Community Structure, Biodiversity, and Ecosystem Services in Treeline Whitebark Pine Communities: Potential Impacts from a Non-Native Pathogen
Abstract
:1. Introduction
| Keystone Interactions and Foundational Functions |
|---|
|
|
|
|
|
|
| Ecosystem services |
|
|
|
|
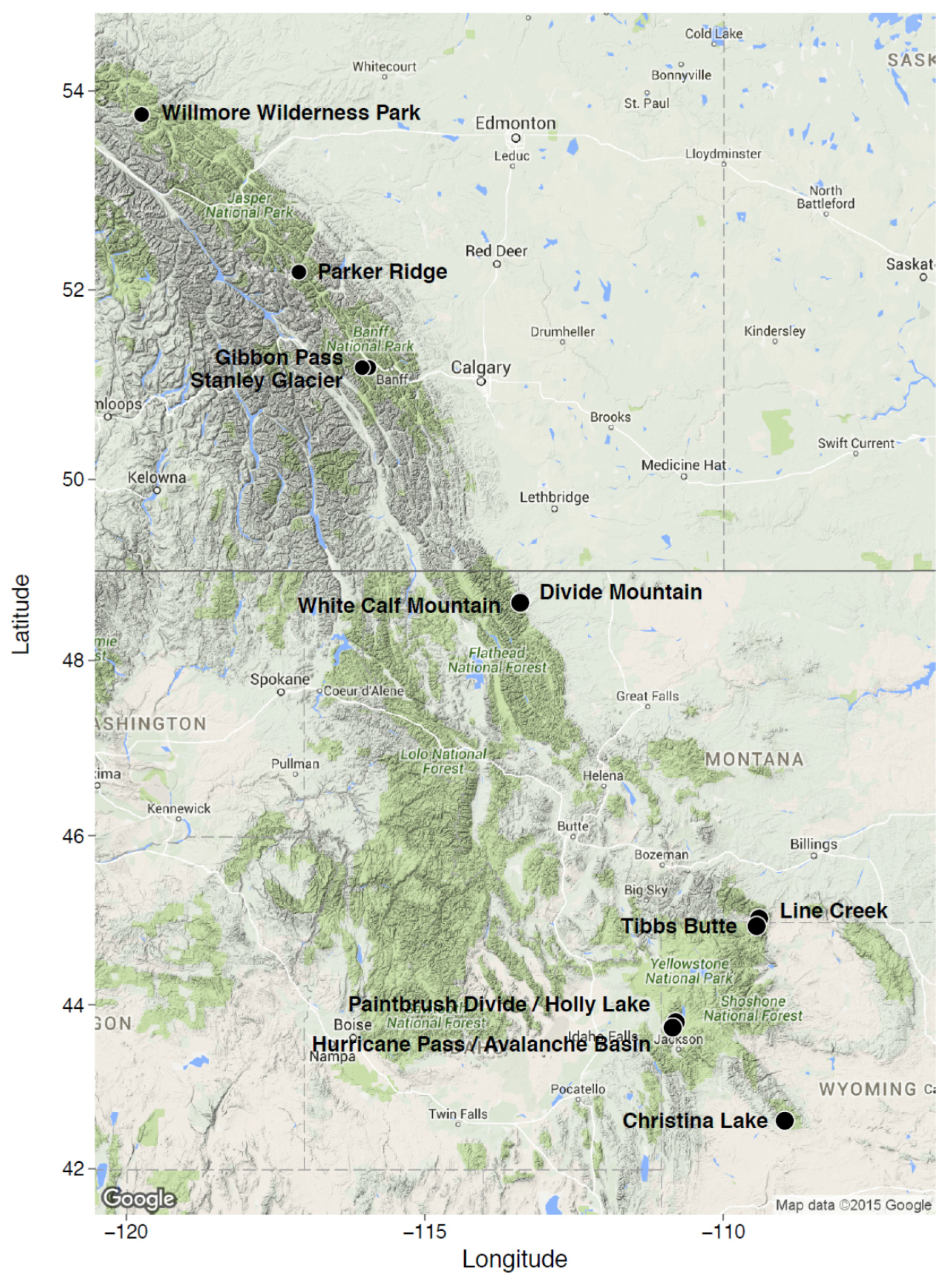
2. Study Areas and Methods
| Study Area | Latitude and Longitude | Elevation m | Aspect | Plot Size, N | Area Sampled; Distribution | Reference |
|---|---|---|---|---|---|---|
| Willmore Wilderness Park; Alberta, Canada | 53°46′0′′ N; 119°44′22′′ W | 1964–2175 | SW, SE, E, NE | 500 m2; N = 11 | 5500 m2; non-random | Tomback and Resler, in preparation [51] |
| Parker Ridge Banff National Park; Alberta, Canada | 52°10′44′′ N; 117°06′24′′ W | 2100 | NE, NW, S, SE, SW | 225 m2; N = 20 | 4500 m2; random | Resler et al. [52]; Resler et al., unpublished data [53] |
| Gibbon Pass; Banff National Park, Alberta, Canada | 51°11′15′′ N; 115°56′12′′ W | 2389–2430 | SE, NE | 500 m2; N = 3 | 1500 m2; non-random | Tomback et al. [45] |
| Stanley Glacier; Banff National Park, Alberta, Canada | 51°11′13′′ N; 116°02′51′′ W | 1969–1997 | SW, NW | 500 m2; N = 1; 250 m2; N = 1 | 750 m2; non-random | Tomback et al. [45] |
| Divide Mountain/White Calf Mountain; Glacier National Park, Blackfeet Indian Reservation, MT | 48°39′25′′ N 113°23′45′′ W; 48°38′20′′ N; 113°24′08′′ W | 1920–2272 | NE, NW, W, SW | 225 m2; N = 30 | 6750 m2; random | Smith-McKenna et al. [48] |
| Line Creek; Custer National Forest, MT | 45°01′47′′ N; 109°24′09′′ W | 2950 | NE | 225 m2; N = 30 | 6750 m2; random | Smith-McKenna et al. [48] |
| Tibbs Butte; Shoshone National Forest, WY | 44°56′28′′ N; 109°26′39.69′′ W | 2983–3238 | E, NE | 225 m2; N = 12 | 2700 m2; random | Wagner et al., in preparation [54] |
| Paintbrush Divide/Holly Lake; Grand Teton National Park, WY | 43°47′34′′ N; 110°47′54′′ W | 3055–3289 | NW, SW, NE | 225 m2; N = 20 | 4500 m2; random | Resler and Shao, unpublished data [55] |
| Hurricane Pass/Avalanche Basin; Grand Teton National Park, WY | 43°43′41′′ N; 110°51′02′′ W | 3045–3204 | NW, SW, NE | 225 m2; N = 20 | 4500 m2; random | Resler and Shao, unpublished data [55] |
| Christina Lake; Shoshone National Forest, WY | 42°35′35′′ N; 108°58′24′′ W | 3200–3400 | SW, SE, NE | 225 m2; N = 30 | 6,750 m2; random | Wagner et al., in preparation [54] |
3. Results
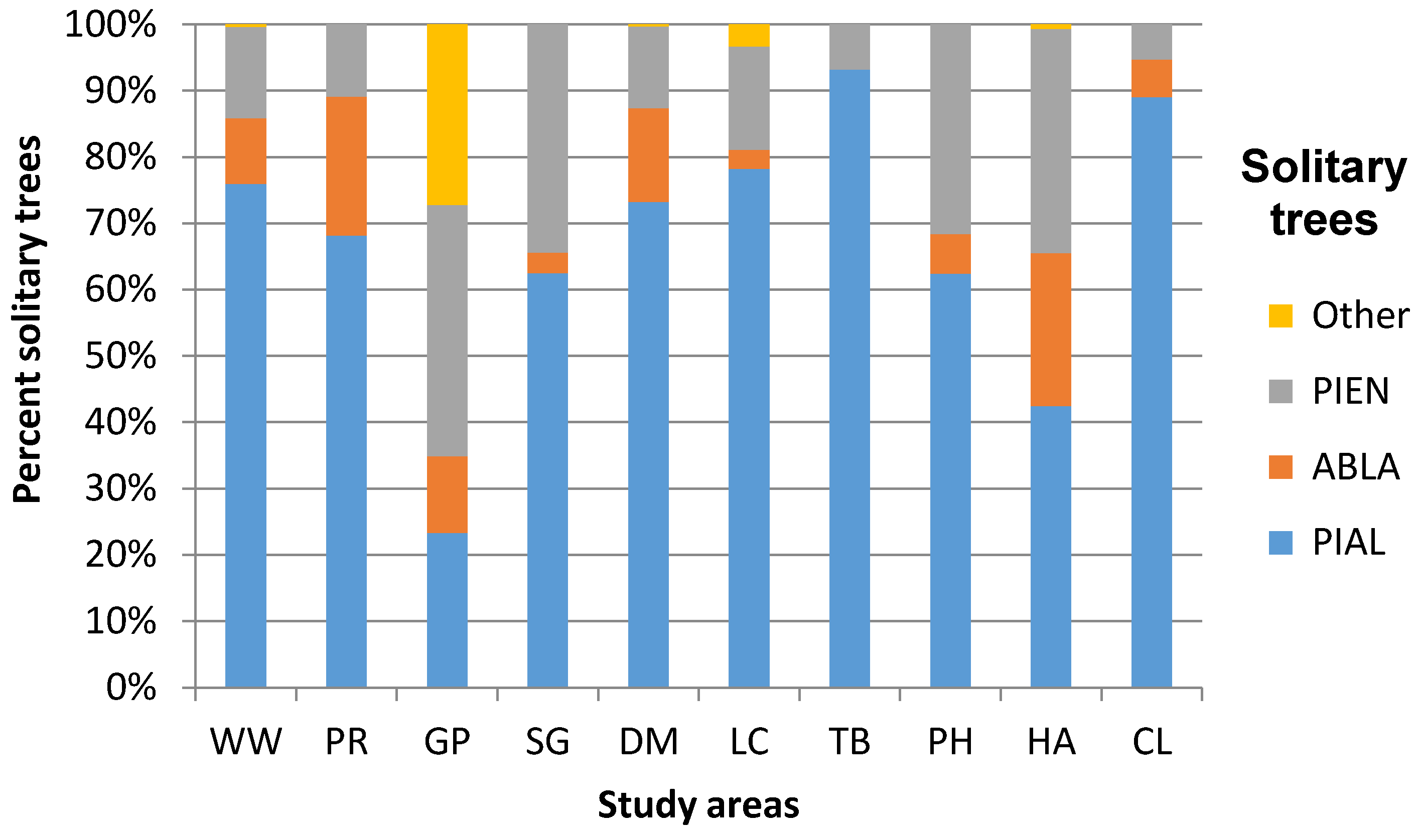
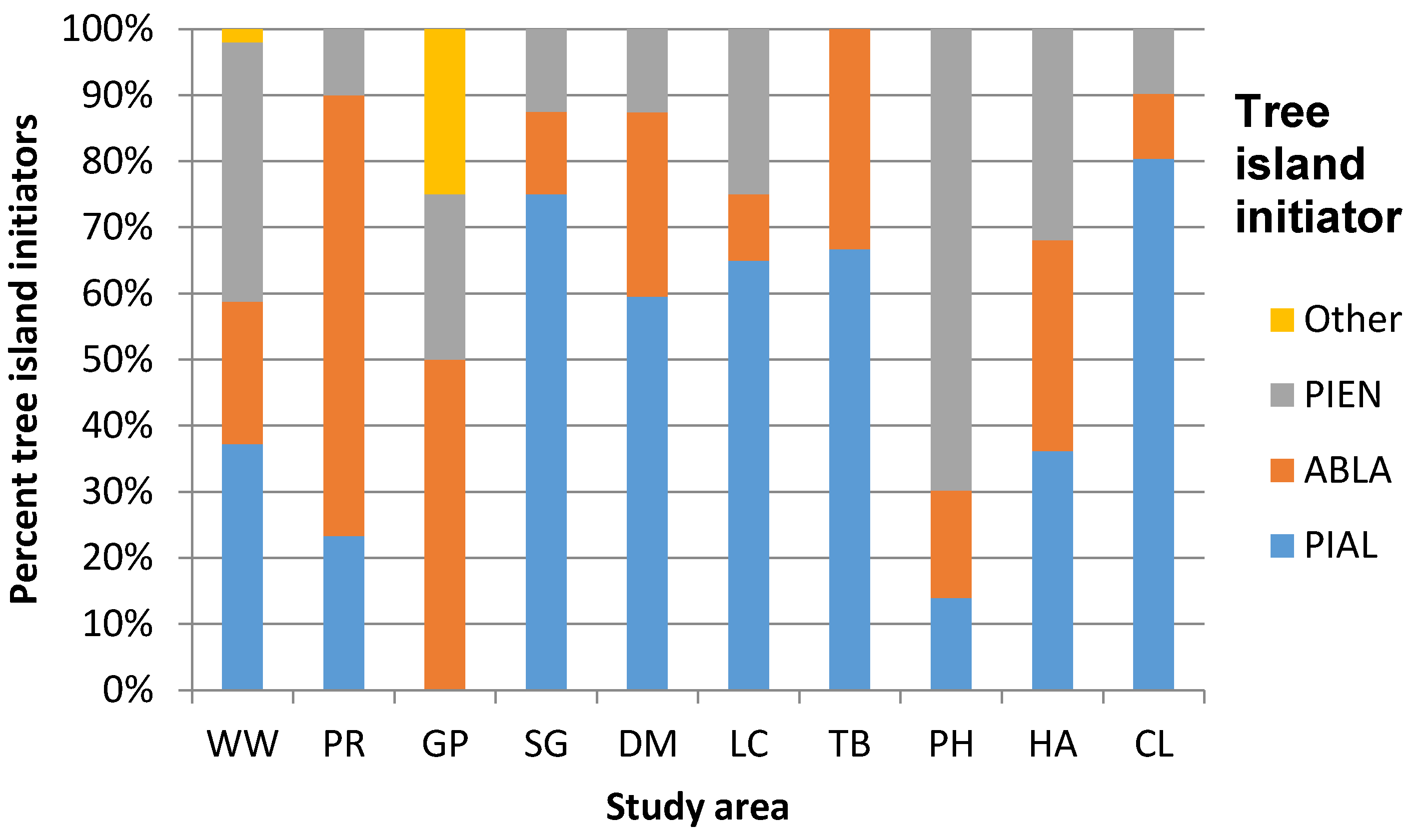
3.1. Geographic Variation in ATE Community Composition and Structure
| Location | Tree Species Richness S | Median, Min, Max, No. Solitary Trees/ha, total Solitary Trees n | Shannon Diversity Index for Solitary Trees | Median, Min, Max, Tree Islands/ha, Total Tree Islands n | Median, Min, Max Length m, Total Tree Islands n | Dominant Understory Species | |
|---|---|---|---|---|---|---|---|
| Solitary | Tree Islands | ||||||
| Willmore Wilderness Park | 4 PIAL ABLA PIEN PICO | 4 PIAL ABLA PIEN PICO | 780.00 140.00 2080.00 n = 485 | 0.724 | 80.00 20.00 200.00 n = 49 | 2.59 0.10 9.60 n = 48 | Dryas integrifolia Vahl Cassiope mertensiana (Bong.) G. Don Empetrum nigrum L. Phyllodoce glanduliflora (Hook.) Coville Rhododendron albiflorum Hook. Menziesia ferruginea Sm. Vaccinium membranaceum Douglas ex Torr. |
