine Fir Has a Thicker Cuticle than Green Needles


4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Appendix
| R | P (No. and Name) | State | Long. | Lat. | Alt. | Color * | D. abietis ** |
|---|---|---|---|---|---|---|---|
| 1 | 1 Spruce Hole | Colorado | −106.417 | 37.1 | 3150 | 3.02(0.13) | 0.54(0.05) |
| 1 | 10 Duck Lake | Utah | −112.733 | 37.53333 | 2770 | 2.38(0.12) | 0.3(0.04) |
| 1 | 11 Big Lake | Utah | −111.95 | 38.65 | 2870 | 3.14(0.13) | 0.33(0.05) |
| 1 | 12 Willow Lake | Utah | −111.367 | 39.13333 | 2900 | 3.62(0.13) | 0.5(0.04) |
| 1 | 13 South Fork | Utah | −111.05 | 40.5 | 2750 | 3.63(0.13) | 0.46(0.05) |
| 1 | 14 Monte Cristo | Utah | −111.5 | 41.35 | 2700 | 3.37(0.14) | 0.49(0.05) |
| 1 | 15 Rabbit Ears Pass | Colorado | −106.55 | 40.36667 | 2900 | 3.41(0.13) | 0.57(0.04) |
| 1 | 16 Crown Point (Fort Collins) | Colorado | −105.7 | 40.61667 | 3150 | 2.82(0.13) | 0.52(0.05) |
| 1 | 17 Seaman Park | Colorado | −107.633 | 39.78333 | 2750 | 2.75(0.12) | 0.42(0.04) |
| 1 | 18 Divide Fork | Colorado | −108.667 | 38.68333 | 2700 | 2.57(0.13) | 0.51(0.04) |
| 1 | 19 The Meadows | Colorado | −108.017 | 37.78333 | 3100 | 2.92(0.14) | 0.72(0.05) |
| 1 | 2 Alamitos Creek (Gravel Pit) | New Mexico | −105.467 | 36.05 | 2900 | 1.41(0.13) | 0.21(0.04) |
| 1 | 20 Wolf Creek Pass | Colorado | −106.783 | 37.46667 | 2900 | 2.66(0.12) | 0.49(0.04) |
| 1 | 21a Apishapa River (Cordova Pass) | Colorado | −105 | 37.41667 | 3000 | 2.49(0.14) | 0.52(0.05) |
| 1 | 21b Apishapa River (Cordova Pass) | Colorado | −105.5 | 37.41667 | 3250 | 2.21(0.15) | 0.65(0.05) |
| 1 | 22 Cerro Pavo | New Mexico | −106.533 | 36.05 | 2900 | 1.94(0.12) | 0.39(0.04) |
| 1 | 23 Church Park | Colorado | −106 | 39.91667 | 2925 | 3.25(0.15) | 0.5(0.06) |
| 1 | 24 Big Lake | Arizona | −109.417 | 33.86667 | 2850 | 1.59(0.14) | 0.14(0.05) |
| 1 | 3 Sandia Crest | New Mexico | −106.45 | 35.21667 | 3100 | 1.67(0.12) | 0.19(0.04) |
| 1 | 4 La Mosca (Mount Taylor) | New Mexico | −107.583 | 35.26667 | 3200 | 1.08(0.12) | 0.08(0.04) |
| 1 | 5a Bearwallow Mtn. | New Mexico | −108.667 | 33.45 | 2950 | 1.79(0.13) | 0.12(0.05) |
| 1 | 5b Sør for Bearwallow Mtn. | New Mexico | −108.667 | 33.45 | 2950 | 1.74(0.13) | 0.21(0.05) |
| 1 | 6 Old Columbine/Mt. Graham | Arizona | −109.9 | 32.7 | 3000 | 1.34(0.13) | 0.15(0.05) |
| 1 | 7 Mount Lemon | Arizona | −110.767 | 32.43333 | 2750 | 1.68(0.13) | 0.19(0.05) |
| 1 | 8a Agassiz Peak | Arizona | −111.7 | 35.33333 | 3000 | 1.45(0.12) | 0.19(0.04) |
| 1 | 8b Agassiz Peak | Arizona | −111.7 | 35.33333 | 3000 | 1.49(0.13) | 0.18(0.04) |
| 1 | 8c Agassiz Peak | Arizona | −111.7 | 35.33333 | 3000 | 1.34(0.12) | 0.15(0.04) |
