Propagation of Native Tree Species to Restore Subtropical Evergreen Broad-Leaved Forests in SW China
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Site
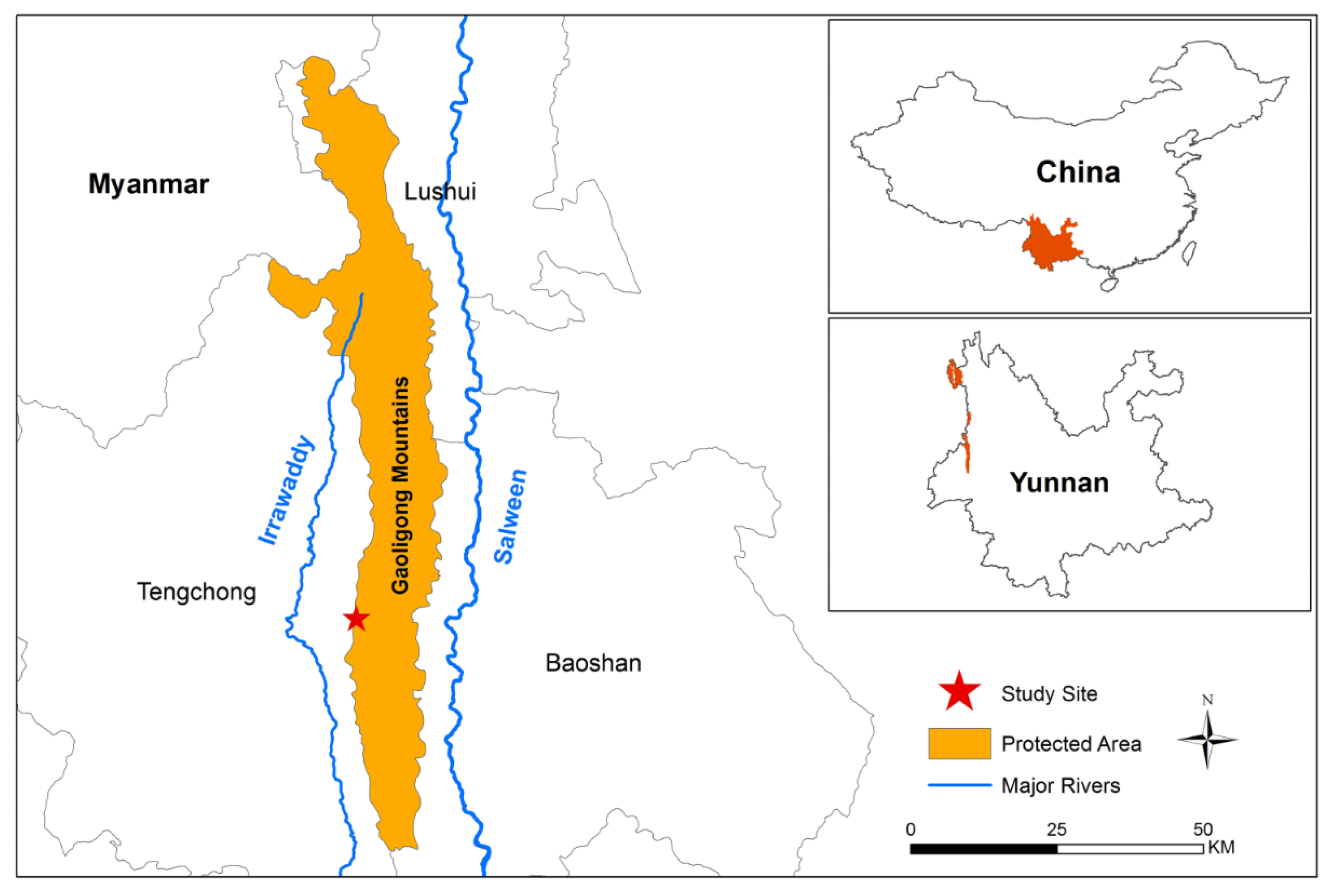
2.2. Species Selection
| Species | Family | Ecology † | Uses † | SCM † |
|---|---|---|---|---|
| Lindera communis Hemsley | Lauraceae | ESC | TB, OL, FW | Nov |
| Lindera thomsonii C. K. Allen | Lauraceae | LSC | TB, OL | Oct |
| Machilus rufipes H. W. Li | Lauraceae | LC | TB | Oct |
| Machilus yunnanensis Lecomte | Lauraceae | LC | TB | Oct |
| Cerasus serrulata Loudon | Rosaceae | ESC | FR, OR | May |
| Cerasus cerasoides S. Y. Sokolov | Rosaceae | ESC | OR | Mar |
| Sorbus corymbifera N. T. Kh’ep & G. P. Yakovlev | Rosaceae | ESC | FW | Sep |
| Padus wilsonii C. K. Schneider | Rosaceae | LSC | TB | Oct |
| Manglietia hookeri Cubitt & W. W. Smith | Magnoliaceae | LC | TB, OR | Oct |
| Michelia doltsopa Buchanan-Hamilton ex Candolle | Magnoliaceae | LSC | TB, OR | Oct |
| Quercus acutissima Carruthers | Fagaceae | EC | TB, FW, FD | Oct |
| Schima wallichii Korthals | Theaceae | LC | TB | Dec |
| Ilex polyneura S. Y. Hu | Aquifoliaceae | LC | TB | Nov |
| Myrsine semiserrata Wallich | Myrsinaceae | ESC | FW | Oct |
| Diospyros kaki var. silvestris Makino | Ebenaceae | ESC | TB, FR | Nov |
| Tetradium ruticarpum T. G. Hartley | Rutaceae | LSC | MD | Nov |
| Heynea trijuga Roxburgh | Meliaceae | LSC | MD | Nov |
| Betula alnoides Buchanan-Hamilton ex D. Don | Betulaceae | EC | TB | Mar |
| Alnus nepalensis D. Don | Betulaceae | EC | TB, FW | Dec |
| Choerospondias axillaris B. L. Burtt & A. W. Hill | Anacardiaceae | LC | TB, FR | Dec |
| Ligustrum lucidum W. T. Aiton | Oleaceae | ESC | OR, MD | Nov |
2.3. Seed Collection
2.4. Species Germination Performance and Seed Storage Experiments
2.5. Seedling Growth
2.6. Seedling Early Survival
2.7. Species Rating System
| Aspect | Categories | Score |
|---|---|---|
| Germination rate | >80% | 3 |
| 50%–80% | 2 | |
| <50% | 1 | |
| Germination time (MLG) | <30 days | 3 |
| 30–90 days | 2 | |
| >90 days | 1 | |
| Seedling height when planting | >30 cm | 3 |
| 15–30 cm | 2 | |
| <15 cm | 1 | |
| Seedling early survival rate | >80% | 3 |
| 50%–80% | 2 | |
| <50% | 1 | |