| Parker Ridge | 3 PIAL ABLA PIEN | 3 PIAL ABLA PIEN | 777.78 88.89 2400.00 n = 321 | 0.830 | 88.89 0 222.22 n = 49 | 2.70 0.04 19.90 n = 49 | Dryas octopetala L. Silene acaulis (L.) Jacq. |
| Gibbon Pass | 4 PIAL ABLA PIEN LALY | 4 PIAL ABLA PIEN LALY | 160.00 120.00 1760.00 n = 103 | 1.312 | 80.00 80.00 100.00 n = 12 | 4.35 0.65 11.60 n = 12 | Dryas octopetala L. Silene acaulis (L.) Jacq. |
| Stanley Glacier | 3 PIAL ABLA PIEN | 3 PIAL ABLA PIEN | 720.00 240.00 1200.00 n = 32 | 0.769 | 130.00 60.00 200.00 n = 8 | 7.34* 1.00 >35.00 n = 8 | Dryas octopetala L. Silene acaulis (L.) Jacq. |
| Divide Mountain | 4 PIAL ABLA PIEN PSME | 4 PIAL ABLA PIEN PSME | 977.78 88.89 3911.11 n = 367 | 0.778 | 111.00 0.00 355.55 n = 84 | 5.40 0.40 >35.00 n = 84 | Dryas octopetala L. Arctostaphylos uva-ursi (L.) Spreng. Hedysarum sulphurescens Rydb. Oxytropis sericea Nutt. Silene acaulis (L.) Jacq. |
| White Calf Mountain | Arctostaphylos uva-ursi (L.) Spreng. Achillea millefolium L. Hedysarum sulphurescens Rydb. Potentilla diversifolia Lehm. | ||||||
| Line Creek | 5 PIAL ABLA PIEN PSME PICO | 5 PIAL ABLA PIEN PSME PICO | 533.33 88.89 1733.33 n = 244 | 0.695 | 88.89 0 444.44 n = 62 | 4.50 0.76 16.88 n = 62 | Agoseris glauca (Pursh) Raf. Aster alpigenus (Torr. & A. Gray) A. Gray Geum rossii (R. Br.) Ser. Potentilla diversifolia Lehm. Lupinus argenteus Pursh |
| Tibbs Butte | 2 PIAL PIEN | 3 PIAL ABLA PIEN | 222.22 44.44 1422.22 n = 88 | 0.249 | 0.00 0.00 88.89 n = 3 | 7.68 0.76 14.07 n = 3 | Geum rossii (R. Br.) Ser. Potentilla diversifolia Lehm. Saxifrage spp. Graminoids |
| Paintbrush Divide/Holly Lake | 3 PIAL ABLA PIEN | 3 PIAL ABLA PIEN | 550.71 0.00 1497.93 n = 282 | 0.828 | 88.11 0.00 308.40 n = 45 | 3.35 0.11 >35.00 n = 45 | Arctostaphylos uva-ursi (L.) Spreng. Myosotis asiatica (Vesterg.) Schischkin & Sergievskaja Senecio spp. Silene acaulis (L.) Jacq. |
| Hurricane Pass/Avalanche Basin | 3 PIAL ABLA PIEN | 4 PIAL ABLA PIEN PSME | 242.31 0.00 793.02 n = 138 | 1.069 | 66.08 0.00 352.45 n = 45 | 7.30 0.98 >35.00 n = 45 | Dryas octopetala L. Senecio spp. Silene acaulis (L.) Jacq. Graminoids |
| Christina Lake | 3 PIAL ABLA PIEN | 3 PIAL ABLA PIEN | 244.44 0.00 2000.00 n = 247 | 0.421 | 0.00 0.00 444.44 n = 41 | 3.39 1.14 29.07 n = 41 | Geum rossii (R. Br.) Ser. Pteryxia hendersonii (J.M. Coult. & Rose) Mathias & Constance Phlox pulvinata (Wherry) Cronquist Silene acaulis (L.) Jacq. |

3.2. Geographic Variation in ATE Understory Community Composition
3.3. Snow Retention As an Ecosystem Service
| Topic | References |
|---|---|
| Algorithms and highway safety research indicate wind distributes snow leeward of objects. | [58,60,61,62] |
| Field measurements demonstrate increased snow accumulation, snow depth, and snow water equivalent leeward of trees. | [42,66,67,68,69,80] |
| Increased snow depth positively correlates with snow water equivalent (SWE). | [69,70] |
| Increased snow depth is associated with delayed runoff initiation and longer snow persistence. | [41,70,71,72] |
| Snow depth and snowpack persistence predict herbaceous vegetation community distribution. | [73,74,75,76] |