| 1 | 9 De Motte | Arizona | −112.117 | 36.38333 | 2650 | 2.4(0.13) | 0.24(0.05) |
| 2 | 22/57 Biri Frøplantasje | Norge | 10.6 | 60.95 | 190 | 2.51(0.13) | 0.85(0.05) |
| 2 | 32 Spruce Mtn. | Idaho | −116.333 | 47.98333 | 1500 | 3.61(0.14) | 0.65(0.06) |
| 2 | 33 Davies Pass | Idaho | −116.167 | 47.1 | 1250 | 3.13(0.13) | 0.8(0.05) |
| 2 | 34 Goose Lake | Idaho | −116.15 | 45.05 | 1950 | 3.16(0.14) | 0.77(0.06) |
| 2 | 35 Deadwood Summit | Idaho | −115.567 | 44.53333 | 2050 | 3.16(0.13) | 0.56(0.05) |
| 2 | 36 Williams Creek Summit | Idaho | −115.083 | 45.08333 | 2050 | 3.12(0.14) | 0.68(0.06) |
| 2 | 37 Big Eightmile | Idaho | −113.567 | 44.6 | 2250 | 3.13(0.14) | 0.82(0.05) |
| 2 | 38 Webster Ridge | Idaho | −111.15 | 42.7 | 2400 | 3.47(0.14) | 0.74(0.06) |
| 2 | 39 Monument Peak | Idaho | −114.233 | 42.13333 | 2400 | 3.29(0.14) | 0.49(0.06) |
| 2 | 40 Santiam Pass | Oregon | −121.867 | 44.41667 | 1500 | 2.45(0.14) | 0.87(0.06) |
| 2 | 41 Still Creek | Oregon | −121.733 | 45.31667 | 1450 | 2.33(0.14) | 0.86(0.06) |
| 2 | 42 Tower Mt. | Oregon | −118.567 | 45.08333 | 1650 | 2.98(0.14) | 0.91(0.06) |
| 2 | 43 Horseshoe Prairie. | Oregon | −118.083 | 45.68333 | 1450 | 3.37(0.14) | 0.79(0.06) |
| 2 | 44 Red Mtn. | Washington | −121.817 | 45.91667 | 1450 | 2.2(0.13) | 0.95(0.05) |
| 2 | 45 Chinook Pass | Washington | −121.483 | 46.9 | 1500 | 2.44(0.13) | 0.8(0.05) |
| 2 | 46 Sugarloaf Peak | Washington | −120.517 | 47.71667 | 1550 | 2.88(0.14) | 0.69(0.06) |
| 2 | 47 Handsqrabble Mtn. | Washington | −118.817 | 48.81667 | 1500 | 3.35(0.14) | 0.87(0.05) |
| 2 | 48 Rattlesnake Creek | Washington | −120.617 | 48.68333 | 1550 | 2.79(0.14) | 0.8(0.06) |
| 2 | 49 Snoqualmie Pass | Washington | −121.483 | 47.45 | 1200 | 3.04(0.13) | 0.37(0.05) |
| 2 | 51 Teton Pass | Wyoming | −110.95 | 43.5 | 2600 | 2.52(0.14) | 0.84(0.06) |
| 2 | 52 Sheep Creek | Wyoming | −110.583 | 43.51667 | 2580 | 3.36(0.14) | 0.62(0.06) |
| 2 | 54 Louis Lake | Wyoming | −108.85 | 42.58333 | 2650 | 3.4(0.14) | 0.51(0.06) |
| 2 | 58 Eagle Peak | Wyoming | −105.517 | 42.26667 | 2350 | 2.77(0.14) | 0.5(0.06) |
| 2 | 59 Munkres Pass | Wyoming | −107.033 | 44.15 | 2850 | 3.53(0.15) | 0.58(0.06) |
| 2 | 60 Wood River | Wyoming | −109.3 | 43.88333 | 2500 | 3.21(0.14) | 0.61(0.06) |
| 2 | 61 Middle Fork, Bridger | Montana | −110.917 | 45.86667 | 2000 | 3.36(0.14) | 0.75(0.06) |
| 2 | 62 Quartz Hill | Montana | −112.933 | 45.7 | 2550 | 2.95(0.13) | 0.86(0.05) |
| 2 | 63 N.Trapper Peak | Montana | −114.267 | 45.83333 | 2000 | 3.21(0.13) | 0.63(0.05) |
| 2 | 64 Lost Park | Montana | −114.483 | 46.65 | 1750 | 3.33(0.14) | 0.91(0.06) |