| Species rating | Excellent | 10–12 |
| Good | 7–9 | |
| Poor | 4–6 |
3. Results
3.1. Species Germination Performance
| Species | GR (%) | MLG (Days) | TD (Days) | SH (cm) | SS (%) | Rating |
|---|---|---|---|---|---|---|
| Lindera communis | 52 (11.7) | 102 | 202 | 19.9 (3.6) | 91.8 | G |
| Lindera thomsonii | 78 (7.4) | 111 | 203 | 20.5 (3.9) | 89.9 | G |
| Machilus rufipes | 96 (10.6) | 94 | 204 | 26.4 (4.4) | 71.8 | G |
| Machilus yunnanensis | 85 (16.0) | 84 | 205 | 18.7 (3.1) | 83.6 | E |
| Cerasus serrulata | 95 (3.1) | 30 | 81 | 28.1 (5.5) | 92.3 | E |
| Cerasus cerasoides | 85 (10.2) | 45 | 122 | 58.9 (6.3) | 86.8 | E |
| Sorbus corymbifera | 96 (5.9) | 46 | 203 | 40.3 (8.8) | 93.8 | E |
| Padus wilsonii | 81 (3.5) | 24 | 204 | 44.7 (11.4) | 70.8 | E |
| Manglietia hookeri | 70 (26.9) | 84 | 220 | 9.9 (3.6) | 39.2 | P |
| Michelia doltsopa | 90 (6.3) | 87 | 218 | 25.1 (4.3) | 90.7 | E |
| Quercus acutissima | 88 (5.2) | 47 | 173 | 46.0 (5.3) | 90.2 | E |
| Schima wallichii | 69 (4.1) | 46 | 134 | 9.4 (2.1) | 35.8 | P |
| Ilex polyneura | 45 (3.4) | 144 | 216 | 4.4 (1.5) | 77.8 | P |
| Myrsine semiserrata | 91 (4.1) | 118 | 219 | 4.9 (1.4) | 36.4 | P |
| Diospyros kaki var. silvestris | 73 (3.3) | 75 | 206 | 31.5 (5.0) | 96.5 | E |
| Tetradium ruticarpum | 75 (1.7) | 32 | 203 | 66.4 (13.1) | 52.5 | G |
| Heynea trijuga | 87 (2.6) | 83 | 217 | 15.5 (3.6) | 89.7 | E |
| Betula alnoides | 54 (3.3) | 41 | 123 | 9.1 (3.2) | 58.3 | G |
| Alnus nepalensis | 48 (21.2) | 82 | 198 | 14.0 (6.2) | 94.3 | G |
| Choerospondias axillaris | 68 (17.5) | 71 | 213 | 81.7 (21.9) | 54.9 | G |
| Ligustrum lucidum | 61 (4.4) | 74 | 201 | 50.7 (7.5) | 92.7 | E |
3.2. Seed Storage
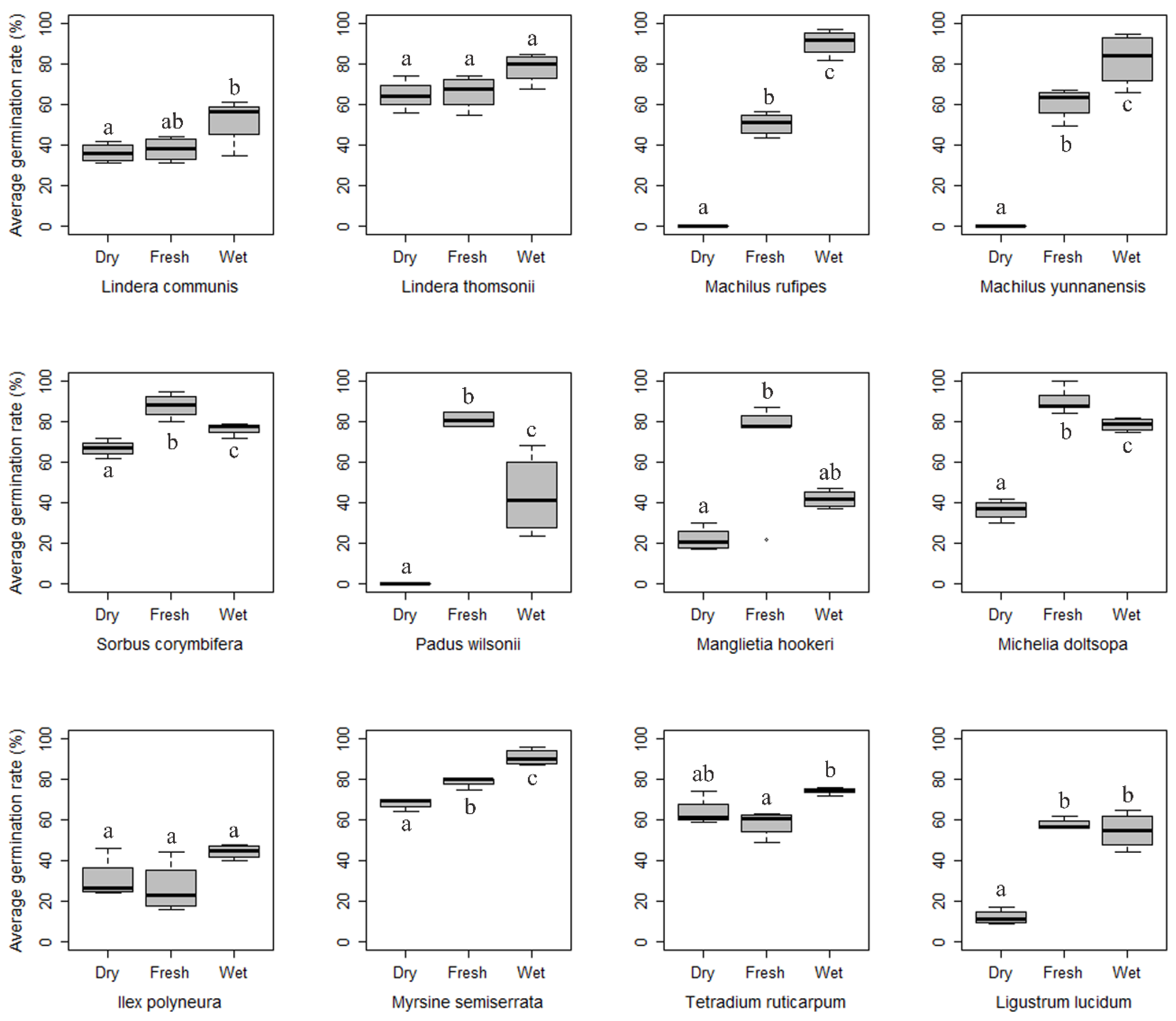
3.3. Seedling Growth Performance
3.4. Seedling Early Survival
3.5. Species Performance
4. Discussion
4.1. Species Germination Performance
4.2. Seed Storage Method
4.3. Seedling Growth Performance
4.4. Seedling Early Survival
4.5. Species Selection Criteria and Species Selected
5. Concluding Remarks
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Li, W.H. Degradation and restoration of forest ecosystems in China. For. Ecol. Manag. 2004, 201, 33–41. [Google Scholar]