| Snow accumulation is positively associated with soil nutrient deposition. | [68,77,78] |
| Field based assessment of a whitebark pine ecosystem indicates vegetation influences snow accumulation and spatial distribution. | [66] |
| Summary of processes | |
| 1. Objects, such as trees and rocks, act as snowtraps, forcing snow redistribution in their lee. | |
| 2. The distribution of trees on the landscape positively influences snow depth. | |
| 3. An increase in snow depth directly increases downstream water yield. | |
| 4. Whitebark pine is abundant at treeline and initiates tree islands, creating snow traps, and thus influences local hydrological processes. | |


3.4. Blister Rust Incidence Regionally and Within Treeline Communities
| Location | Overall Infection Incidence; Mortality Notes | Reference |
|---|---|---|
| Subalpine: Greater Yellowstone Area | 20%–30% mortality indicated | [83] |
| Subalpine: Canadian Rocky Mountains | 52% 28% of trees, primarily BR | [81] |
| Treeline: east slope Glacier National Park: Divide Mountain and Lee Ridge | 33.7% multiple symptoms; 24.3% confirmed cankered; mortality in both study areas: 6 dead trees on 3 transects ; 6 dead trees on 4 transects; confirmed BR | [38] |
| Treeline: Willmore Wilderness Park | 1.1% (confirmed cankers) | Tomback and Resler, in preparation [51] |
| Treeline: Glacier National park, 6 study areas | 47% (0%–100% per plot) | [48] |
| Treeline: Divide Mountain; Line Creek | 23.6% (confirmed cankers); 19.2% (confirmed cankers) | [49] |
| Treeline: Gibbon Pass; | 0% | [45] |
| Stanley Glacier; | 16.2% (multiple symptoms); 10.8% (confirmed cankers); | |
| Tibbs Butte | 0% (on transects; presence off transects) | |
| Treeline: Paintbrush Divide/Holly Lake; Hurricane Pass/Avalanche Basin | 17.2%; 14.9% | Resler and Shao unpublished data [55] |
| Treeline: Tibbs Butte; Christina Lake | <10% blister rust in both areas | Wagner et al., in preparation [54] |
4. Discussion
4.1. Potential Impacts to ATE Whitebark Pine Communities from White Pine Blister Rust
4.2. Geographic Variation in ATE Whitebark Pine Communities
4.3. The role of Whitebark Pine Communities In Snow Retention
4.4. Management Implications
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Pan, Y.; Birdsey, R.A.; Phillips, O.L.; Jackson, R.B. The structure, distribution, and biomass of the world’s forests. Annu. Rev. Ecol. Syst. 2013, 44, 593–622. [Google Scholar] [CrossRef]
- Ricketts, T.H.; Dinerstein, E.; Olson, D.M.; Loucks, C.J.; Eichbaum, W.; DellaSala, D.; Kavanagh, K.; Hedao, P.; Hurley, P.T.; Carney, K.M.; et al. Terrestrial Ecoregions of North America: A Conservation Assessment; Island Press: Washington, WA, USA; Covelo, CA, USA, 1999. [Google Scholar]
- Habeck, J.R.; Mutch, R.W. Fire-dependent forests in the northern Rocky Mountains. Quat. Res. 1973, 3, 408–424. [Google Scholar] [CrossRef]
- Romme, W.H.; Despain, D.G. The long history of fire in the Greater Yellowstone Ecosystem. West. Wildlands 1989, 15, 10–17. [Google Scholar]
- Fischer, W.C.; Clayton, B.D. Fire Ecology of Montana Forest Habitat types East of the Continental Divide; General Technical Report INT-141; USDA Forest Service Intermountain Forest and Range Experiment Station: Ogden, UT, USA, 1983. [Google Scholar]