| 2 | 65 Marcum Mtn. | Montana | −112.9 | 46.88333 | 1500 | 3.5(0.14) | 0.8(0.06) |
| 2 | 66 Jeffersons Creek | Montana | −110.6 | 46.91667 | 2200 | 3.06(0.13) | 0.71(0.05) |
| 2 | 69 Whitefish Range | Montana | −114.383 | 48.53333 | 1800 | 3.35(0.13) | 0.84(0.05) |
| 2 | 70 Quartz Mtn. | Montana | −115.667 | 48.55 | 1750 | 3.38(0.14) | 0.8(0.06) |
| 3 | 22/47 Grizzly Lake | Br.Columbia | −126.917 | 54.4 | 1000 | 2.3(0.14) | 0.74(0.06) |
| 3 | 22/53 Inzana Lake | Br.Columbia | −124.867 | 55.05 | 1100 | 3(0.14) | 0.8(0.06) |
| 3 | 22/54 Spring Mt. | Br.Columbia | −122.083 | 53.78333 | 1220 | 2.97(0.14) | 0.67(0.06) |
| 3 | 22/55 Blue Joint | Br.Columbia | −118.517 | 49.55 | 1850 | 3.12(0.14) | 0.93(0.06) |
| 3 | 25 Cunningham Creek | Br.Columbia | −121.333 | 52.98333 | 1300 | 3.23(0.14) | 0.9(0.06) |
| 3 | 26 Cedar Creek | Br.Columbia | −121.467 | 52.55 | 1150 | 3.12(0.14) | 0.69(0.06) |
| 3 | 27 McGillvray Lake | Br.Columbia | −119.833 | 50.86667 | 1400 | 3.29(0.14) | 0.84(0.06) |
| 3 | 28 Sun Peaks | Br.Columbia | −119.917 | 50.88333 | 1200 | 3.37(0.14) | 0.9(0.06) |
| 3 | 29 Pennask Mtn. | Br.Columbia | −120.017 | 49.91667 | 1700 | 2.85(0.14) | 0.81(0.06) |
| 3 | 30 Upper Coldwater | Br.Columbia | −121.083 | 49.65 | 1200 | 2.79(0.14) | 0.78(0.06) |
| 3 | 31 Kootenai Pass | Br.Columbia | −117.033 | 49.06667 | 1650 | 3.19(0.14) | 0.92(0.06) |
| 3 | 67 Summit Lake | Alberta | −114.033 | 49.01667 | 1900 | 2.97(0.14) | 0.81(0.06) |
| 3 | 68 N. Racehorse Creek | Alberta | −114.333 | 49.66667 | 1700 | 3.09(0.14) | 0.86(0.06) |
| 3 | 71 Grassie Mtn. | Br.Columbia | −124.667 | 49.13333 | 1150 | 1.53(0.14) | 0.34(0.06) |
| 3 | 72 Duffy Lake | Br.Columbia | −122.417 | 50.38333 | 1500 | 2.42(0.14) | 0.86(0.06) |
References
- Solheim, H.; Skage, J.O. Losses caused by the needle blight fungus Delphinella abietis in a greenery trial of Noble fir in western Norway. For. Pathol. 2002, 32, 373–377. [Google Scholar] [CrossRef]
- Talgø, V.; Stensvand, A. The fungi Delphinella abietis and Herpotrichia parasitica cause severe foliage damage on fir (Abies spp.) in Christmas tree plantations in western Norway. In Proceedings of the 8th International Christmas Tree Research & Extension Conference, Bogense, Denmark, 13–17 August 2007; Thomsen, I.M., Rasmusen, H.N., Sørensen, J.M., Eds.; Working Paper. Forest & Landscape Denmark, University of Copenhagen: Copenhagen, Denmark, 2008; Volume 26, pp. 49–54. [Google Scholar]
- Strande, J.-A. Producing Christmas trees in “the land of the midnight sun”. In the 12th International Christmas Tree Research and Extension Conference, Honne, Norway, 6–11 September 2015; Talgø, V., Fløistad, I.S., Eds.; NIBIO: Ås, Norway, 2015; Volume 1, p. 16. [Google Scholar]