- Liu, J.; Li, S.; Ouyang, Z.; Tam, C.; Chen, X. Ecological and socioeconomic effects of China’s policies for ecosystem services. Proc. Natl. Acad. Sci. USA 2008, 105, 9477–9482. [Google Scholar] [CrossRef] [PubMed]
- Stone, R. Nursing China’s ailing forests back to health. Science 2009, 325, 556–558. [Google Scholar] [CrossRef] [PubMed]
- Xu, J. China’s new forests aren’t as green as they seem. Nature 2011, 477, 371. [Google Scholar] [CrossRef] [PubMed]
- Zhai, D.-L.; Xu, J.-C.; Dai, Z.-C.; Cannon, C.H.; Grumbine, R. Increasing tree cover while losing diverse natural forests in tropical Hainan, China. Reg. Environ. Chang. 2014, 14, 611–621. [Google Scholar] [CrossRef]
- Cao, S.; Sun, G.; Zhang, Z.; Chen, L.; Feng, Q.; Fu, B.; McNulty, S.; Shankman, D.; Tang, J.; Wang, Y. Greening China naturally. Ambio 2011, 40, 828–831. [Google Scholar] [CrossRef] [PubMed]
- McNamara, S.; Tinh, D.V.; Erskine, P.D.; Lamb, D.; Yates, D.; Brown, S. Rehabilitating degraded forest land in central Vietnam with mixed native species plantings. For. Ecol. Manag. 2006, 233, 358–365. [Google Scholar] [CrossRef]
- Shono, K.; Cadaweng, E.A.; Durst, P.B. Application of assisted natural regeneration to restore degraded tropical forestlands. Restor. Ecol. 2007, 15, 620–626. [Google Scholar] [CrossRef]
- Raman, T.; Mudappa, D.; Kapoor, V. Restoring rainforest fragments: Survival of mixed-native species seedlings under contrasting site conditions in the western Ghats, India. Restor. Ecol. 2009, 17, 137–147. [Google Scholar] [CrossRef]
- Elliott, S.; Kuarak, C.; Navakitbumrung, P.; Zangkum, S.; Anusarnsunthorn, V.; Blakesley, D. Propagating framework trees to restore seasonally dry tropical forest in northern Thailand. New For. 2002, 23, 63–70. [Google Scholar] [CrossRef]
- Meli, P.; Martínez-Ramos, M.; Rey-Benayas, J.M.; Carabias, J. Combining ecological, social and technical criteria to select species for forest restoration. Appl. Veg. Sci. 2014, 17, 744–753. [Google Scholar] [CrossRef] [Green Version]
- Lamb, D. Ecological restoration. In Regreening the Bare Hills: Tropical Forest Restoration in the Asia-Pacific Region; Lamb, D., Ed.; Springer: New York, NY, USA, 2011; Volume 8, pp. 325–358. [Google Scholar]
- Shono, K.; Davies, S.J.; Chua, Y. Performance of 45 native tree species on degraded lands in Singapore. J. Trop. For. Sci. 2007, 19, 25–34. [Google Scholar]