- Turner, M.G.; Hargrove, W.W.; Gardner, R.H.; Romme, W.H. Effects of fire on landscape heterogeneity in Yellowstone National Park, Wyoming. J. Veg. Sci. 1994, 5, 731–742. [Google Scholar] [CrossRef]
- Noss, R.F. Indicators for monitoring biodiversity: A hierarchical approach. Conserv. Biol. 1990, 4, 355–364. [Google Scholar] [CrossRef]
- Pederson, G.T.; Graumlich, L.J.; Fagre, D.B.; Kipfer, T.; Muhlfeld, C.C. A century of climate and ecosystem change in Western Montana: What do temperature trends portend? Clim. Chang. 2011, 98, 133–154. [Google Scholar] [CrossRef]
- Westerling, A.L.; Hidalgo, H.G.; Cayan, D.R.; Swetnam, T.W. Warming and earlier spring increase western U.S. forest wildfire activity. Science 2006, 313, 940–943. [Google Scholar] [CrossRef] [PubMed]
- Van Mantgem, P.J.; Stephenson, N.L.; Byrne, J.C.; Daniels, L.D.; Franklin, J.F.; Fulé, P.Z.; Harmon, M.E.; Larson, A.J.; Smith, J.M.; Taylor, A.H.; et al. Widespread increase of tree mortality rates in the western United States. Science 2009, 323, 521–524. [Google Scholar] [CrossRef] [PubMed]
- Raffa, K.F.; Aukema, B.H.; Bentz, B.J.; Carroll, A.L.; Hicke, J.A.; Turner, M.G.; Romme, W.H. Cross-scale drivers of natural disturbances prone to anthropogenic amplification: The dynamics of bark beetle eruptions. BioScience 2008, 58, 501–517. [Google Scholar] [CrossRef]
- Weed, A.S.; Ayres, M.P.; Hicke, J.A. Consequences of climate change for biotic disturbances in North American forests. Ecol. Monogr. 2013, 83, 441–470. [Google Scholar] [CrossRef]
- Roy, B.A.; Alexander, H.M.; Davidson, J.; Campbell, F.T.; Burdon, J.J.; Sniezko, R.; Brasier, C. Increasing forest loss worldwide from invasive pests requires new trade regulations. Front. Ecol. Environ. 2014, 12, 457–465. [Google Scholar] [CrossRef]
- Millar, C.I.; Stephenson, N.L. Temperate forest health in an era of emerging megadisturbance. Science 2015, 349, 823–826. [Google Scholar] [CrossRef] [PubMed]
- Tomback, D.F.; Achuff, P. Blister rust and western forest biodiversity: Ecology, values and outlook for white pines. For. Pathol. 2010, 40, 186–225. [Google Scholar] [CrossRef]
- Tomback, D.F.; Achuff, P.; Schoettle, A.W.; Schwandt, J.; Mastrogiuseppe, R.J. The Magnificent High-Elevation Five-Needle White Pines: Ecological Roles and Future Outlook. In Proceedings High-Five Symposium: The Future of High-Elevation Five-Needle White Pines in Western North America; Keane, R.E., Tomback, D.F., Murray, M.P., Smith, C.M., Eds.; USDA Forest Service, Rocky Mountain Research Station: Fort Collins, CO, USA, 2011; pp. 2–28. [Google Scholar]
- U.S. Fish and Wildlife Service. Endangered and threatened wildlife and plants; 12-month finding on a petition to list Pinus albicaulis as Endangered or Threatened with critical habitat. Fed. Regist. 2011, 76, 42631–42654. [Google Scholar]
- Government of Canada. Order amending Schedule 1 to the Species at Risk Act. Canada Gazette. 2012. Part II. Vol. 146. No. 14, SOR/2012–113. Available online: http://www.sararegistry.gc.ca/virtual_sara/files/orders/g2–14614i_e.pdf (accessed on 20 June 2012).