- Talgø, V. Norwegian Institute of Bioeconomy Research (NIBIO), Ås, Norway. Unpublished work. 2006. [Google Scholar]
- Rostrup, E. Plantepatologi Haandbog i Læren om Plantesygdomme for Landbrugere, Havebrugere og Skovbrugere; Det Nordiske Forlag: Copenhagen, Denmark, 1902; p. 579. (In Danish) [Google Scholar]
- Jørstad, I. Melding om soppsykdommer på skogtrærne i årene 1931-1935. In Særtrykk av Beretning om det Norske Skogvesen for 1935, Avgitt av Skogdirektøren; Tandberg & Jensens bogtrykkeri: Oslo, Norway, 1936; pp. 83–100. (In Norwegian) [Google Scholar]
- Solheim, H. Edelgranskuddsoppen, en trussel for edelgrandyrking? Nor. Pyntegrønt 1999, 6, 14–16. (In Norwegian) [Google Scholar]
- Cech, T.; (the Federal Research and Training Centre for Forests, Vienna, Austria). Personal communication, 2006.
- Cannon, P. Delphinella Abietis; Fungi of Great Britain and Ireland: Kew, London, UK, 2011; Available online: http://fungi.myspecies.info/taxonomy/term/4775/descriptions (accessed on 9 December 2015).
- Funk, A. Foliar Fungi of Western Trees; NOR-X-286; Canadian Forestry Service: Victoria, BC, Canada, 1985; p. 142. [Google Scholar]
- Merrill, W; Wenner, N.G.; Kelly, R. Delphinella balsameae Tip Blight of Abies lasiocarpa in Vermont. Plant Dis. 1997, 81, 229. [Google Scholar] [CrossRef]
- Chastagner, G.A.; Riley, K.; Eikemo, H.; Talgø, V. Delphinella shoot blight and Grovesiella canker on Abies lasiocarpa in western USA. In Proceedings of the 12th International Christmas Tree Research and Extension Conference, Honne, Norway, 6–11 September 2015; Talgø, V., Fløistad, I.S., Eds.; NIBIO: Ås, Norway, 2015; Volume 1, p. 57. [Google Scholar]
- Talgø, V.; Børja, I.; Fløistad, I.S.; Stensvand, A. Omfattande skade av edelgranskotsjuke i 2011 (Extensive damage by Delphinella shoot blight in 2011). Nåledrys 2012, 80, 26–27. (In Norwegian) [Google Scholar]
- Talgø, V. Diseases and Disorders on fir (Abies spp.) Grown as Christmas Trees, Boughs, and Landscape Plants in Norway; from Seed to Site. Ph.D. Thesis, Norwegian University of Life Science, Ås, Norway, 2009; p. 174. [Google Scholar]
- White, T.J.; Bruns, T.D.; Lee, S.; Taylor, J. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In PCR Protocols: A Guide to Methods and Applications; Innis, M.A., Gelfand, D.H., Sninsky, J.J., White, T.J., Eds.; Academic Press: New York, NY, USA, 1990; pp. 315–322. [Google Scholar]
- Ellis, M.B.; Ellis, J.P. Microfungi on Land Plants. An Identification Handbook, Enlarged version; The Richmond Publishing Co. Ltd.: Slough, UK, 1997; p. 868. [Google Scholar]