- Doust, S.J.; Erskine, P.D.; Lamb, D. Restoring rainforest species by direct seeding: Tree seedling establishment and growth performance on degraded land in the wet tropics of Australia. For. Ecol. Manag. 2008, 256, 1178–1188. [Google Scholar] [CrossRef]
- Blakesley, D.; Hardwick, K.; Elliott, S. Research needs for restoring tropical forests in Southeast Asia for wildlife conservation: Framework species selection and seed propagation. New For. 2002, 24, 165–174. [Google Scholar] [CrossRef]
- Wydhayagarn, C.; Elliott, S.; Wangpakapattanawong, P. Bird communities and seedling recruitment in restoring seasonally dry forest using the framework species method in northern Thailand. New For. 2009, 38, 81–97. [Google Scholar] [CrossRef]
- Padilla, F.M.; Pugnaire, F.I. The role of nurse plants in the restoration of degraded environments. Front. Ecol. Environ. 2006, 4, 196–202. [Google Scholar] [CrossRef]
- Blakesley, D.; Elliott, S.; Kuarak, C.; Navakitbumrung, P.; Zangkum, S.; Anusarnsunthorn, V. Propagating framework tree species to restore seasonally dry tropical forest: Implications of seasonal seed dispersal and dormancy. For. Ecol. Manag. 2002, 164, 31–38. [Google Scholar] [CrossRef]
- Knowles, O.H.; Parrotta, J.A. Amazonian forest restoration: An innovative system for native species selection based on phenological data and field performance indices. Commonw. For. Rev. 1995, 230–243. [Google Scholar]
- Broadhurst, L.M.; Lowe, A.; Coates, D.J.; Cunningham, S.A.; McDonald, M.; Vesk, P.A.; Yates, C. Seed supply for broadscale restoration: Maximizing evolutionary potential. Evol. Appl. 2008, 1, 587–597. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Yang, H.; Jamnadass, R.; Xu, J.; Yang, Y. Decentralization of tree seedling supply systems for afforestation in the west of Yunnan Province, China. Small-Scale For. 2012, 11, 147–166. [Google Scholar] [CrossRef]
- Ciccarese, L.; Mattsson, A.; Pettenella, D. Ecosystem services from forest restoration: Thinking ahead. New For. 2012, 43, 543–560. [Google Scholar] [CrossRef]
- Wang, X.-H.; Kent, M.; Fang, X.-F. Evergreen broad-leaved forest in eastern China: Its ecology and conservation and the importance of resprouting in forest restoration. For. Ecol. Manag. 2007, 245, 76–87. [Google Scholar] [CrossRef]