- Tomback, D.F.; Linhart, Y.B. The evolution of bird-dispersed pines. Evolut. Ecol. 1990, 4, 185–219. [Google Scholar] [CrossRef]
- Tomback, D.F. Clark’s Nutcracker: Agent of regeneration. In Whitebark Pine Communities: Ecology and Restoration; Tomback, D.F., Arno, S.F., Keane, R.E., Eds.; Island Press: Washington, DC, USA, 2001; pp. 89–104. [Google Scholar]
- Tomback, D.F. The impact of seed dispersal by Clark’s nutcracker on whitebark pine: Multi-Scale perspective on a high mountain mutualism. In Mountain Ecosystems: Studies in Treeline Ecology; Broll, G., Keplin, B., Eds.; Springer: Berlin, Germany, 2005; pp. 181–201. [Google Scholar]
- Tomback, D.F.; Anderies, A.J.; Carsey, K.S.; Powell, M.L.; Mellmann-Brown, S. Delayed seed germination in whitebark pine and regeneration patterns following the Yellowstone fires. Ecology 2001, 82, 2587–2600. [Google Scholar] [CrossRef]
- Gernandt, D.S.; Geada Lόpez, G.G.; Ortiz Garcia, S.; Liston, A. Phylogeny and classification of Pinus. Taxon 2005, 54, 29–42. [Google Scholar] [CrossRef]
- Arno, S.F.; Hoff, R.J. Pinus albicaulis Engelm. Whitebark pine. In Silvics of North America; Conifers; Burns, R.P., Honkala, B.H., Eds.; USDA Forest Service: Washington, DC, USA, 1990; Volume 1, pp. 268–279. [Google Scholar]
- McCune, B. Ecological diversity in North American pines. Am. J. Bot. 1988, 75, 353–368. [Google Scholar] [CrossRef]
- Arno, S.F. Community types and natural disturbance processes. In Whitebark Pine Communities: Ecology and Restoration; Tomback, D.F., Arno, S.F., Keane, R.E., Eds.; Island Press: Washington, WA, USA, 2001; pp. 74–88. [Google Scholar]
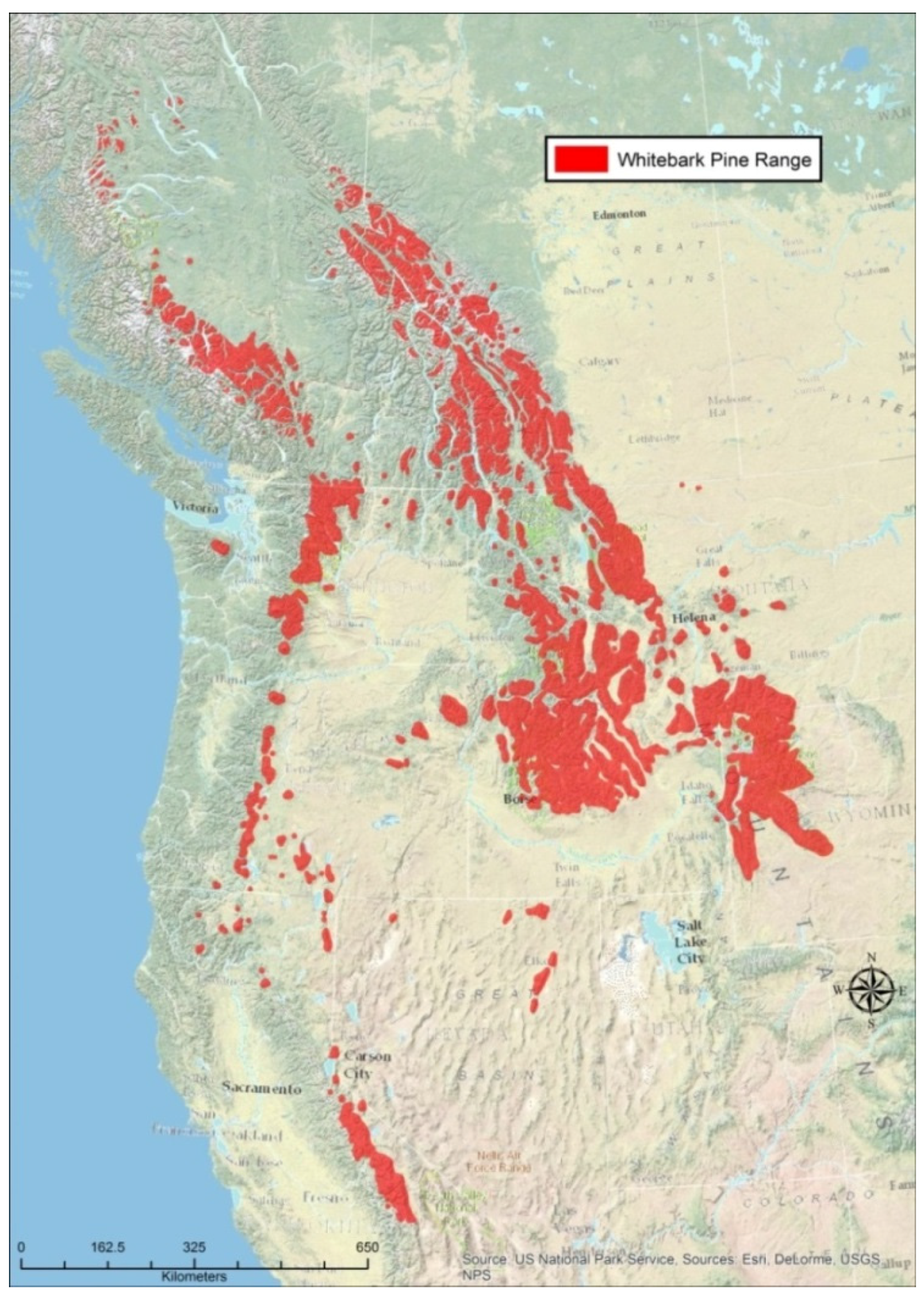
- Whitebark Pine Ecosystem Foundation. Rangewide Map for Whitebark Pine. Whitebark Pine Ecosystem Foundation: 2015. Available online: http://Whitebarkfound.org/wp-content/uploads/2014/05/whitebark-pine-range-colour-2014.jpg (accessed on 10 August 2015).