- Talgø, V.; Brodal, G.; Klemsdal, S.S.; Stensvand, A. Seed borne fungi on Abies spp. Seed Sci. Technol. 2010, 38, 477–493. [Google Scholar] [CrossRef]
- Fløistad, I.S.; Nyeggen, H.; Skage, J.O. Field trial with Abies lasiocarpa progenies for Christmas tree production in Southern Norway. In Proceedings of the the 12th International Christmas Tree Research and Extension Conference, Honne, Norway, 6–11 September 2015; Talgø, V., Fløistad, I.S., Eds.; NIBIO: Ås, Norway, 2015; Volume 1, p. 20. [Google Scholar]
- Skage, J.O.; Nyeggen, H.; Østgård, Å. Utvikling av plantemateriale med fjelledelgran (Abies lasiocarpa) til produksjon av juletrær. Oppdragsrapp. Skog Landsk. 2012, 12, 13. (In Norwegian) [Google Scholar]
- SAS Institute Inc. SAS/STAT 9.4 User’s Guide; SAS Institute Inc.: Cary, NC, USA, 2012. [Google Scholar]
- Talgø, V.; Chastagner, G.A.; Thomsen, I.M.; Cech, T.; Riley, K.; Lange, K.; Klemsdal, S.S.; Stensvand, A. Sydowia polyspora associated with current season needle necrosis (CSNN) on true fir (Abies spp.). Fungal Biol. 2010, 114, 545–554. [Google Scholar] [CrossRef] [PubMed]
- Talgø, V.; Dobson, A.; Slørstad, T.; Brurberg, M.B.; Stensvand, A. Sclerophoma-skade på juletre (Sclerophoma shoot blight on Christmas trees). Nåledrys 2011, 75, 28–31. [Google Scholar]
- Talgø, V.; Brodal, G. Norwegian Institute of Bioeconomy Research (NIBIO). Ås, Norway. Unpublished work. 2009. [Google Scholar]
- Thomsen, I.M.; Nielsen, U.B.; Proschowsky, G.F.; Brodal, G.; Talgø, V. Frøsmitte med Neonectria og Sydowia (Seed borne inoculum of Sydowia and Neonectria). Nåledrys 2015, 91, 45–52. [Google Scholar]
- Yeats, T.H.; Rose, J.K.C. The formation and function of plant cuticles. Plant Physiol. 2013, 163, 5–20. [Google Scholar] [CrossRef] [PubMed]
- Talgø, V. Norwegian Institute of Bioeconomy Research (NIBIO). Ås, Norway. Unpublished work. 2013. [Google Scholar]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Talgø, V.; Skage, J.-O.; Steffenrem, A.; Junker, C.; Eikemo, H.; Brurberg, M.B.; Johnskås, O.R. Delphinella Shoot Blight on Abies lasiocarpa Provenances in Norway. Forests 2016, 7, 7. https://doi.org/10.3390/f7010007
Talgø V, Skage J-O, Steffenrem A, Junker C, Eikemo H, Brurberg MB, Johnskås OR. Delphinella Shoot Blight on Abies lasiocarpa Provenances in Norway. Forests. 2016; 7(1):7. https://doi.org/10.3390/f7010007
Chicago/Turabian StyleTalgø, Venche, Jan-Ole Skage, Arne Steffenrem, Corina Junker, Håvard Eikemo, May Bente Brurberg, and Odd Ragnar Johnskås. 2016. "Delphinella Shoot Blight on Abies lasiocarpa Provenances in Norway" Forests 7, no. 1: 7. https://doi.org/10.3390/f7010007