- Li, X.-S.; Liu, W.-Y.; Chen, J.-W.; Tang, C.Q.; Yuan, C.-M. Regeneration pattern of primary forest species across forest-field gradients in the subtropical mountains of Southwestern China. J. Plant Res. 2010, 123, 751–762. [Google Scholar] [CrossRef] [PubMed]
- Tang, C.Q. Subtropical montane evergreen broad-leaved forests of Yunnan, China: Diversity, succession dynamics, human influence. Front. Earth Sci. China 2010, 4, 22–32. [Google Scholar] [CrossRef]
- Ranjitkar, S.; Sujakhu, N.M.; Lu, Y.; Wang, Q.; Wang, M.; He, J.; Mortimer, P.E.; Xu, J.; Kindt, R.; Zomer, R.J. Climate modelling for agroforestry species selection in Yunnan Province, China. Environ. Model. Softw. 2016, 75, 263–272. [Google Scholar] [CrossRef]
- Li, X.; Liu, W.; Tang, C.Q. The role of the soil seed and seedling bank in the regeneration of diverse plant communities in the subtropical Ailao Mountains, Southwest China. Ecol. Res. 2010, 25, 1171–1182. [Google Scholar] [CrossRef]
- Yang, Q.-H.; Wei, X.; Zeng, X.-L.; Ye, W.-H.; Yin, X.-J.; Zhang-Ming, W.; Jiang, Y.-S. Seed biology and germination ecophysiology of Camellia nitidissima. For. Ecol. Manag. 2008, 255, 113–118. [Google Scholar] [CrossRef]
- Zheng, Y.; Sun, W.; Zhou, Y.; Coombs, D. Variation in seed and seedling traits among natural populations of Trigonobalanus doichangensis (A. Camus) Forman (Fagaceae), a rare and endangered plant in southwest China. New For. 2009, 37, 285–294. [Google Scholar] [CrossRef]
- Weyerhaeuser, H.; Wilkes, A.; Kahrl, F. Local impacts and responses to regional forest conservation and rehabilitation programs in China’s northwest Yunnan province. Agric. Syst. 2005, 85, 234–253. [Google Scholar] [CrossRef]
- Yang, X.; Bauhus, J.; Both, S.; Fang, T.; Haerdtle, W.; Kroeber, W.; Ma, K.; Nadrowski, K.; Pei, K.; Scherer-Lorenzen, M.; et al. Establishment success in a forest biodiversity and ecosystem functioning experiment in subtropical China (BEF-China). Eur. J. For. Res. 2013, 132, 593–606. [Google Scholar] [CrossRef] [Green Version]
- Khurana, E.; Singh, J. Ecology of seed and seedling growth for conservation and restoration of tropical dry forest: A review. Environ. Conserv. 2001, 28, 39–52. [Google Scholar] [CrossRef]