- Tomback, D.F.; Arno, S.F.; Keane, R.E. The compelling case for management intervention. In Whitebark Pine Communities: Ecology and Restoration; Tomback, D.F., Arno, S.F., Keane, R.E., Eds.; Island Press: Washington, DC, USA, 2001; pp. 3–25. [Google Scholar]
- Dayton, P.K. Towards an Understanding of Community Resilience and the Potential Effects of Enrichments to the Benthos at McMurdo Sound, Antarctica. In Proceedings of the Colloquium on Conservation Problems in Antarctica; Parker, B.C., Ed.; Allen Press: Lawrence, KS, USA, 1972; pp. 81–96. [Google Scholar]
- Ellison, A.M.; Bank, M.S.; Clinton, B.D.; Colburn, E.A.; Elliott, K.; Ford, C.R.; Foster, D.R.; Kloeppel, B.D.; Knoepp, J.D.; Lovett, G.M.; et al. Loss of foundation species: Consequences for the structure and dynamics of forested ecosystems. Front. Ecol. Environ. 2005, 3, 479–486. [Google Scholar] [CrossRef]
- Mills, L.S.; Soulé, M.E.; Doak, D.F. The keystone-species concept in ecology and conservation. BioScience 1993, 43, 219–224. [Google Scholar] [CrossRef]
- Soulé, M.E.; Estes, J.A.; Berger, J.; Martinez del Rio, C. Ecological effectiveness: Conservation goals for interactive species. Conserv. Biol. 2003, 17, 1238–1250. [Google Scholar] [CrossRef]
- Costanza, R.; d’Arge, R.; de Groot, R.; Farber, S.; Grasso, M.; Hannon, B.; Limburg, K.; Naeem, S.; O’Neill, R.V.; Paruelo, J.; et al. The value of the world’s ecosystem services and natural capital. Nature 1997, 387, 253–260. [Google Scholar] [CrossRef]
- Millenium Ecosystem Assessment. Ecosystem and Human Well-being: Synthesis; Island Press: Washington, DC, USA, 2005. [Google Scholar]
- Farnes, P.E. SNOTEL and Snow Course Data: Describing the Hydrology of Whitebark Pine Ecosystems. In Proceedings—Symposium on Whitebark Pine Ecosystems: Ecology and Management of a High-Mountain Resource; Schmidt, W.C., McDonald, K.J., Eds.; General Technical Report INT-270; USDA Forest Service, Intermountain Research Station: Ogden, UT, USA, 1990; pp. 302–304. [Google Scholar]
- Callaway, R.M. Competition and facilitation on elevation gradients in subalpine forests of the northern Rocky Mountains, USA. Oikos 1998, 2, 561–573. [Google Scholar] [CrossRef]
- Tomback, D.F.; Kendall, K.C. Biodiversity losses: The downward spiral. In Whitebark Pine Communities: Ecology and Restoration; Tomback, D.F., Arno, S.F., Keane, R.E., Eds.; Island Press: Washington, DC, USA, 2001; pp. 243–262. [Google Scholar]
- Resler, L.M.; Tomback, D.F. Blister rust prevalence in krummholz whitebark pine: Implications for treeline dynamics. Arct. Antarct. Alp. Res. 2008, 40, 161–170. [Google Scholar] [CrossRef]
- Moerman, D.E. Native American Ethnobotany; Timber Press: Portland, OR, USA, 1998. [Google Scholar]
- Moerman, D.E. Native American Medicinal Plants: An Ethnobotany dictionary; Timber Press: Portland, OR, USA, 2009. [Google Scholar]
- Marsh, P.; Quinton, B.; Pomeroy, J. Hydrological Processes and Runoff at the Arctic Treeline in Northwestern Canada. In Proceedings of the Tenth International Northern Research Basins Symposium and Workshop, Norway, 1994; Sand, K., Killingtveit, A., Eds.; SINTF: Trondheim, Norway, 1995; pp. 368–397. [Google Scholar]
- Hiemstra, C.A.; Liston, G.E.; Reiners, W.A. Snow redistribution by wind and interactions with vegetation at upper treeline in the Medicine Bow Mountains, Wyoming, USA. Arct. Antarct. Alp. Res. 2002, 34, 262–273. [Google Scholar] [CrossRef]
- Barnett, T.P.; Adam, J.C.; Lettenmaier, D.P. Potential impacts of a warming climate on water availability in snow-dominated regions. Nature 2005, 438, 303–309. [Google Scholar] [CrossRef] [PubMed]
- Viviroli, D.; Dürr, H.H.; Messerli, B.; Meybeck, M. Mountains of the world, water towers for humanity, typology, mapping, and global significance. Water Resour. Res. 2007, 43, W07447. [Google Scholar] [CrossRef]
- Tomback, D.F.; Chipman, K.G.; Resler, L.M.; Smith-McKenna, E.K.; Smith, C.M. Relative abundance and functional role of whitebark pine at treeline in the northern Rocky Mountains. Arct. Antarct. Alp. Res. 2014, 46, 407–418. [Google Scholar] [CrossRef]
- Blakeslee, S.C. Assessing Whitebark Pine Vigor and Facilitation Roles in the Alpine Treeline Ecotone; Masters of Science, University of Colorado Denver: Denver, CO, USA, 2012. [Google Scholar]
- Pyatt, J.C.; Tomback, D.F.; Blakeslee, S.C.; Wunder, M.B.; Resler, L.M.; Boggs, L.A.; Bevency, H.D. The importance of conifers for facilitation at treeline: Comparing biophysical characteristics of leeward microsites in whitebark pine communities. Arct. Antarct. Alp. Res. 2016. in review. [Google Scholar]
- Smith, E.K.; Resler, L.M.; Vance, E.A.; Carstensen, L.W., Jr.; Kolivras, K.N. Blister rust incidence in treeline whitebark pine, Glacier National Park, USA: Environmental and topographic influences. Arct. Antarct. Alp. Res. 2011, 43, 107–117. [Google Scholar] [CrossRef]
- Smith-McKenna, E.K.; Resler, L.M.; Tomback, D.F.; Zhang, H.; Malanson, G.P. Topographic influences on the distribution of white pine blister rust in Pinus albicaulis treeline communities. Écoscience 2013, 20, 215–229. [Google Scholar] [CrossRef]
- R Core Team. R: A language and environment for statistical computing. R Foundation for Statistical Computing: Vienna, Austria, 2015; Version 3.22; Available online: https://www.R-project.org/ (accessed on 2 October 2015).