- Smith, N.; Zahid, D.M.; Ashwath, N.; Midmore, D.J. Seed ecology and successional status of 27 tropical rainforest cabinet timber species from Queensland. For. Ecol. Manag. 2008, 256, 1031–1038. [Google Scholar] [CrossRef]
- Kindt, R.; Barnekow-Lillesø, J.; Mbora, A.; Muriuki, J.; Carsan, S.; Wambugu, C.; Beniest, J.; Aithal, A.; Awimbo, J.; Rao, S. Tree Seeds for Farmers: A Toolkit and Reference Source; World Agroforestry Centre: Nairobi, Kenya, 2006. [Google Scholar]
- Ranjitkar, S.; Luedeling, E.; Shrestha, K.K.; Guan, K.; Xu, J. Flowering phenology of tree rhododendron along an elevation gradient in two sites in the Eastern Himalayas. Int. J. Biometeorol. 2013, 57, 225–240. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Guo, H.; Dao, Z. Flora of Gaoligong Mountains; Science Press: Beijing, China, 2000. [Google Scholar]
- Myers, N.; Mittermeier, R.A.; Mittermeier, C.G.; da Fonseca, G.A.B.; Kent, J. Biodiversity hotspots for conservation priorities. Nature 2000, 403, 853–858. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.Y.; Raven, P.H.; Hong, D.Y. Flora of China (Vol. 1–25); Science Press: Beijing, China; Missouri Botanical Garden Press: St. Louis, MO, USA, 2014; Available online: http://www.efloras.org (accessed on 10 February 2014).
- Du, Y.; Mi, X.; Liu, X.; Chen, L.; Ma, K. Seed dispersal phenology and dispersal syndromes in a subtropical broad-leaved forest of China. For. Ecol. Manag. 2009, 258, 1147–1152. [Google Scholar] [CrossRef]
- Shen, H. Seed collecting, processing, storage and transportation. In Nursery Stock Growing; Chinese forestry Press: Beijing, China, 2009. (In Chinese) [Google Scholar]
- Hagen, D. Propagation of native arctic and alpine species with a restoration potential. Polar Res. 2002, 21, 37–47. [Google Scholar] [CrossRef]
- Sautu, A.; Baskin, J.M.; Baskin, C.C.; Condit, R. Studies on the seed biology of 100 native species of trees in a seasonal moist tropical forest, Panama, Central America. For. Ecol. Manag. 2006, 234, 245–263. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R foundation for Statistical Computing: Vienna, Austria, 2012; Available online: http://www.R-project.org/ (accessed on 12 May 2015).