- Tomback, D.F.; Resler, L.M. Structure and composition of Rocky Mountain treeline whitebark pine communities at their northern boundary. University of Colorado Denver: Denver, CO, USA, 2015; in preparation. [Google Scholar]
- Resler, L.M.; Shao, Y.; Tomback, D.F.; Malanson, G.P. Predicting the functional role and occurrence of whitebark pine (Pinus albicaulis) at alpine treeline: Model accuracy and variable importance. Ann. Assoc. Am. Geogr. 2014. [Google Scholar] [CrossRef]
- Resler, L.M; Tomback, D.F.; Malanson, G.P. Treeline community structure and composition on Parker Ridge, Banff National Park. Virginia Tech: Blacksburg, VA, USA, unpublished data; 2015. [Google Scholar]
- Wagner, A.C.; Tomback, D.F.; Pansing, E.R. Structure and composition of treeline communities at whitebark pine’s (Pinus albicaulis) southern distributional limit in the Rocky Mountains. University of Colorado: Denver, CO, USA, 2015; in preparation. [Google Scholar]
- Resler, L.M.; Shao, Y. Assessing whitebark pine treeline communities and blister rust infection in Grand Teton National Park. Virginia Tech: Blacksburg, VA, USA, unpublished data; 2015. [Google Scholar]
- Arno, S.F.; Hammerly, R.P. Timberline: Mountain and Arctic Forest Frontiers; Timber Press: Portland, OR, USA, 1984. [Google Scholar]
- Finklin, A. A Climate handbook for Glacier National Park—With data for Waterton Lakes National Park; General Technical Report GTR INT-204; USDA Forest Service, Intermountain Research Station: Ogden, UT, USA, 1986. [Google Scholar]
- Mellor, M. Blowing snow. USA Cold Reg. Res. Eng. Lab. Monogr. 1965, III-A3c, 1–52. [Google Scholar]
- Finney, E.A. Snow control on the highways. In Michigan Engineering Experimental Station, Bulletin; Michigan State College: Lansing, MI, USA, 1934; Volume 75, pp. 1–81. [Google Scholar]
- Calkins, D.J. Simulated snowdrift patterns. USA Cold Reg. Res. Eng. Lab. Spec. Rep. 1975, 219, 1–16. [Google Scholar]
- Kind, R.J. Snow Drifting. In Handbook of Snow; Gray, D.M., Male, D.H., Eds.; Pergamon Press: New York, NY, USA, 1981; pp. 338–358. [Google Scholar]
- Daly, C. Snow distribution patterns in the alpine krummholz zone. Prog. Phys. Geogr. 1984, 8, 157–175. [Google Scholar] [CrossRef]
- Allen, J.R.L. Current Ripples; North Holland Publishing Company: Amsterdam, Netherlands, 1968. [Google Scholar]
- Kobayashi, D. Studies of snow transport in low-level drifting snow. Inst. Low Temp. Sci. Rep. 1972, 231, 1–58. [Google Scholar]
- Sturges, D.L. Snow fencing to increase streamflow: Preliminary results. West. Snow Confer. Proc. 1986, 54, 18–28. Available online: http://www.westernsnowconference.org/sites/westernsnowconference.org/PDFs/1986Sturges.pdf (accessed on 21 December 2015). [Google Scholar]
- Geddes, C.A.; Brown, D.G.; Fagre, D.B. Topography and vegetation as predictors of snow water equivalent across the Alpine Treeline Ecotone at Lee Ridge, Glacier National Park, Montana, USA. Arct. Alp. Res. 2005, 37, 19–205. [Google Scholar]
- Vajda, A.; Venäläinen, A.; Hänninen, P.; Sutinen, R. Effect of vegetation on snow cover at the northern timberline: A case study in Finnish Lapland. Silva Fenn. 2006, 40, 195–207. [Google Scholar] [CrossRef]
- Liptzin, D.; Seastedt, T. Patterns of snow, deposition, and soil nutrients at multiple spatial scales at a Rocky Mountain tree line ecotone. J. Geophys. Res. 2009, 114, G04002. [Google Scholar] [CrossRef]
- Jonas, T.; Marty, C.; Magnusson, J. Estimating the snow water equivalent from snow depth measurements in the Swiss Alps. J. Hydrol. 2009, 378, 161–167. [Google Scholar] [CrossRef]
- Anderton, S.P.; White, S.M.; Alvera, B. Evaluation of spatial variability in snow water equivalent for a high mountain catchment. Hydrol. Process. 2004, 19, 435–453. [Google Scholar] [CrossRef]
- Pomeroy, J.W.; Brun, E. Physical Properties of Snow. In Snow Ecology: An Interdisciplinary Examination of Snow-Covered Ecosystems; Jones, H.G., Pomeroy, J.W., Walker, D.A., Hoham, R.W., Eds.; Cambria University Press: Cambridge, UK, 2001; pp. 45–126. [Google Scholar]
- Tyler, S.; Burak, S.A.; McNamara, J.P.; Lamontagne, A.; Selker, J.S.; Dozier, J. Spatially distributed temperatures at the base of two mountain snowpacks measured with fiber-optic sensors. J. Glaciol. 2008, 54, 673–679. [Google Scholar] [CrossRef] [Green Version]
- Billings, W.D.; Bliss, L.C. An alpine snowbank environment and its effects on vegetation, plant development, and productivity. Ecology 1959, 40, 388–397. [Google Scholar] [CrossRef]
- Billings, W.D. Vegetational pattern near alpine timberline as affected by fire-snowdrift interactions. Vegetatio 1969, 19, 192–207. [Google Scholar] [CrossRef]
- Walker, D.A.; Halfpenny, J.C.; Walker, M.D.; Wessman, C.A. Long-term studies of snow-vegetation interactions. BioScience 1993, 43, 287–301. [Google Scholar] [CrossRef]
- Odland, A.; Munkejord, H.K. Plants as indicators of snow layer duration in southern Norwegian mountains. Ecol. Indic. 2008, 8, 57–68. [Google Scholar] [CrossRef]
- Fahnestock, J.; Povrik, K.; Welker, J. Ecological significance of litter redistribution by wind and snow in arctic landscapes. Ecography 2008, 23, 623–631. [Google Scholar] [CrossRef]