- Elliott, S.; Navakitbumrung, P.; Kuarak, C.; Zangkum, S.; Anusarnsunthorn, V.; Blakesley, D. Selecting framework tree species for restoring seasonally dry tropical forests in northern Thailand based on field performance. For. Ecol. Manag. 2003, 184, 177–191. [Google Scholar] [CrossRef]
- Tunjai, P.; Elliott, S. Effects of seed traits on the success of direct seeding for restoring southern Thailand’s lowland evergreen forest ecosystem. New For. 2012, 43, 319–333. [Google Scholar] [CrossRef]
- Jiang, X.L.; Kuang, X.L.; Sun, Y.Y. Seed germination trials of Michelia doltsopa. For. Sci. Technol. 2014, 39, 5–6. (In Chinese) [Google Scholar]
- Yao, G.N.; Wu, Y.X.; Bai, R.F.; Song, S.H.; Zheng, X.D.; Huang, C.Y. Effects on treatment methods of insect-killing and storage for Quercus acutissima seeds. Liaoning For. Sci. Technol. 2004, 6, 9–11, (In Chinese with English Abstract). [Google Scholar]
- Lang, S.R.; Gao, Y.C.; Zhao, H.; Wang, X.F.; Liu, X.P. Dormancy and germination characteristics of Lindera communis seed. J. Beijing For. Univ. 2011, 6, 124–129, (In Chinese with English Abstract). [Google Scholar]
- Chen, Y.B.; Li, G.Y.; Xu, J.M.; Yang, J.S.; Zhang, B.H.; Chen, X.L. The nursing and cultivation technique of Choerospondias axillaries. J. Anhui Agric. Sci. 2014, 42, 2975–2976, (In Chinese with English Abstract). [Google Scholar]
- Wang, Y.P.; Wang, C.W.; Yang, H.T.; He, J.P.; Guo, M.; He, Z.J.; He, J.W. Study on the seeds seedling-raising technology of winter cherry. Seed 2015, 3, 117–119. (In Chinese) [Google Scholar]
- Yang, B.; Chen, H.W.; Shi, F.Q.; Chen, W. Current studies on some aspects of Alnus nepalensis tree in China. J. West China For. Sci. 2011, 3, 86–89, (In Chinese with English Abstract). [Google Scholar]
- Baskin, C.C.; Baskin, J.M. Seeds: Ecology, Biogeography, and Evolution of Dormancy and Germination; Academic Press: San Diego, CA, USA, 2014. [Google Scholar]
- He, Y. Study on dormancy and germination of Ilex fargesii seed. J. Zhejiang For. Sci. Technol. 2008, 28, 63–65, (In Chinese with English Abstract). [Google Scholar]
- Baskin, C.C.; Baskin, J.M. Advances in understanding seed dormancy at the whole-seed level: An ecological, biogeographical and phylogenetic perspective. Acta Bot. Yunnanica 2008, 30, 279–294. [Google Scholar]
- Blakesley, D.; Anusarnsunthorn, V.; Kerby, J.; Navakitbumrung, P.; Kuarak, C.; Zangkum, S.; Hardwick, K.; Elliott, S. Nursery technology and tree species selection for restoring forest biodiversity in northern Thailand. In Forest Restoration for Wildlife Conservation; Elliott, S., Kerby, J., Eds.; International Tropical Timber Organisation and The Forest Restoration Research Unit, Chiang Mai University: Chiang Mai, Thailand, 2000; pp. 207–220. [Google Scholar]
- Wang, W.B.; Jing, Y.B.; Yang, D.J.; Wang, D.M.; Jiang, Y.D. Seed collection and treatment of 7 indigenous broad-leaved species in tropical Yunnan. J. West China For. Sci. 2008, 2, 17–20, (In Chinese with English Abstract). [Google Scholar]
- Jaenicke, H. Good Tree Nursery Practices: Practical Guidelines for Research Nurseries; World Agroforestry Centre: Nairobi, Kenya, 1999. [Google Scholar]
- Thomas, E.; Jalonen, R.; Loo, J.; Boshier, D.; Gallo, L.; Cavers, S.; Bordács, S.; Smith, P.; Bozzano, M. Genetic considerations in ecosystem restoration using native tree species. For. Ecol. Manag. 2014, 333, 66–75. [Google Scholar] [CrossRef]
- Nussbaum, R.; Anderson, J.; Spencer, T. Factors limiting the growth of indigenous tree seedlings planted on degraded rainforest soils in Sabah, Malaysia. For. Ecol. Manag. 1995, 74, 149–159. [Google Scholar] [CrossRef]
- Harris, J. Soil microbial communities and restoration ecology: Facilitators or followers? Science 2009, 325, 573–574. [Google Scholar] [CrossRef] [PubMed]
- Román-Dañobeytia, F.J.; Levy-Tacher, S.I.; Aronson, J.; Rodrigues, R.R.; Castellanos-Albores, J. Testing the performance of fourteen native tropical tree species in two abandoned pastures of the lacandon rainforest region of Chiapas, Mexico. Restor. Ecol. 2012, 20, 378–386. [Google Scholar] [CrossRef]
- Reubens, B.; Moeremans, C.; Poesen, J.; Nyssen, J.; Tewoldeberhan, S.; Franzel, S.; Deckers, J.; Orwa, C.; Muys, B. Tree species selection for land rehabilitation in Ethiopia: From fragmented knowledge to an integrated multi-criteria decision approach. Agrofor. Syst. 2011, 82, 303–330. [Google Scholar] [CrossRef] [Green Version]
- Cramer, J.M.; Mesquita, R.C.; Bruce Williamson, G. Forest fragmentation differentially affects seed dispersal of large and small-seeded tropical trees. Biol. Conserv. 2007, 137, 415–423. [Google Scholar] [CrossRef]
- Cole, R.J.; Holl, K.D.; Keene, C.; Zahawi, R.A. Direct seeding of late-successional trees to restore tropical montane forest. For. Ecol. Manag. 2011, 261, 1590–1597. [Google Scholar] [CrossRef]
- Suárez, A.; Williams-Linera, G.; Trejo, C.; Valdez-Hernández, J.I.; Cetina-Alcalá, V.M.; Vibrans, H. Local knowledge helps select species for forest restoration in a tropical dry forest of central Veracruz, Mexico. Agrofor. Syst. 2012, 85, 35–55. [Google Scholar] [CrossRef]
- Gregorio, N.; Herbohn, J.; Harrison, S.; Smith, C. A systems approach to improving the quality of tree seedlings for agroforestry, tree farming and reforestation in the philippines. Land Use Policy 2015, 47, 29–41. [Google Scholar] [CrossRef]
- Harrison, S.; Gregorio, N.; Herbohn, J. A critical overview of forestry seedling production policies and practices in relation to smallholder forestry in developing countries. Small-Scale For. 2008, 7, 207–223. [Google Scholar] [CrossRef]
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lu, Y.; Ranjitkar, S.; Xu, J.-C.; Ou, X.-K.; Zhou, Y.-Z.; Ye, J.-F.; Wu, X.-F.; Weyerhaeuser, H.; He, J. Propagation of Native Tree Species to Restore Subtropical Evergreen Broad-Leaved Forests in SW China. Forests 2016, 7, 12. https://doi.org/10.3390/f7010012
Lu Y, Ranjitkar S, Xu J-C, Ou X-K, Zhou Y-Z, Ye J-F, Wu X-F, Weyerhaeuser H, He J. Propagation of Native Tree Species to Restore Subtropical Evergreen Broad-Leaved Forests in SW China. Forests. 2016; 7(1):12. https://doi.org/10.3390/f7010012
Chicago/Turabian StyleLu, Yang, Sailesh Ranjitkar, Jian-Chu Xu, Xiao-Kun Ou, Ying-Zai Zhou, Jian-Fang Ye, Xun-Feng Wu, Horst Weyerhaeuser, and Jun He. 2016. "Propagation of Native Tree Species to Restore Subtropical Evergreen Broad-Leaved Forests in SW China" Forests 7, no. 1: 12. https://doi.org/10.3390/f7010012