- Nardi, A.; Bowman, W. Hot spots of inorganic nitrogen availability in an alpine-subalpine ecosystem, Colorado Front Range. Ecosystems 2011, 14, 848–863. [Google Scholar] [CrossRef]
- Tomback, D.F.; Resler, L.M. Invasive pathogens at alpine treeline: Consequences for treeline dynamics. Phys. Geogr. 2007, 28, 397–418. [Google Scholar] [CrossRef]
- Hiemstra, C.A.; Liston, G.E.; Reiners, W.A. Observing, modelling, and validating snow redistribution by wind in a Wyoming upper treeline landscape. Ecol. Model. 2006, 197, 35–51. [Google Scholar] [CrossRef]
- Smith, C.M.; Wilson, B.; Rasheed, S.; Walker, R.C.; Carolin, T.; Shepherd, R. Whitebark pine and white pine blister rust in the Rocky Mountains of Canada and northern Montana. Can. J. For. Res. 2008, 38, 982–985. [Google Scholar] [CrossRef]
- Keane, R.E.; Tomback, D.F.; Aubry, C.A.; Bower, A.D.; Campbell, E.M.; Cripps, C.L.; Jenkins, M.B.; Mahalovich, M.F.; Manning, M.; McKinney, S.T.; et al. A Range-Wide Restoration Strategy for Whitebark Pine (Pinus albicaulis); General Technical Report RMRS-GTR-279; USDA Forest Service: Fort Collins, CO, USA, 2012. [Google Scholar]
- GYWPMWG (Greater Yellowstone Whitebark Pine Monitoring Working Group). Monitoring Whitebark Pine in the Greater Yellowstone Ecosystem; 2012 Annual Report, Natural Resource Data Series NPS/GRYN/NRDS—2013/498; Chambers, N., Ed.; U.S. Department of the Interior, National Park Service, Natural Resource Stewardship and Science: Fort Collins, CO, USA, 2013. [Google Scholar]
- Smith, C.M.; Shepherd, B.; Gillies, C.; Stuart-Smith, J. Changes in blister rust infection and mortality in whitebark pine over time. Can. J. For. Res. 2013, 43, 90–96. [Google Scholar] [CrossRef]
- McDonald, G.I.; Hoff, R.J. Blister rust: An introduced plague. In Whitebark Pine Communities: Ecology and Restoration; Tomback, D.F., Arno, S.F., Keane, R.E., Eds.; Island Press: Washington, DC, USA, 2001; pp. 193–220. [Google Scholar]
- Geils, B.W.; Hummer, K.E.; Hunt, R.S. White pines, Ribes, and blister rust: A review and synthesis. For. Pathol. 2010, 40, 147–185. [Google Scholar] [CrossRef]
- Tomback, D.F. Post-fire regeneration of krummholz whitebark pine: A consequence of nutcracker seed caching. Madroño 1986, 33, 100–110. [Google Scholar]
- McKinney, S.T.; Fiedler, C.E.; Tomback, D.F. Invasive pathogen threatens bird-pine mutualism: Implications for sustaining a high-elevation ecosystem. Ecol. Appl. 2009, 19, 597–607. [Google Scholar] [CrossRef] [PubMed]
- Barringer, L.; Tomback, D.F.; Wunder, M.B.; McKinney, S.T. Whitebark pine stand condition, tree abundance, and cone production as predictors of visitation by Clark’s Nutcracker. PLoS ONE 2012, 7, e37663. [Google Scholar] [CrossRef] [PubMed]
- Malanson, G.P.; Butler, D.R.; Fagre, D.B.; Walsh, S.J.; Tomback, D.F.; Daniels, L.D.; Resler, L.M.; Smith, W.K.; Weiss, D.J.; Peterson, D.L.; et al. Alpine treeline of western North America: Linking organism-to-landscape dynamics. Phys. Geogr. 2007, 28, 378–396. [Google Scholar] [CrossRef]
- Smith-McKenna, E.K.; Malanson, G.P.; Resler, L.M.; Carstensen, L.W.; Prisley, S.P.; Tomback, D.F. Cascading effects of feedbacks, disease, and climate change on alpine treeline dynamics. Environ. Model. Softw. 2014, 62, 85–96. [Google Scholar] [CrossRef]
- Primack, R.B. Essentials of Conservation Biology, 6th ed.; Sinauer: Sunderland, MA, USA, 2014. [Google Scholar]
- Environment Canada. Recovery Strategy for Whitebark Pine (Pinus albicaulis) in Canada [Draft]. In Species at Risk Act Recovery Strategy Series; Environment Canada: Ottawa, ON, Canada, 2015. [Google Scholar]
- Keane, R.E.; Holsinger, L.; Mahalovich, M.F.; Tomback, D.F. Restoring Whitebark Pine in the Face of Climate Change; General Technical Report RMRS-GTR-XXX; USDA Forest Service, Rocky Mountain Research Station: Fort Collins, CO, USA, 2016; in press. [Google Scholar]
- Keane, R.E.; Parsons, R. Restoring whitebark pine forests of the Northern Rocky Mountains, USA. Ecol. Restor. 2010, 28, 56–70. [Google Scholar] [CrossRef]
- Pansing, E.R.; Tomback, D.F.; Wunder, M.B.; French, J.P.; Wagner, A.C. Simulated seed dispersal reveals geographic and community-based patterns of seed pilferage, germination, and seedling survival in whitebark pine (Pinus albicaulis). Oecologia 2016, submitted. [Google Scholar]
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tomback, D.F.; Resler, L.M.; Keane, R.E.; Pansing, E.R.; Andrade, A.J.; Wagner, A.C. Community Structure, Biodiversity, and Ecosystem Services in Treeline Whitebark Pine Communities: Potential Impacts from a Non-Native Pathogen. Forests 2016, 7, 21. https://doi.org/10.3390/f7010021
Tomback DF, Resler LM, Keane RE, Pansing ER, Andrade AJ, Wagner AC. Community Structure, Biodiversity, and Ecosystem Services in Treeline Whitebark Pine Communities: Potential Impacts from a Non-Native Pathogen. Forests. 2016; 7(1):21. https://doi.org/10.3390/f7010021
Chicago/Turabian StyleTomback, Diana F., Lynn M. Resler, Robert E. Keane, Elizabeth R. Pansing, Andrew J. Andrade, and Aaron C. Wagner. 2016. "Community Structure, Biodiversity, and Ecosystem Services in Treeline Whitebark Pine Communities: Potential Impacts from a Non-Native Pathogen" Forests 7, no. 1: 21. https://doi.org/10.3390/f7010021







